Published online Sep 28, 2011. doi: 10.3748/wjg.v17.i36.4143
Revised: January 19, 2011
Accepted: January 26, 2011
Published online: September 28, 2011
AIM: To evaluate the comparative therapeutic efficacy of radiofrequency ablation (RFA) and hepatic resection (HR) for solitary colorectal liver metastases (CLM).
METHODS: A literature search was performed to identify comparative studies reporting outcomes for both RFA and HR for solitary CLM. Pooled odds ratios (OR) with 95% confidence intervals (95% CI) were calculated using either the fixed effects model or random effects model.
RESULTS: Seven nonrandomized controlled trials studies were included in this analysis. These studies included a total of 847 patients: 273 treated with RFA and 574 treated with HR. The 5 years overall survival rates in the HR group were significantly better than those in the RFA group (OR: 0.41, 95% CI: 0.22-0.90, P = 0.008). RFA had a higher rate of local intrahepatic recurrence compared to HR (OR: 4.89, 95% CI: 1.73-13.87, P = 0.003). No differences were found between the two groups with respect to postoperative morbidity and mortality.
CONCLUSION: HR was superior to RFA in the treatment of patients with solitary CLM. However, the findings have to be carefully interpreted due to the lower level of evidence.
-
Citation: Wu YZ, Li B, Wang T, Wang SJ, Zhou YM. Radiofrequency ablation
vs hepatic resection for solitary colorectal liver metastasis: A meta-analysis. World J Gastroenterol 2011; 17(36): 4143-4148 - URL: https://www.wjgnet.com/1007-9327/full/v17/i36/4143.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i36.4143
Colorectal cancer continues to be one of the most com-mon human malignancies, afflicting nearly one million individuals worldwide every year[1]. Approximately 50% of patients with colorectal cancer develop hepatic metastases during the course of their disease. Survival without treatment is very limited, with a median of 7.4 to 11 mo[2]. Hepatic resection (HR) is the only chance of cure for patients with colorectal liver metastases (CLM) and 5 years survival rates after radical resection are about 27%-58%[3]. However, the great majority of patients with CLM present with unresectable disease, mainly due to the extent or distribution of their disease, or concurrent medical disability, so only up to 20% of patients are candidates for HR[4,5]. So, many nonsurgical ablative methods have been developed. The most widely utilized modality is radiofrequency ablation (RFA), which includes generation of high-frequency alternating current which causes ionic agitation and conversion to heat, with subsequent evaporation of intracellular water which leads to irreversible cellular changes, including intracellular protein denaturation, melting of membrane lipid bilayers, and coagulative necrosis of individual tumor cells.
Although RFA has established its role in the treatment algorithm of patients with inoperable CLM as a safe, well tolerated, easily repeated and less invasive procedure[2,6,7], the therapeutic efficacy of RFA for those with resectable CLM remains controversial, especially for solitary lesions. For example, Oshowo et al[8] reported equivalent median (41 mo vs 37 mo) and 3 years overall survival rates (55.4% vs 52.6%) between HR and RFA groups, whereas White et al[3] reported better 5 years (71% vs 27%) and overall median survival (56 mo vs 36 mo) for resection vs RFA.
Meta-analysis can be used to evaluate the existing literature in both a qualitative and quantitative way by comparing and integrating the results of different studies and taking into account variations in characteristics that can influence the overall estimate of the outcome of interest[9]. Therefore, we evaluated the available evidence comparing the clinical efficacy and safety of RFA and HR for treatment of solitary CLM using meta-analysis.
A MEDLINE, EMBASE, OVID, and Cochrane database search was performed on all studies between 1996 and 2010 to compare RFA and HR for solitary CLM. The following MeSH search headings were used: “colorectal liver metastases”, “hepatic resection”, “radiofrequency ablation” and “comparative study”. Only studies on humans and in English language were considered for inclusion. Reference lists of all retrieved articles were manually searched for additional studies.
Two reviewers (BL and TW, respectively) independently extracted the following parameters from each study: (1) first author and year of publication; (2) number of patients, patients’ characteristics, study design; and lastly (3) treatment outcome. All relevant text, tables and figures were reviewed for data extraction. Discrepancies between the two reviewers were resolved by discussion and consensus.
For inclusion in the meta-analysis, a study had to fulfill the following criteria: (1) compare the initial therapy effects of RFA and HR for the treatment of solitary CLM; (2) report on at least one of the outcome measures mentioned below; (3) clearly document indications for RFA and HR; and (4) if dual (or multiple) studies were reported by the same institution and/or authors, the one of higher quality or the most recent publication was included in the analysis.
Abstracts, letters, editorials and expert opinions, reviews without original data, case reports and studies lacking control groups were excluded. The following studies were also excluded: (1) those dealing with multiple CLM; (2) those with no clearly reported outcomes of interest; and (3) those evaluating patients with primary liver cancer.
The primary outcome was efficacy, including 5 years overall survival, local intrahepatic recurrence or 5 years disease-free survival. The secondary outcome was safety, including the morbidity and mortality.
The meta-analysis was performed using the Review Manager (RevMan) software, version 4.2.7. We analysed dichotomous variables using estimation of odds ratios (OR) with a 95% confidence interval (95% CI). Pooled effect was calculated using either the fixed effects model or random effects model. Heterogeneity was evaluated by χ2 and I2. We considered heterogeneity to be present if the I2 statistic was > 50%. P < 0.05 was considered significant.
After initial screening, 13 potentially relevant clinical trials were identified[3,8,10-20]. Of these, in three trials including patients with multiple metastases, it was impossible to extract or calculate the appropriate data regarding solitary CLM[10-12], two trials included patients with non-colorectal cancer[13,14], and one trial lacked information concerning 5 years overall survival[15]; all 6 studies were excluded. Finally, a total of 7 nonrandomized studies published between 2003 and 2009 matched the inclusion criteria and were therefore included[3,8,16-20].
The characteristics of these 7 studies are summarized in Table 1. The 7 studies included a total of 847 patients: 273 in the RFA group and 574 in the HR group. Four studies were conducted in United States[3,16,17,20], two in Korea[18,19], and one in United Kingdom[3]. The sample size of each study varied from 45 to 192 patients. The proportion of men ranged from 46.6% to 66.6%. Median duration of follow-up ranged from 17 to 68 mo.
| Author/(yr) | Country | Group | n | M/F | Mean age (yr) | Mean tumor size (cm) | Median follow-up (mo) |
| Oshowo[8] | United Kingdom | RFA | 25 | 11/14 | 57 (34-80) | 3 (1-10)1 | 37 (9-67) |
| 2003 | HR | 20 | 10/10 | 63 (52-77) | 4 (2-7) | 41 (0-97) | |
| Aloia[16] | United State | RFA | 30 | 23/7 | - | 3.0 (1.0-7.0)1 | 31.3 (4-138) |
| 2006 | HR | 150 | 85/65 | - | 3.5 (0.5-17.0) | 31.3 (4-138) | |
| White[3] | United States | RFA | 22 | 8/14 | 62 ± 7.5 | 2.4 ± 1.0 | 17 |
| 2007 | HR | 30 | 20/10 | 63 ± 9.6 | 2.7 ± 1.1 | 68 | |
| Berber[17] | United States | RFA | 68 | 43/25 | 67 ± 1.4 | 3.7 ± 0.2 | 23 (2-86) |
| 2008 | HR | 90 | 57/33 | 63.7 ± 1.3 | 3.8 ± 0.2 | 33 (2-132) | |
| Lee[18] | Korea | RFA | 37 | 26/11 | 59.0 (28-75)1 | 2.25 (0.8-5.0) | 48.2 (0.9-133.9) |
| 2008 | HR | 116 | 76/40 | 58.0 (26-79) | 3.29 (0.5-18.0) | 48.2 (0.9-133.9) | |
| Hur[19] | Korea | RFA | 25 | 15/10 | 62.6 (33-82) | 2.5 (0.8-3.6) | 42 (13-120) |
| 2009 | HR | 42 | 27/15 | 58 (42-75) | 2.8 (0.6-8) | 42 (13-120) | |
| Reuter[20] | United States | RFA | 66 | 46/20 | 63.5 | 3.2 | 20 |
| 2009 | HR | 126 | 69/57 | 61.9 | 5.3 | 20 |
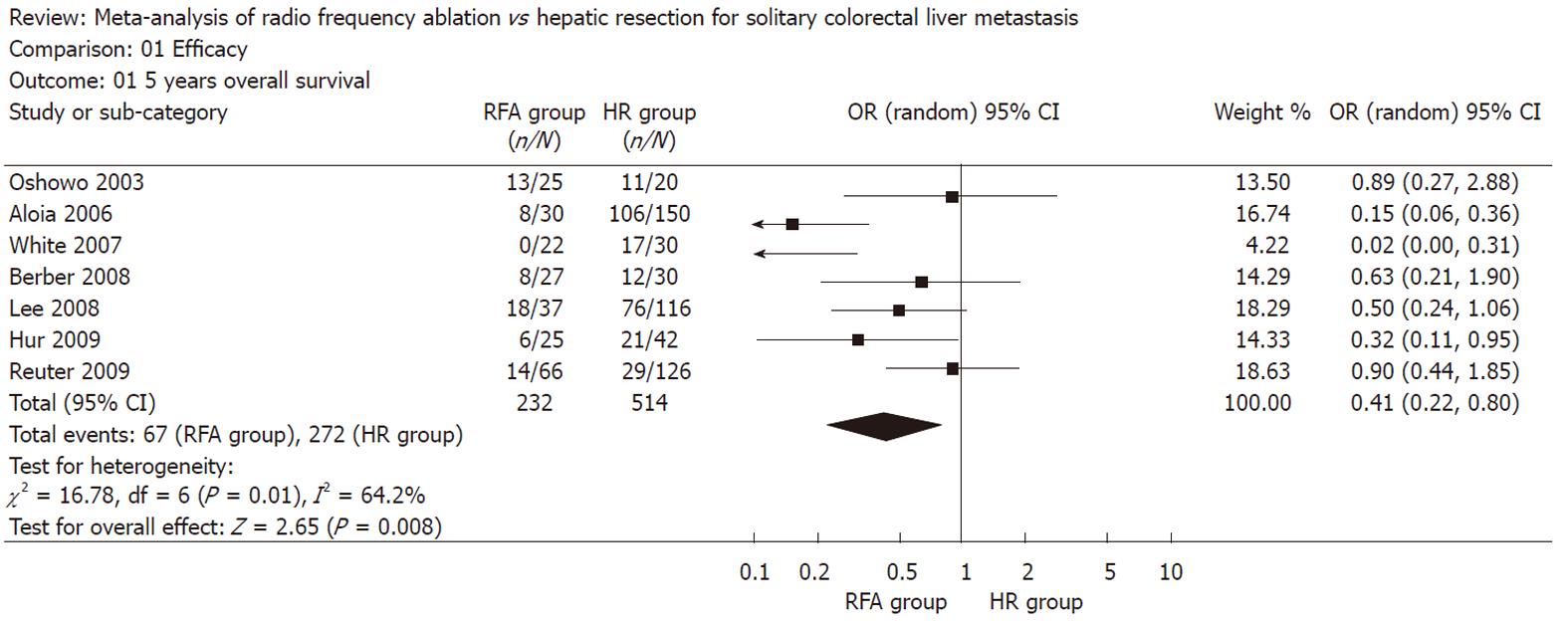
The pooled analysis of the 7 studies furnishing data demonstrated a significant improvement in 5 years overall survival favoring HR over RFA (OR: 0.41, 95% CI: 0.22-0.90, P = 0.008, I2 = 64.2%) (Figure 1).
Six trials investigated local intrahepatic recurren-ce[3,16-20]. Local recurrence was more frequently observed after RFA than after HR (OR: 4.89, 95% CI: 1.73-13.87, P = 0.003, I2 = 77.3%) (Figure 2).
Only two studies reported on 5 years disease-free survival. Aloia et al[16] reported that 5 years disease-free survival rates were higher after HR compared with RFA (50% vs 0%), whereas Lee et al[18] reported equivalent results between two groups (25.7% vs 30.1%). We did not perform an analysis because of the small number of trials included in the review.
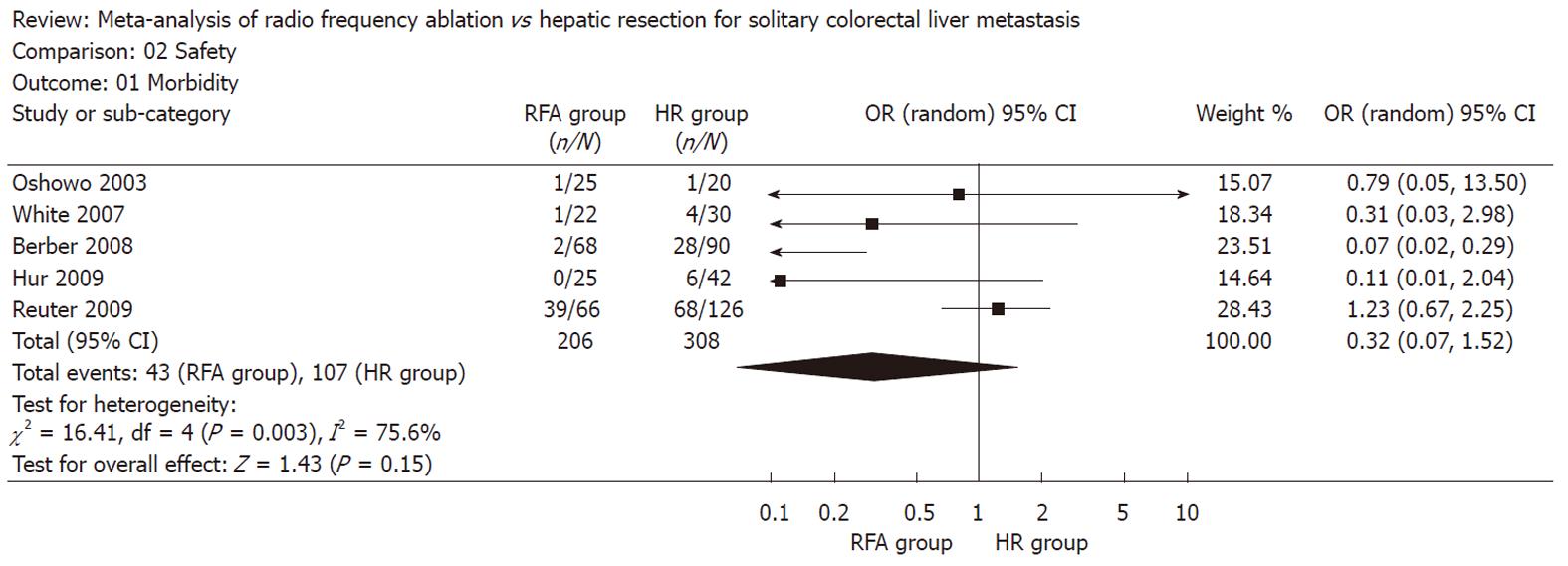
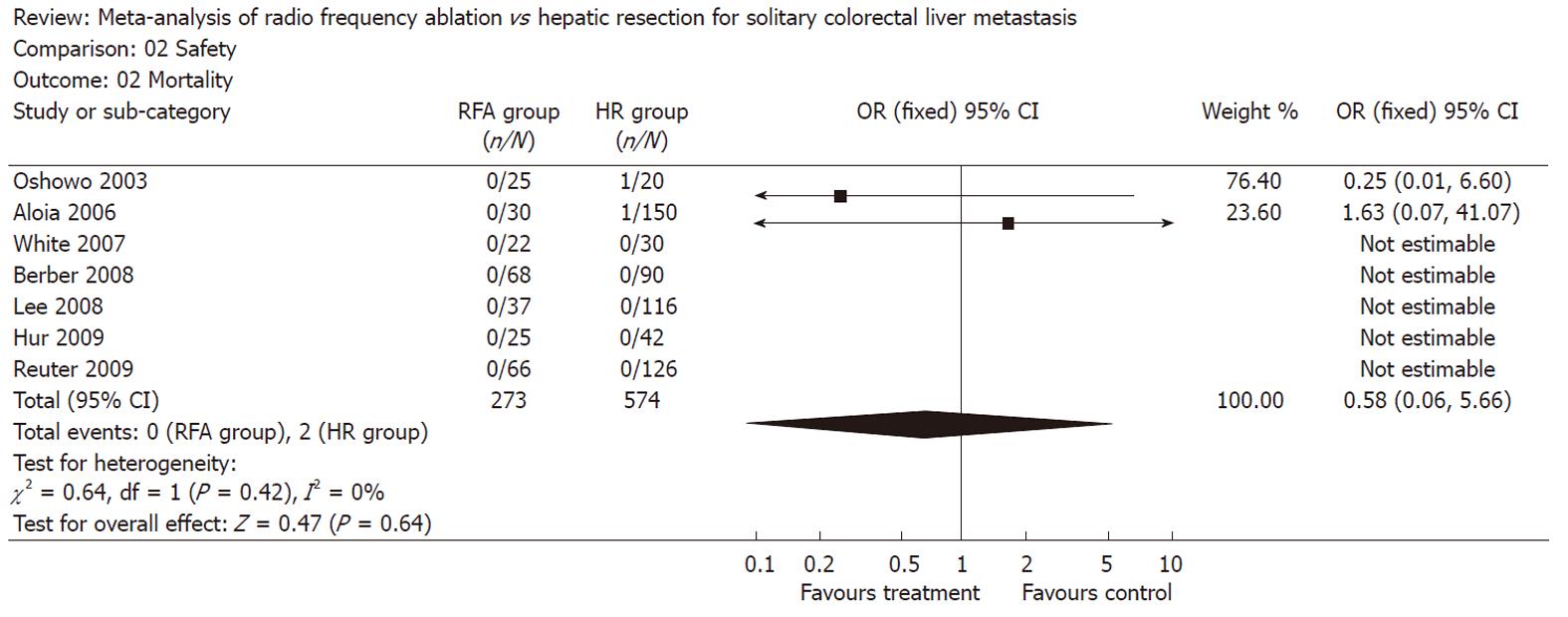
There was no statistically significant difference in the postoperative morbidity (five trials reported this data[3,8,17,19,20], OR: 0.32, 95% CI: 0.07-1.52, P = 0.15, I2 = 75.6%) and mortality (all trials reported this data, OR: 0.58, 95% CI: 0.06-5.66, P = 0.64, I2 = 0%) between the two groups (Figures 3 and 4). There were no deaths reported in the RFA group, and 2 in the HR group.
This meta-analysis shows that the HR treatment group had better 5 years survival outcomes than the RFA treatment group for solitary CLM. The major contributing factor for this finding may be the higher local recurrence rate after RFA. In addition to being more likely to have a recurrence, RFA patients also recurred earlier than resection patients[3,20]. This could be due to incomplete ablation secondary to lesion size, heat sink effect, or the limitations of the modality[20]. Resection of the entire area of preexisting tumor is more oncologically sound than attempting thermal destruction of a frequently ill defined region in the liver[21]. This may explain the better outcomes following HR.
In a mouse xenograft model of CLM, von Breitenbuch et al[22] revealed that RFA led to an increased survival of residual neoplastic cells and significantly promoted the proliferation of neoplastic cells. Recently, Nijkamp et al[23] found that RFA treatment resulted in a highly localized hypoxia-driven acceleration of tumor growth occurring in the transition zone between necrosis induced by RFA and the normal liver tissue, and that the stimulated outgrowth of perilesional micrometastases is associated with profound and chronic microvascular disturbances, chronic tissue and tumor hypoxia, and stabilization of hypoxia-inducible factor (HIF)-1a and HIF-2a. These experimental findings may further explain the better outcome after RFA compared with HR in current study.
For liver metastases ≤ 3 cm, Mulier and colleagues found that local recurrence after RFA is extremely low in a recent review, and the authors proposed a randomized trial comparing resection and RFA for resectable CLM ≤ 3 cm is warranted[24]. However, in a study of 79 patients with solitary CLM ≤ 3 cm, RFA treatment resulted in a higher local recurrence rate than HR treatment (31% vs 3%, respectively). RFA was also associated with a marked decrease in the 5 years survival rate and the 5 years local recurrence-free rate compared with those of HR (18% vs 72% and 66% vs 97%, respectively)[16]. Similarly, another study of 60 patients showed that both time to recurrence after treatment of liver metastases and overall survival were significantly shorter, and marginal recurrence significantly more frequent, in the RFA group[15]. Although Hur et al[19] reported equivalent 5 years survival rates (56.1% vs 55.4%) and local recurrence-free survival rates (95.7% vs 85.6%) between HR and RFA groups in patients with tumors ≤ 3 cm, it must be noted that the limited number of patients (n = 38) in their study might have insufficient power to detect any differences.
In that review, Mulier et al[24] stated that the two randomized clinical trials[25,26] showed equivalent survival after percutaneous RFA and surgical resection for small HCC will encourage the use of RFA for resectable CLM. However, in one of the two studies, 19 of 90 patients (21%) who were randomized for RFA converted to HR[25]. More importantly, a recently published meta-analysis and a randomized clinical trial both found that HR was superior to RFA in the treatment of patients with small HCC with respect to survival and local control of the disease[27,28]. Thus, we agree with the idea proposed by Curley that “it is not yet time for a randomized clinical trial comparing resection with RFA for resectable CLM.”
The results of this meta-analysis should be interpre-ted with caution for several reasons. First, all of data in the present study comes from nonrandomized studies, and the overall level of clinical evidence is low. Second, there is important heterogeneity between two groups, because it was not possible to match patients characteristics in all studies. We applied a random effect model to take between study variation into consideration. This does not necessarily rule out the effect of heterogeneity between studies, but one may expect a very limited influence. Finally, potential publication bias might be present due to the small number of trials included in the current study.
In summary, HR was superior to RFA in the treatment of patients with solitary CLM. RFA should be reserved for patients who are not optimal candidates for resection, rather than being used as a first-line therapeutic option. However, the findings have to be carefully interpreted due to the lower level of evidence.
Hepatic metastases are the commonest cause of morbidity and death of patients with colorectal cancer. Survival without treatment is very limited, with a median of 7.4 to 11 mo. Hepatic resection (HR) is the only chance of cure for patients with colorectal liver metastases (CLM) and 5 years survival rates after radical resection are about 27%-58%. Unfortunately, only up to 20% of patients are candidates for HR.
Radiofrequency ablation (RFA) is an established effective nonsurgical ablative method for treatment of inoperable CLM, but its therapeutic efficacy for resectable CLM remains controversial, especially for solitary lesions.
This meta-analysis shows for the first time that HR was superior to RFA in the treatment of patients with solitary CLM with respect to survival and local control of the disease.
The results suggest that RFA should be reserved for patients with solitary CLM who are not optimal candidates for resection, rather than being used as a first-line therapeutic option.
The article is a well written, well analysed one that is worth publishing.
Peer reviewer: Selin Kapan, Dr., Associate Professor of General Surgery, Dr., Sadi Konuk Training and Research Hospital, Department of General Surgery, Kucukcekmece, Istanbul 34150, Turkey
S- Editor Tian L L- Editor O’Neill M E- Editor Zhang DN
| 1. | El-Tawil AM. Colorectal cancer and pollution. World J Gastroenterol. 2010;16:3475-3477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Gillams AR, Lees WR. Radiofrequency ablation of colorectal liver metastases. Abdom Imaging. 2005;30:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | White RR, Avital I, Sofocleous CT, Brown KT, Brody LA, Covey A, Getrajdman GI, Jarnagin WR, Dematteo RP, Fong Y. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg. 2007;11:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 4. | Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59-71. [PubMed] |
| 5. | Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, Majno P, Engerran L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509-520; discussion 520-522. [PubMed] |
| 6. | Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159-166. [PubMed] |
| 7. | Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the "test-of-time approach". Cancer. 2003;97:3027-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 221] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90:1240-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 197] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Aziz O, Constantinides V, Tekkis PP, Athanasiou T, Purkayastha S, Paraskeva P, Darzi AW, Heriot AG. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol. 2006;13:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 10. | Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-825; discussion 825-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1364] [Cited by in RCA: 1291] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 11. | Otto G, Düber C, Hoppe-Lotichius M, König J, Heise M, Pitton MB. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg. 2010;251:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Gleisner AL, Choti MA, Assumpcao L, Nathan H, Schulick RD, Pawlik TM. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg. 2008;143:1204-1212. [PubMed] |
| 13. | Leblanc F, Fonck M, Brunet R, Becouarn Y, Mathoulin-Pélissier S, Evrard S. Comparison of hepatic recurrences after resection or intraoperative radiofrequency ablation indicated by size and topographical characteristics of the metastases. Eur J Surg Oncol. 2008;34:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Chow DH, Sinn LH, Ng KK, Lam CM, Yuen J, Fan ST, Poon RT. Radiofrequency ablation for hepatocellular carcinoma and metastatic liver tumors: a comparative study. J Surg Oncol. 2006;94:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Park IJ, Kim HC, Yu CS, Kim PN, Won HJ, Kim JC. Radiofrequency ablation for metachronous liver metastasis from colorectal cancer after curative surgery. Ann Surg Oncol. 2008;15:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, Curley SA, Zorzi D, Abdalla EK. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460-466; discussion 466-467. [PubMed] |
| 17. | Berber E, Tsinberg M, Tellioglu G, Simpfendorfer CH, Siperstein AE. Resection versus laparoscopic radiofrequency thermal ablation of solitary colorectal liver metastasis. J Gastrointest Surg. 2008;12:1967-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Lee WS, Yun SH, Chun HK, Lee WY, Kim SJ, Choi SH, Heo JS, Joh JW, Choi D, Kim SH. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol. 2008;42:945-949. [PubMed] |
| 19. | Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, Cho CH, Ko HK, Lee JT, Kim NK. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg. 2009;197:728-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Reuter NP, Woodall CE, Scoggins CR, McMasters KM, Martin RC. Radiofrequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J Gastrointest Surg. 2009;13:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Curley SA. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann Surg Oncol. 2008;15:11-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | von Breitenbuch P, Köhl G, Guba M, Geissler E, Jauch KW, Steinbauer M. Thermoablation of colorectal liver metastases promotes proliferation of residual intrahepatic neoplastic cells. Surgery. 2005;138:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Nijkamp MW, van der Bilt JD, de Bruijn MT, Molenaar IQ, Voest EE, van Diest PJ, Kranenburg O, Borel Rinkes IH. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg. 2009;249:814-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Mulier S, Ni Y, Jamart J, Michel L, Marchal G, Ruers T. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann Surg Oncol. 2008;15:144-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1104] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 26. | Lü MD, Kuang M, Liang LJ, Xie XY, Peng BG, Liu GJ, Li DM, Lai JM, Li SQ. [Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial]. Zhonghua YiXue ZaZhi. 2006;86:801-805. [PubMed] |
| 27. | Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, Yang J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 642] [Article Influence: 42.8] [Reference Citation Analysis (0)] |