Published online Oct 21, 2011. doi: 10.3748/wjg.v17.i39.4389
Revised: May 6, 2011
Accepted: May 13, 2011
Published online: October 21, 2011
AIM: To investigate the anti-tumor effects of Paris chinensis dioscin (PCD) and mechanisms regarding cell cycle regulation and apoptosis in human gastric cancer SGC-7901 cells.
METHODS: Cell viability was analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay. Cell apoptosis was evaluated by flow cytometry and laser scanning confocal microscope (LSCM) using Annexin-V/propidium iodide (PI) staining, and the cell cycle was evaluated using PI staining with flow cytometry. Intracellular calcium ions were detected under fluorescence microscope. The expression of cell cycle and apoptosis-related proteins cyclin B1, CDK1, cytochrome C and caspase-3 was measured by immunohistochemical staining.
RESULTS: PCD had an anti-proliferation effect on human gastric cancer SGC-7901 cells in a dose- and time-dependent manner. After treatment of SGC-7901 cells with PCD, apoptosis appeared in SGC-7901 cells. Morphological changes typical of apoptosis were also observed with LSCM by Annexin V/PI staining, and the cell number of the G0/G1 phase was decreased, while the number of cells in the G2/M phase was increased. Cell cycle-related proteins, such as cyclin B1 and CDK1, were all down-regulated, but caspase-3 and cytochrome C were up-regulated. Moreover, intracellular calcium accumulation occurred in PCD-treated cells.
CONCLUSION: G2/M phase arrest and apoptosis induced by PCD are associated with the inhibition of CDK-activating kinase activity and the activation of Ca2+-related mitochondrion pathway in SGC-7901 cells.
-
Citation: Gao LL, Li FR, Jiao P, Yang MF, Zhou XJ, Si YH, Jiang WJ, Zheng TT.
Paris chinensis dioscin induces G2/M cell cycle arrest and apoptosis in human gastric cancer SGC-7901 cells. World J Gastroenterol 2011; 17(39): 4389-4395 - URL: https://www.wjgnet.com/1007-9327/full/v17/i39/4389.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i39.4389
Gastric cancer is the most common cause of death from cancer in China[1,2]. Recent evidence suggests that cell apoptosis is closely related to the occurrence, progress and metastasis of tumors[3-5]. The mechanisms of apoptosis in tumor cells is an important field of study for tumor treatment and molecular cancer biology[6].
The progression of cells through the cell cycle is tightly controlled by the sequential activation and inactivation of a family of serine-threonine kinases known as the cyclin-dependent kinases (CDKs). In particular, CDK1 controls progression from the S phase through G2 and into the M phase. Similarly, progression from the G1 to S phase is controlled sequentially by CDK4/6 and CDK2. CDK activity is regulated by binding to cyclin partners and the action of endogenous inhibitory peptides[7,8].
Loss of cell cycle control, leading to uncontrolled proliferation, is common in cancer. Therefore, the identification of potent and selective cyclin dependent kinase inhibitors is a priority for anti-cancer drug discovery.
Paris chinensis (Liliaceae) is distributed in many regions of the world, such as India, China, Vietnam, and Germany. As a traditional Chinese medicine, it grows wildly throughout South China and has been used mainly as a folk remedy for treatment of abscesses, throat swelling and pain, thanatophidia bites, contused wounds and convulsions[9] for centuries. It is also the major component of the famous Chinese patent medicine Yunnan Baiyao Powder and snake-bite therapeutics. It also has been used to treat liver cancer in China for many decades[10-12]. The active components of Paris chinensis are the saponin steroids polyphyllin D, dioscin, and balanitin 7. Among its three chemical constituents, polyphyllin D has been previously reported[13-15] to circumvent drug resistance and elicit apoptosis in HepG2 and R-HepG2 cells via mitochondrial damage. However, as there has been no documentation of the use of the other important steroid saponin dioscin in the treatment of cancer, its mechanisms in human gastric cancer cells remain unknown.
Therefore, the aim of the present study was to evaluate the effects of Paris chinensis dioscin(PCD)on human gastric cancer SGC-7901 cells and the signaling pathways involved in PCD-induced apoptosis.
PCD with a purity of 99% was purchased from Yuancheng Science and Technology Corporation (Wuhan, China). RPMI-1640 medium, 4-hydroxyethyl piperazine ethanesulfonic acid (HEPES), fetal calf serum and trypsogen were purchased from Gibco BRL Life Tech-nologies Inc. (Grand Island, New York, United States). 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT), penicillin, streptomycin and trypsin were purchased from Amresco Chemical Co. Ltd. (United States). Sodium dodecyl sul-fate-polyacrylamide gel electrophoresis (SDS-PAGE) reagents were purchased from Sigma (St. Louis, United States). The fluorescent probe Fluo-3/AM is a product of Molecular Probes Incorporated (United States). The Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was purchased from BD Biosciences (United States). The primary antibodies for cyclinB1, CDK1, caspase-3, cytochrome C and β-actin and the secondary antibody were acquired from Santa Cruz Biotechnology. Fetal bovine serum (FBS) was purchased from Hyclone (United States), and all chemicals were of analytical grade and were obtained from Tianjin Chemical Reagents Co. Ltd. (Tianjin, China).
SGC-7901 cells were obtained from the Chinese Type Culture Collection (Shanghai Institute of Cell Biology, Chinese Academy of Science, Shanghai, China). SGC-7901 cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37°C in a humidified atmosphere of 95% air and 5% CO2; the medium was changed every other day. When the cultures were 80%-90% confluent, the SGC-7901 cells were washed with phosphate-buffered saline (PBS), detached with 0.25% trypsin, centrifuged and re-plated onto 96- or 24-well plates at an appropriate density according to each experimental scale.
The cultured cells at the exponential growth phase were harvested from the culture flasks by trypsin and then resuspended in fresh medium. The cell suspensions were dispensed into a 96-well microplate at 100 μL/well and incubated in an incubator with 5% CO2 at 37°C. After 24 h, 200 μL of various concentrations (0-500 μg/mL) of PCD were added and incubated for 12, 24, 36, 48, 60 and 72 h to evaluate their anti-proliferation effects on SGC-7901. The cell proliferation in the microplate was determined using the MTT assay[16] after incubation. Twenty microliters of PBS solution containing 5 mg/mL MTT was added to each well. After incubation for 4 h, the cells from each well were solubilized with 100 μL DMSO for optical density determination at 570 nm. Cell proliferation activity was expressed as the percentage of MTT counts of treated cells relative to those of the control (% of control). The IC50 was taken as the concentration that caused 50% inhibition of cell viabilities and was calculated by the Logit method.
The SGC-7901 cells were seeded into six-well plates (2.0 × 105 cells/well) and incubated in RPMI-1640 at 37°C in an atmosphere of 5% CO2 for 24 h. The cells were treated with several concentrations of PCD. After incubation for 24 h, cellular morphology was observed under a phase contrast microscope (Nikon, Japan). The photographs were taken at a magnification × 40.
SGC-7901 cells (2 × 106 cells/mL) in 100-mm culture dishes were incubated with PCD for 24 h, then harvested by trypsinization and fixed with 90% ice-cold ethanol. The fixed cells were incubated with a staining solution containing 0.2% NP-40, RNase A (30 μg/mL), and propidium iodide (PI) (50 μg/mL) in a phosphate-citrate buffer (pH 7.2). Cellular DNA content was analyzed by flow cytometry (BD FACS Calibur, United States). At least 10 000 cells were used for each analysis, and the results were displayed as histograms.
SGC-7901 cells were cultured in RPMI-1640 with 10% fetal bovine serum. Before the cell density was modulated to 1 × 105 cells, cell synchronization was conducted to force the cells to the G0 phase via a serum-free culture for 12 h, and the cells were washed twice with PBS before being suspended in a binding buffer (10 mmol/L HEPES pH 7.4, 140 mmol/L NaOH, and 2.5 mmol/L CaCl2). Five microliters of fluorescein isothiocyanate (FITC)-labeled Annexin V was mixed with 100 μL cell suspensions containing 1 × 105 cells, and the cells were incubated at room temperature for 5 min. Thereafter, 50 μL PI solution (10 μg/mL) was added to the cells, followed by an additional 5-min incubation. The scatter parameters of the cells (20 000 cells per experiment) were analyzed using a FACS flow cytometer and Cell Quest analysis software (Becton-Dickinson, CA). Four cell populations were identified according to the following interpretations: viable population in the lower-left quadrant (low-PI and FITC signals), early apoptotic population in the lower-right quadrant (low-PI and high-FITC signals), necrotic population in the upper-left quadrant (high-PI and low-FITC signals), and late apoptotic or necrotic population in the upper-right quadrant (high-PI and high-FITC signals).
At this point, cells treated in the manner described above were examined on a glass slide using a laser-scanning confocal microscope (LSCM) (Bio-Rad Radiance2100, United States) with 488-nm excitation and 525-nm emission wavelengths. Bright green fluorescence was manifested in membranes of the cells undergoing prophase apoptosis because of Annexin V-FITC staining, while nuclear cardinal red fluorescence was associated with advanced stage apoptosis because of PI staining.
The intracellular calcium ion ([Ca2+]i) was measured as previously described[17]. After confluence, SGC-7901 cells on a coverslip were loaded by the [Ca2+]i indicator Fluo-3/AM in HEPES solution at 37°C in the dark for 30 min. HEPES solution contains (concentration in mmol/L): NaCl 118, KCl 4.8, CaCl2 2.5, KH2PO4 1.2, HEPES 5, and glucose 10. The pH was brought to 7.4 with NaOH. The final concentration of Fluo-3/AM was 5 μmol/L. After loading with Fluo-3/AM, a fluorescence image of [Ca2+]i was taken using a laser-scanning confocal microscope (Bio-Rad Radiance2100, United States) at 600 ×, and qualitative changes of [Ca2+]i were inferred from the fluorescence intensity using SimplePCI imaging systems (Simple PCI, Compix Inc., United States).
Twenty μg of protein in each 20-μL sample was electrophoresed through 10% SDS-PAGE gels as previously described[18]. Separated proteins were incubated with primary antibodies overnight at 4 °C, transferred to nitrocellulose membranes, and blocked with a 5% skim milk solution. They were incubated with secondary antibodies for 1 h at 37 °C. Each antigen-antibody complex was visualized by enhanced chemiluminescence Western blotting detection kits (Amersham Pharmacia Biotech, Piscataway, NJ), and band densities were determined using Chemi Doc Software (BioRad); β-Actin was used as a loading for normalization.
All experiments were repeated three times. The results of multiple experiments are given as the mean ± SE. Statistical analysis was performed using the statistical software package SPSS 13.0 (SPSS). A P value of 0.05 (two-sided) was considered statistically significant.
As shown in Figure 1A, vehicle-treated SGC-7901 cells (control) grew well with clear skeletons, whereas cells treated with PCD exhibited cytoplasmic shrinkage and either detached from each other, floated in the medium, or became distorted and blurry under a phase contrast microscope. The number of sloughed cells increased with increasing drug concentrations. The MTT assay showed that PCD significantly inhibited the viability of SGC-7901 cells (Figure 1B). The cells were incubated in the absence or presence of various concentrations of PCD for specified time periods, and the IC50 values were 13.77 ± 0.18, 8.73 ± 0.41, and 3.62 ±0 .29 mg/mL for 24, 48 and 72 h, respectively. The MTT assay showed that PCD decreased the viability of SGC-7901 cells in a concentration- and time-dependent manner (P < 0.05 and P < 0.01, respectively).
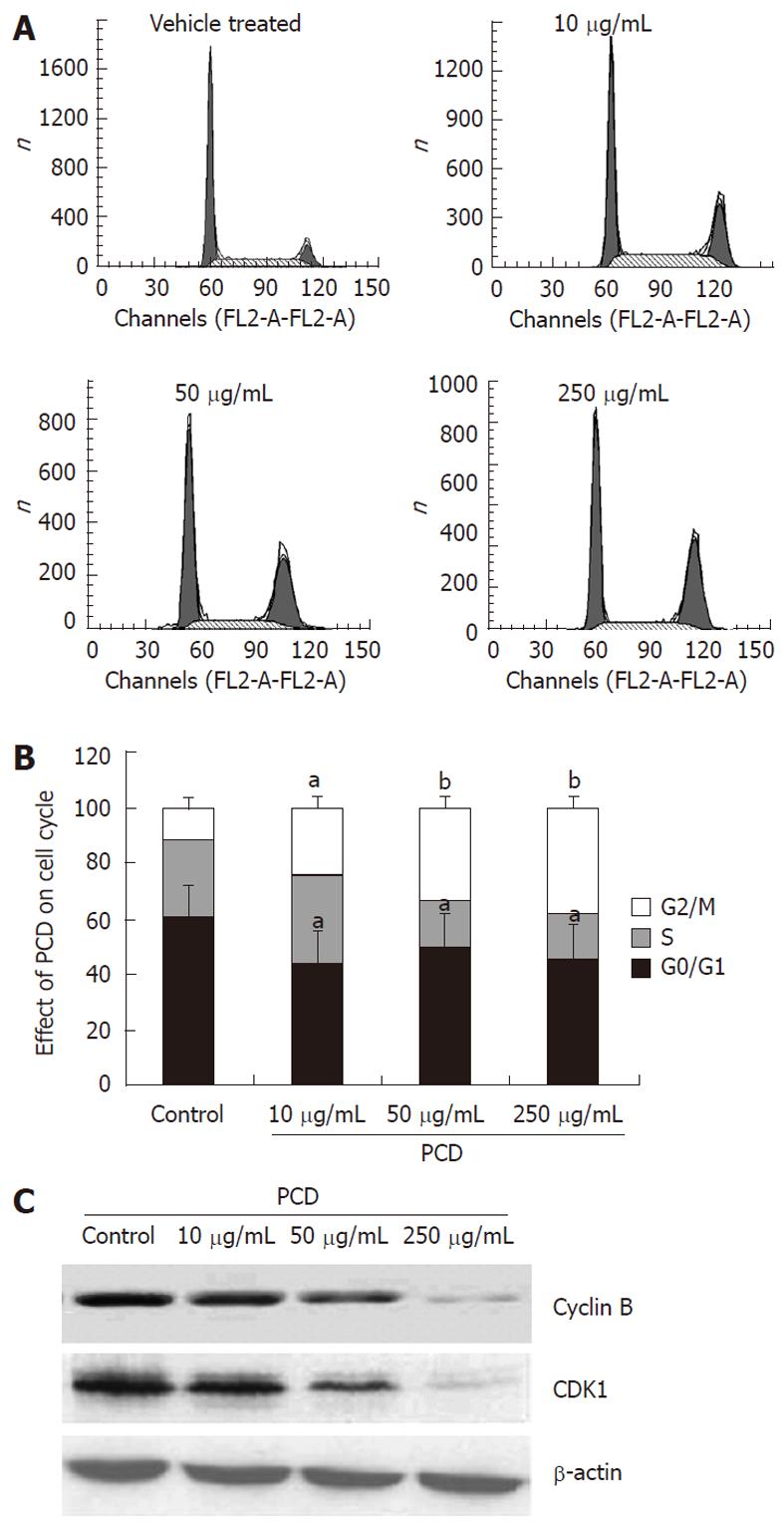
To investigate whether PCD affects the cell cycle of SGC-7901 cells, the cell cycle distribution of synchronized cells treated with or without PCD were analyzed by measuring the DNA content with PI after exposure to PCD for 24 h. As shown in Figure 2B, compared to vehicle treatment (59.26% ± 5.12%), PCD treatment reduced the percentage of the cells in the G1 phase to 43.58% ± 1.79%, 49.58% ± 1.79% and 45.58% ± 1.79%, respectively (P < 0.05). The percentage of G2/M cells was 12.48% ± 1.71% in control cells and increased to 24.48% ± 1.62%, 33.00% ± 3.16% and 38.32% ± 3.90% in the cells treated with 10, 50 and 250 μg/mL of PCD for 24 h, respectively. These results showed that PCD exerted its effect of G2/M phase cell cycle arrest rather than S phase arrest induction in SGC-7901 cells, which contributed to the effects of PCD on decreasing viability against SGC-7901 cells.
To gain insight into the mechanism of G2/M phase cell cycle arrest induced by PCD, we examined the expression of cyclins B and CDK1, which are closely related to G2/M cell cycle progression, using the Western blot assay. As shown in Figure 2C, the expression of cyclin B1 and CDK1 was decreased after PCD treatment for 24 h.
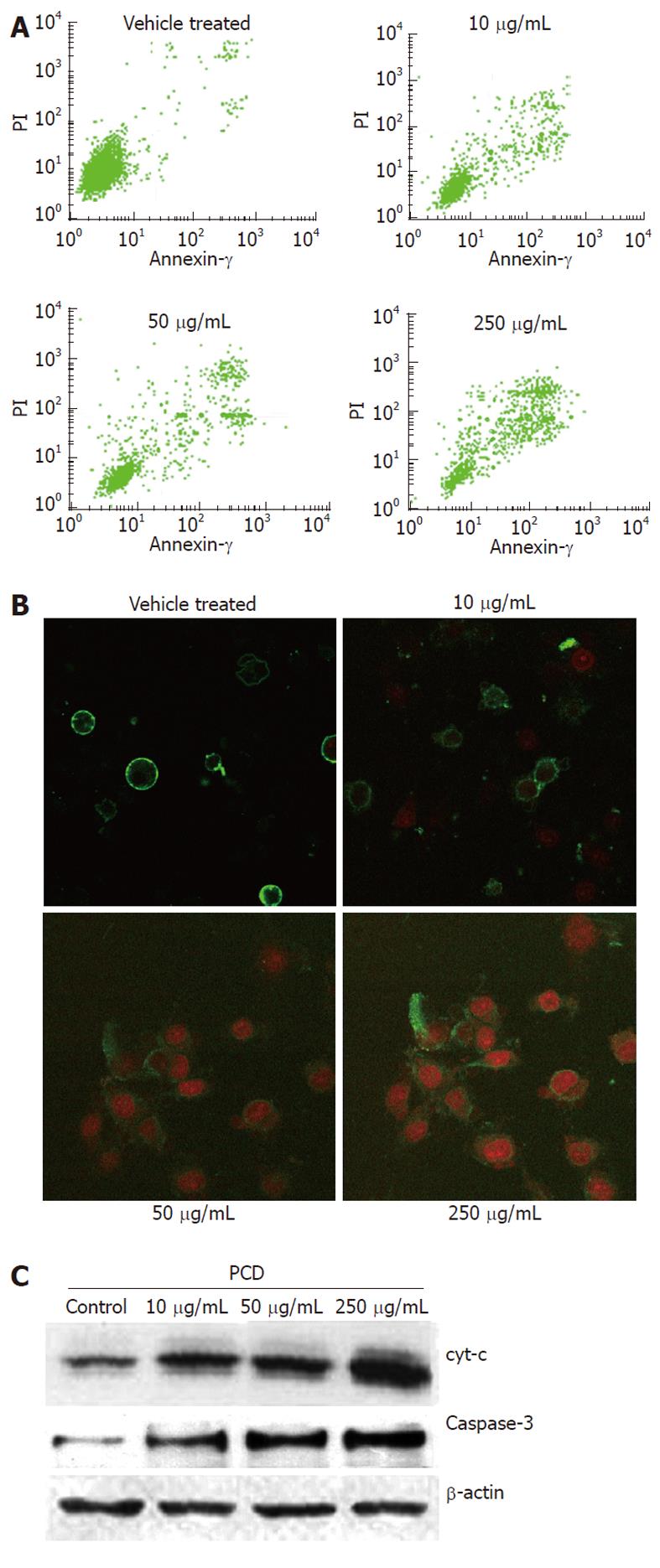
To identify whether PCD induces apoptosis, the treated cells were also stained with Annexin V-FITC/PI, and the population of apoptotic cells was analyzed by flow cytometry. As seen in Figure 3A, the drug treatment significantly increased the proportion of apoptotic cells. In the vehicle treated cells, 12.04% ± 1.62% were positive for Annexin V-FITC staining, while PCD treatment resulted in increases of 17.18% ± 2.58%, 24.75% ± 2.72% and 54.91% ± 3.35% in apoptosis when cells were treated with PCD (P < 0.05 and P < 0.01, respectively). These results demonstrate the ability of PCD to induce apoptosis in SGC-7901 cells. The morphologic changes of cells treated in the manner described above were also observed under LSCM by Annexin V/PI staining. As shown in Figure 3B, typical morphological changes, such as the formation of apoptotic bodies, appeared after the cells were treated for 24 h with 250 μg/mL PCD, whereas the vehicle-treated cells did not show evident apoptotic morphological changes.
To determine whether apoptosis induced by PCD was due to a mitochondrial-dependent caspase pathway, we further tested whether cytochrome C could be released from the mitochondria into the cytoplasm. We next investigated the levels of cytochrome C and caspase-3, which was the core protein in the caspase cascade in the soluble cytosolic fractions of SGC-7901 cells, after PCD treatment for 24 h. Figure 3C shows that PCD increased the level of cytochrome C released into the cytosol, and the expression of caspase-3 was increased after PCD treatment for 24 h compared with the vehicle-treated cells (P < 0.05), which indicated that PCD increased the caspase-3 level in SGC-7901 cells. Moreover, cells treated with PCD exhibited a dose-dependent increase at this level (P < 0.05).
To explore whether PCD-induced apoptosis involved [Ca2+]i, we used the [Ca2+]i indicator Fluo-3/AM to detect [Ca2+]i changes after PCD treatment with various densities. As shown in Figure 4, [Ca2+]i fluorescence intensity in the group treated with 250 μg/mL PCD was higher than in the vehicle-treated and lower concentration groups (P < 0.01), and PCD treatment with 10, 50 and 250 μg/mL induced an increase by 41% ± 4.72%, 66% ± 5.61%, and 86% ± 7.25% vs the vehicle-treated cells (25.33% ± 2.17%) (P < 0.01, n = 6) in Fluo-3/AM fluorescence intensity after 24 h treatment, respectively. These results suggest that the PCD can induce a dose-dependent [Ca2+]i influx and might induce apoptosis or necrosis that follows via calcium ion overload.
Natural products with anticancer properties could be valuable substances in cancer treatment, and this study examined the effect of PCD and its underlying mechanisms on the inhibition of tumor cell proliferation. In this study, we assessed the inhibitory effects and molecular mechanisms of PCD using human gastric cancer SGC-7901 cells. MTT showed that PCD inhibited the growth of SGC-7901 cells in both time-dependent and concentration-dependent manners (Figure 1B). To determine whether the cytotoxic activity of PCD was due to apoptosis, SGC-7901 human stomach carcinoma cells were treated for 24 h with various concentrations of PCD. Not only were morphological changes such as cytoplasmic shrinkage, detachment from each other, floating in the medium, distortion and some blurring under a fluorescence microscope observed (Figure 1A), but marked chromatin condensation and apoptotic body formation in PCD-treated cells were also observed in cells stained with Annexin V-FITC/PI using an LSCM (Figure 3B). Flow cytometry with Annexin V-FITC/PI staining showed that the drug treatment significantly increased the proportion of apoptotic cells, confirming that PCD induced apoptosis in SGC-7901 cells. Dysregulation of the cell cycle mechanism has also been shown to play an important role in the growth of various types of cancer cells, and the induction of cancer cell apoptosis is recognized as an important target in cancer therapy. In this study, PCD-inhibited SGC-7901 cell proliferation resulted partly from an accumulation of cells in the G2/M phase of the cell cycle. The G2/M phase is associated with DNA synthesis and the mitotic preparation period, which plays a crucial role in cell cycle progression. The complex formation of cyclins with CDKs results in an active agent that phosphorylates substrates involved in cell cycle progression[19]. The mitosis-promoting factor, which comprises a complex of CDK1 and cyclin B, is thought to be the key controller of the progression from G2 to mitosis[20-22]. In this study, PCD induced G2/M phase cell cycle arrest (Figure 2A and B), the cells of G2/M phase were present at 3.2 folds of the typical concentration after 24 h treatment, and Cyclins B1 and CDK1 were downregulated (Figure 2C), indicating that cell cycle-related proteins were involved in the PCD-induced cell cycle arrest in SGC-7901 cells.
The accumulated data suggest that the mitochondria-initiated death pathway plays an important role in triggering apoptosis in response to those stimuli. In the mitochondria-initiated death pathway, mitochondria undergoing permeability transition release apoptogenic proteins such as cytochrome C or apoptosis-inducing factor from the mitochondrial inter- membrane space into the cytosol. Released cytochrome C can activate caspase-9, and activated caspase-9 in turn cleaves and activates executioner caspase-3.
The apoptotic process is preceded by the collapse of the mitochondrial potential, the opening of a multi-protein structure named the permeability transition pore, which could be triggered by multiple stimuli such as changes in Ca2+, oxygen radicals, pH, swelling of the matrix and rupture of the outer membrane with ensuing changes in the permeability of the outer mitochondrial membrane, and release of apoptogenic factors including cytochrome C from mitochondria[23-26]. Changes in cell cycle arrest and apoptosis are listed below.
In this study, Western blot showed that cytochrome C increased in cytoplasm accompanying caspase-3 upregulation after PCD treatment (Figure 3C), indicating that the mitochondrial apoptotic pathway played a pivotal role in PCD-induced apoptosis of SGC-7901 cells.
Aside from the mechanisms described above, Ca2+ plays a critical role in this process, and intracellular Ca2+ overload appears to mediate the lethal effects of receptor overactivation[27].
Ca2+ overload has even been suggested to be the final common pathway for all types of cell death. Over the last few years, several studies have shown that increases of cytosolic Ca2+ concentration ([Ca2+]c) occur, both at early and late stages of the apoptotic pathway[28-33].
More specifically, it has been suggested that both Ca2+ release from the endoplasmic reticulum (ER) and capacitive Ca2+ influx through Ca2+ release-activated Ca2+ channels are apoptogenic[34-36]. There are also data suggesting that very high intracellular Ca2+ levels can promote cell death through necrosis, whereas lower intracellular Ca2+ increases induced by milder insults promote cell death through apoptosis[37,38]. In this study, the [Ca2+]i fluorescence intensity of cells loaded with Fluo-3/AM under a fluorescence microscope in the group treated with 250 μg/mL PCD was obviously higher than in the control and lower concentration groups (Figure 4).
Corbiere et al[39] reported that diosgenin-induced apoptosis in different human cancer cells is caspase-3-dependent and is concomitant with a fall in the mitochondrial membrane potential. We characterized the mechanisms by which PCD exerts its inhibitory effects on SGC-7901 cells by inducing G2/M cell cycle arrest and Ca2+ - cytochrome C- apoptosis.
Therefore, our results suggest that PCD may be a potential candidate as a novel therapeutic agent originating from a natural source, and the induction of apoptosis by PCD in other cancer cell lines is the subject of on-going investigations.
Gastric cancer is the most leading cause of death from cancer in China and majority in the world. Currently, no effective treatment is available. Therefore, there is a critical need to develop effective chemotherapeutic strategies for gastric cancer.
There has been no documentation of the use of the other important steroid saponin dioscin in the treatment of cancer, its mechanisms in human gastric cancer cells remain unknown.
This is the first report on the anti-proliferation, induction of apoptosis by Paris chinensis dioscin (PCD) on human gastric cancer SGC-7901 cells. The authors characterized the mechanisms by which PCD exerts its inhibitory effects on SGC-7901 cells by inducing G2/M cell cycle arrest and Ca2+ - cytochrome C- apoptosis.
PCD might be useful as an adjuvant drug in human gastric cancer treatment.
Paris chinensis (Liliaceae) is a Traditional Chinese Medicine and has been used mainly as a folk remedy for treatment of thanatophidia bites and convulsions for centuries. The active components of Paris chinensis are the saponin steroids polyphyllin D, dioscin, and balanitin 7.
This is an interesting and good paper mainly due to its potential clinical application.
Peer reviewers: Dr. Lucia Ricci Vitiani, Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, Viale Regina Elena, 299, Rome 00161, Italy; Dr. Jianyuan Chai, Assistant Professor, Research (09-151), VA Long Beach Healthcare System, 5901 E. 7th St, Long Beach, CA 90822, United States
S- Editor Tian L L- Editor Ma JY E- Editor Xiong L
| 1. | Sun X, Mu R, Zhou Y, Dai X, Qiao Y, Zhang S, Huangfu X, Sun J, Li L, Lu F. 1990-1992 mortality of stomach cancer in China. Zhonghua Zhongliu Zazhi. 2002;24:4-8. [PubMed] |
| 2. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 3. | Aneja R, Liu M, Yates C, Gao J, Dong X, Zhou B, Vangapandu SN, Zhou J, Joshi HC. Multidrug resistance-associated protein-overexpressing teniposide-resistant human lymphomas undergo apoptosis by a tubulin-binding agent. Cancer Res. 2008;68:1495-1503. [PubMed] |
| 4. | Kim EH, Yoon MJ, Kim SU, Kwon TK, Sohn S, Choi KS. Arsenic trioxide sensitizes human glioma cells, but not normal astrocytes, to TRAIL-induced apoptosis via CCAAT/enhancer-binding protein homologous protein-dependent DR5 up-regulation. Cancer Res. 2008;68:266-275. [PubMed] |
| 5. | Hung JH, Lu YS, Wang YC, Ma YH, Wang DS, Kulp SK, Muthusamy N, Byrd JC, Cheng AL, Chen CS. FTY720 induces apoptosis in hepatocellular carcinoma cells through activation of protein kinase C delta signaling. Cancer Res. 2008;68:1204-1212. [PubMed] |
| 6. | Sutter AP, Fechner H. Gene therapy for gastric cancer: is it promising? World J Gastroenterol. 2006;12:380-387. [PubMed] |
| 7. | Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441-470. [PubMed] |
| 9. | Pharmacopoeia Commission of the People¡¯s Republic of China: The Pharmacopoeia of the People¡¯s Republic of China. Beijing: People¡¯s Medical Publishing House, Chemical Industry Press 1990; . |
| 10. | Lee MS, Yuet-Wa JC, Kong SK, Yu B, Eng-Choon VO, Nai-Ching HW, Chung-Wai TM, Fung KP. Effects of polyphyllin D, a steroidal saponin in Paris polyphylla, in growth inhibition of human breast cancer cells and in xenograft. Cancer Biol Ther. 2005;4:1248-1254. [RCA] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Shoemaker M, Hamilton B, Dairkee SH, Cohen I, Campbell MJ. In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother Res. 2005;19:649-651. [PubMed] |
| 12. | Sun J, Liu BR, Hu WJ, Yu LX, Qian XP. In vitro anticancer activity of aqueous extracts and ethanol extracts of fifteen traditional Chinese medicines on human digestive tumor cell lines. Phytother Res. 2007;21:1102-1104. [PubMed] |
| 13. | Deng S, Yu B, Hui Y, Yu H, Han X. Synthesis of three diosgenyl saponins: dioscin, polyphyllin D, and balanitin 7. Carbohydr Res. 1999;317:53-62. [PubMed] |
| 14. | Li B, Yu B, Hui Y, Li M, Han X, Fung KP. An improved synthesis of the saponin, polyphyllin D. Carbohydr Res. 2001;331:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Cheung JY, Ong RC, Suen YK, Ooi V, Wong HN, Mak TC, Fung KP, Yu B, Kong SK. Polyphyllin D is a potent apoptosis inducer in drug-resistant HepG2 cells. Cancer Lett. 2005;217:203-211. [PubMed] |
| 16. | Chang CY, Huang ZN, Yu HH, Chang LH, Li SL, Chen YP, Lee KY, Chuu JJ. The adjuvant effects of Antrodia Camphorata extracts combined with anti-tumor agents on multidrug resistant human hepatoma cells. J Ethnopharmacol. 2008;118:387-395. [PubMed] |
| 17. | Li XT, Wang YL, Wang JX, Yang SJ. Effects of tetrandrine on cytosolic free calcium in cultured rat myocardial cells. Zhongguo Yao Li Xue Bao. 1996;17:55-58. [PubMed] |
| 18. | Rasmussen HE, Blobaum KR, Park YK, Ehlers SJ, Lu F, Lee JY. Lipid extract of Nostoc commune var. sphaeroides Kutzing, a blue-green alga, inhibits the activation of sterol regulatory element binding proteins in HepG2 cells. J Nutr. 2008;138:476-481. [PubMed] |
| 19. | Yu J, Guo QL, You QD, Zhao L, Gu HY, Yang Y, Zhang HW, Tan Z, Wang X. Gambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis. 2007;28:632-638. [PubMed] |
| 20. | Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer. 2008;60 Suppl 1:51-60. [PubMed] |
| 21. | Dvory-Sobol H, Cohen-Noyman E, Kazanov D, Figer A, Birkenfeld S, Madar-Shapiro L, Benamouzig R, Arber N. Celecoxib leads to G2/M arrest by induction of p21 and down-regulation of cyclin B1 expression in a p53-independent manner. Eur J Cancer. 2006;42:422-426. [PubMed] |
| 22. | Dorée M, Hunt T. From Cdc2 to Cdk1: when did the cell cycle kinase join its cyclin partner? J Cell Sci. 2002;115:2461-2464. [PubMed] |
| 23. | Petronilli V, Nicolli A, Costantini P, Colonna R, Bernardi P. Regulation of the permeability transition pore, a voltage-dependent mitochondrial channel inhibited by cyclosporin A. Biochim Biophys Acta. 1994;1187:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Skulachev VP. Why are mitochondria involved in apoptosis? Permeability transition pores and apoptosis as selective mechanisms to eliminate superoxide-producing mitochondria and cell. FEBS Lett. 1996;397:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 263] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Bernardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronilli V, Scorrano L. The mitochondrial permeability transition. Biofactors. 1998;8:273-281. [PubMed] |
| 26. | Petit PX, Goubern M, Diolez P, Susin SA, Zamzami N, Kroemer G. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition. FEBS Lett. 1998;426:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261-1276. [PubMed] |
| 28. | Martikainen P, Kyprianou N, Tucker RW, Isaacs JT. Programmed death of nonproliferating androgen-independent prostatic cancer cells. Cancer Res. 1991;51:4693-4700. [PubMed] |
| 29. | Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51:293-308. [PubMed] |
| 30. | Zirpel L, Lippe WR, Rubel EW. Activity-dependent regulation of [Ca2+]i in avian cochlear nucleus neurons: roles of protein kinases A and C and relation to cell death. J Neurophysiol. 1998;79:2288-2302. [PubMed] |
| 31. | Tombal B, Denmeade SR, Isaacs JT. Assessment and validation of a microinjection method for kinetic analysis of [Ca2+]i in individual cells undergoing apoptosis. Cell Calcium. 1999;25:19-28. [PubMed] |
| 32. | Lynch K, Fernandez G, Pappalardo A, Peluso JJ. Basic fibroblast growth factor inhibits apoptosis of spontaneously immortalized granulosa cells by regulating intracellular free calcium levels through a protein kinase Cdelta-dependent pathway. Endocrinology. 2000;141:4209-4217. [PubMed] |
| 33. | Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhães PJ, Di Virgilio F, Pozzan T. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619-8627. [PubMed] |
| 34. | Jiang S, Chow SC, Nicotera P, Orrenius S. Intracellular Ca2+ signals activate apoptosis in thymocytes: studies using the Ca(2+)-ATPase inhibitor thapsigargin. Exp Cell Res. 1994;212:84-92. [PubMed] |
| 35. | Wertz IE, Dixit VM. Characterization of calcium release-activated apoptosis of LNCaP prostate cancer cells. J Biol Chem. 2000;275:11470-11477. [PubMed] |
| 36. | Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690-2701. [PubMed] |
| 37. | Choi DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995;18:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |