Published online Aug 7, 2011. doi: 10.3748/wjg.v17.i29.3453
Revised: November 15, 2010
Accepted: November 22, 2010
Published online: August 7, 2011
AIM: To investigate recurrent variceal hemorrhage and long-term survival rates of patients treated with partial proximal splenorenal venous shunt.
METHODS: Patients with variceal hemorrhage who were treated with small-diameter proximal splenorenal venous shunt in Ruijin Hospital between 1996 and 2009 were included in this study. Shunt diameter was determined before operation using Duplex Doppler ultrasonography. Peri-operative and long-term results in term of rehemorrhage, encephalopathy and mortality were followed up.
RESULTS: Ninety-eight patients with Child A and B variceal hemorrhage received small-diameter proximal splenorenal venous shunt with a diameter of 7-10 mm. After operation, the patients’ mean free portal pressure (P < 0.01) and the flow rate of main portal vein (P < 0.01) decreased significantly compared with that before operation. The rates of rebleeding and mortality were 6.12% (6 cases) and 2.04% (2 cases), respectively. Ninety-one patients were followed up for 7 mo-14 years (median, 48.57 mo). Long-term rates of rehemorrhage and encephalopathy were 4.40% (4 cases) and 3.30% (3 cases), respectively. Thirteen patients (14.29%) died mainly due to progressive hepatic dysfunction. Five- and ten-year survival rates were 82.12% and 71.24%, respectively.
CONCLUSION: Small-diameter proximal splenorenal venous shunt affords protection against variceal rehemorrhage with a low occurrence of encephalopathy in patients with normal liver function.
- Citation: Chen H, Yang WP, Yan JQ, Li QY, Ma D, Li HW. Long-term results of small-diameter proximal splenorenal venous shunt: A retrospective study. World J Gastroenterol 2011; 17(29): 3453-3458
- URL: https://www.wjgnet.com/1007-9327/full/v17/i29/3453.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i29.3453
Total portacaval shunt provides an excellent therapeutic effect for hemorrhage due to esophageal and/or gastric varices as portal venous pressure decreases to a normal range. However, there is a deleterious effect on liver function due to the complete loss of prograde portal flow which is accompanied with a high encephalopathy rate[1]. To reduce the complications caused by total portacaval shunt, partial portacaval shunt has been developed since the 1980s in an attempt to effectively decompress the portal vein while maintaining prograde portal flow to the liver to diminish postoperative encephalopathy. Several centers have validated the hemodynamic advantage of partial portacaval shunts, which significantly reduced the episodes of encephalopathy compared with the shunts that totally divert portal flow[2-5]. The theory of the small-diameter portacaval shunt is based on that variceal hemorrhage will not occur if the pressure gradient between the portal system and the systemic caval system is about 12 mmHg (about 16.3 cmH2O)[6,7]. The most popular small-diameter portacaval H-graft decompressive shunt proposed by Sarfeh et al[1] involves placement of an 8-mm diameter polytetrafluoroethylene (Gore-Tex) graft between the portal vein and inferior vena cava.
The operation of partial portosystemic shunt was initiated in the 1990s in our center. In 1991, we firstly investigated the relationship between portal venous diameter (PVD), free portal pressure (FPP) and collateral venous diameters in percutaneous transhepatic portography (PTP)[8], and found that FPP could decrease to 2.64 kPa (26.94 cmH2O) when the portosystemic shunt diameter (SD) was 67% of PVD. In the studies of Rousselot et al[9] and Burcharth et al[10], no bleeding episodes due to gastroesophageal varices occurred if FPP was 2.64 kPa (26.94 cmH2O). This data outclasses the normal range of PVP (1-5 mmHg, 6.79-13.58 cmH2O). According to the data from PTP, the suitable SD could be determined before operation. However, PTP is a kind of traumatic examination and not suitable for every patient. Thus, Duplex Doppler ultrasonography was introduced to determine SD before the shunting[11]. We had established an equation to calculate SD based on portal venous flow, superior mesenteric venous flow and PVD[11]. Since 1996, proximal splenorenal venous shunting with predicted portal SD has been carried out in 98 patients with hypersplenism and esophageal variceal hemorrhage. This is a retrospective analysis of our experience with proximal splenorenal venous shunting with small stoma.
Patients with hepatic cirrhosis and portal hypertension received proximal splenorenal venous shunt in the Department of Surgery of Ruijin Hospital, Shanghai Jiaotong University School of Medicine. The study design was approved by the independent ethics committee of Ruijin Hospital. Inclusion criteria consisted of Child A or B (score less than 7 points)[12], prograde hepatic portal flow, splenomegaly and hypersplenism, absence of refractory ascites, absence of encephalopathy, absence of portal thrombosis, and hemorrhage due to esophageal and/or gastric varices or portal gastropathy.
Preoperational examinations for clinical conditions and laboratory tests including blood cell counts, hepatic function and blood coagulation, were made in all patients. Esophageal variceal hemorrhage was confirmed by endoscopic examination. Encephalopathy was assessed from stage 0 to 4 by West Haven classification system[13]. Ascites was classified as absent, moderate (clinically evident, but well-controlled with fluid restriction and oral diuretics), or severe (abdominal distention refractory to fluid restriction and maximal diuretic therapy). The diagnosis of hypersplenism was established with the presence of splenomegaly and significant reduction of blood cell counts. The patients were staged by Child classification and preshunt model for end-stage liver disease (MELD) score. Hemodynamics of portal venous system was documented by Duplex Doppler ultrasonography including portal vein, splenic vein and superior mesenteric vein.
In our previous study[11], an equation was established to calculate the SD according to PVD, PVF and splenic venous flow (SVF) of Duplex Doppler ultrasonography before operation, i.e. SD (mm) = PVD (mm) × [1 - SVF (mL/min)/PVF (mL/min)]1/4× 67%.
The procedure of operation included splenectomy and small-diameter proximal splenorenal venous shunting. In brief, left Kocher incision at upper abdomen was undertaken in all patients. Splenectomy was performed and followed by the exposure of splenic vein and left renal vein. The diameter of anastomosis was determined by the equation mentioned above. If the diameter of splenic veous stump was equal to pre-calculated SD, end-to-side anastomosis would be performed, otherwise, side-to-side anastomosis would be performed. Continuous suture was adopted using 5-0 Gore-Tex or 5-0 polypropolene. Free portal venous pressure was determined before splenectomy, and before and after shunting.
Surgical complication, rehemorrhage, encephalopathy and operative mortality were recorded within 30 d after operation. Duplex Doppler ultrasonography was used to examine the portal venous system just before hospital discharge. After discharge, the following clinical manifestations were monitored: rehemorrhage, encephalopathy, hepatic dysfunction or failure and the occurrence of hepatocellular carcinoma. Hematemesis and/or melana were considered as rehemorrhage. Encephalopathy was assessed as mentioned above.
SPSS 13.0 software for Windows was used for statistical analysis. Data were expressed as the mean ± SD and differences were considered significant at P < 0.05. Repeated measure analysis was performed to compare the change of FPP before and after shunting. Statistical significance of hemodynamic changes of portal vein before and after shunting was determined using paired t test. Survival probabilities of patients and survival curves were determined by life table analysis using the Kaplan-Meier method. A comparison of survival probabilities between Child A and Child B, or MELD (4-6) and MELD (7-10) was made using Wilcoxon (Gehan) statistics.
From May 1996 through March 2009, 98 patients, aged 19-73 years, received small-diameter proximal splenorenal venous shunt in our department. The preoperative clinical data of the patients and laboratory tests are shown in Table 1. As a result, 88.78% of hepatic cirrhosis was due to hepatitis B infection, and the rest was due to various reasons including blood fluke (5), cryptogenic causes (3), alcohol intake (2) and hepatitis C (1). Splenomegaly and hypersplenism were observed in all patients while no ascites and encephalopathy were found before shunting.
| Items | Total No. of patients (98) |
| Age (yr) | 45.1 ± 9.1 (19-73) |
| Sex | |
| Male | 80 (71.43) |
| Female | 18 (18.37) |
| Cause of cirrhosis | |
| Hepatitis B | 87 (88.78) |
| Others | 11 (11.22) |
| Esophageal and/or gastric variceal bleeding | 98 (100) |
| White cell count (× 109/L) | 2.7 ± 1.3 |
| Red cell count (× 1012/L) | 3.63 ± 0.68 |
| Hemoglobin (g/L) | 97.58 ± 22.55 |
| Blood platelets count (× 109/L) | 57.53 ± 26.28 |
| Serum total bilirubin (mmol/L) | 20.47 ± 8.05 |
| Serum albumin (g/L) | 36.23 ± 4.14 |
| Child classification | |
| A | 75 (76.53) |
| B | 23 (23.47) |
| MELD score | 6.87 ± 1.25 |
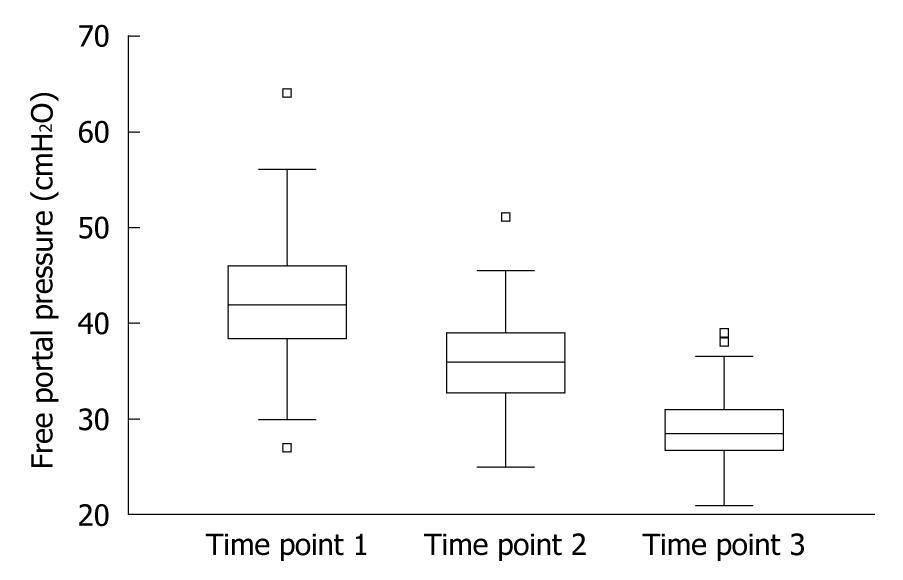
Pre-calculated SD was 7 mm in 3 patients, 8 mm in 54 patients, 9 mm in 23 patients and 10 mm in 18 patients, respectively. After splenectomy, reduction of mean FPP was 14.97% (P < 0.01) (Table 2). After shunting, the mean of free portal pressure decreased to 29.18 cmH2O (range, 21-39 cmH2O) with a rate of 31.53% (P < 0.01) (Table 2, Figure 1).
After operation, Duplex Doppler ultrasonography was undertaken in 50 patients. The diameter and flow rate of main portal vein were decreased significantly after shunting compared with that before operation (P < 0.01) (Table 3). The flow velocity of portal vein was also reduced but not significantly (P = 0.088).
Postoperative complications were observed in 13 patients (13.54%) during hospitalization. Six patients (6.12%) had gastrointestinal bleeding after shunting, including hematemesis in three and melana in three. The cause of rehemorrhage was portal venous thrombosis in 4 patients. Rebleeding was controlled by conservative treatment or endoscopic sclerosing therapy, and venous thrombosis was treated by thrombolytic treatment. Abdominal bleeding occurred in 5 patients (5.1%). Among them, one patient received surgical treatment while the others recovered after conservative treatment. Spontaneous hemothorax and severe ascites were observed each in one patient (1.02%), who was recovered after expectant therapy.
Seven patients were lost to follow-up after discharge. The other 91 patients were followed up for a median period of 48.57 mo (range, 7 mo to 14 years).
After discharge, 4 patients (4.40%) had rehemorrhage including hematemesis in two and melana in two. Encephalopathy was observed in three patients (3.30%), one of them received liver transplantation and the others received conservative treatment.
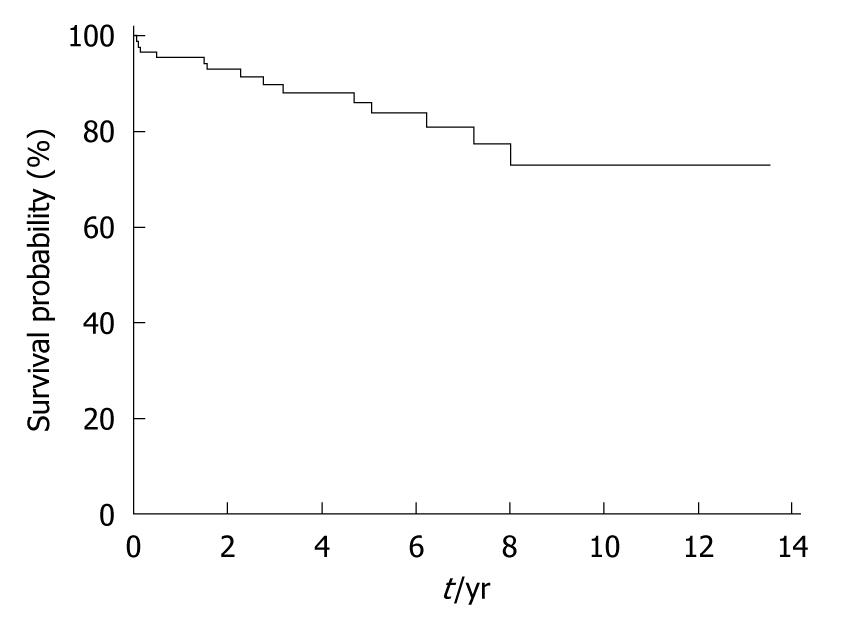
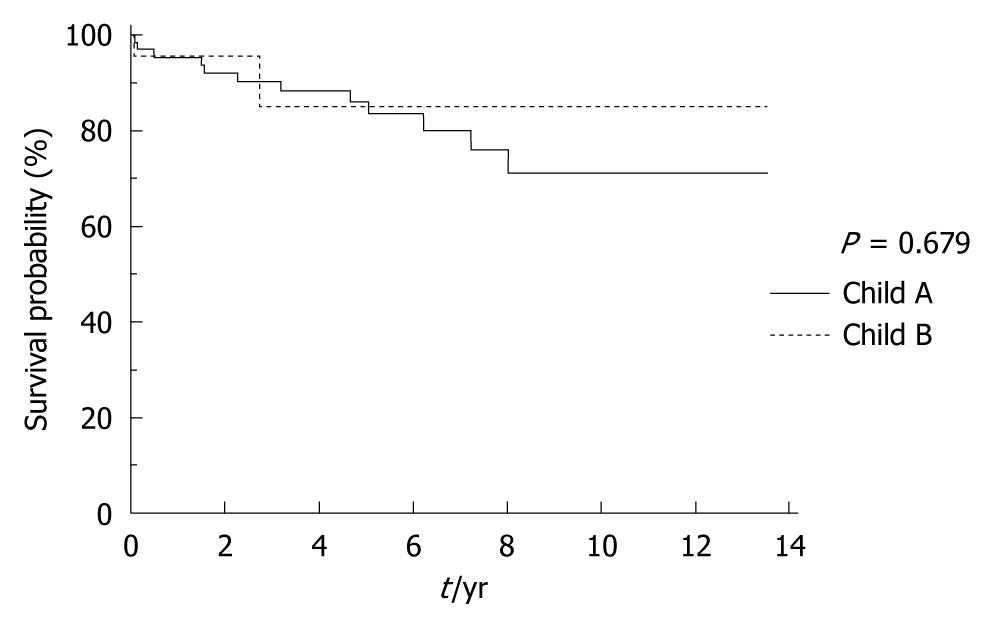
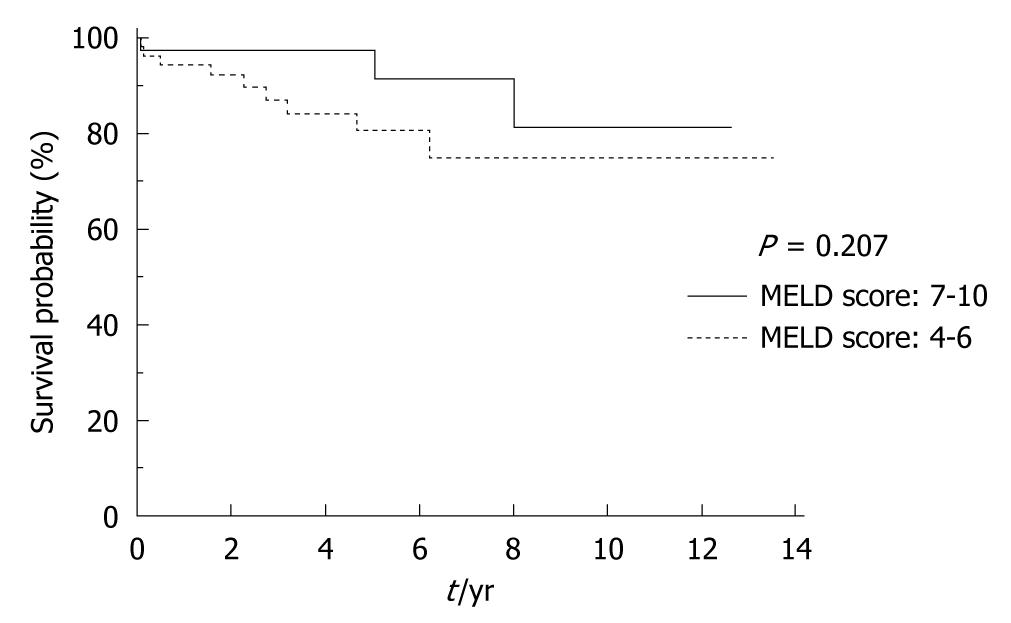
The 30-d perioperative mortality was 2.04% (2). One patient died of hematemesis after 28 d and another one died of abdominal bleeding and disseminated intravascular coagulopathy. Seven patients (7.27%) died 1 year after operation. The 5-year and 10-year mortality rates increased to 16.57% (15) and 27.64% (25) respectively after operation (Table 4, Figure 2). Among the 13 deaths, 8 patients died of progressive hepatic failure, and 5 died of hepatocellular carcinoma. One patient died of uncontrolled gastrointestinal rehemorrhage, and one died of unknown cause. For patients with Child A variceal hemorrhage, the survival after shunting was similar to those with Child B variceal hemorrhage (P > 0.05) (Figure 3). The long-term survival rate in patients with variceal hemorrhage with MELD score 7-10 was similar to those with MELD score 4-6 as well (Figure 4).
| Years after shunting | Cumulative survival (%) | Cumulative mortality (%) | Death (n) |
| 0 | 95.43 | 4.57 | 4 |
| 1 | 92.81 | 7.19 | 2 |
| 2 | 88.21 | 11.79 | 3 |
| 3 | 86.45 | 13.55 | 1 |
| 4 | 84.44 | 15.56 | 1 |
| 5 | 82.12 | 17.88 | 1 |
| 6 | 79.29 | 20.71 | 1 |
| 7 | 75.69 | 24.31 | 1 |
| 8 | 71.24 | 28.76 | 1 |
| 9 | 71.24 | 28.76 | 0 |
| 10 | 71.24 | 28.76 | 0 |
| 11 | 71.24 | 28.76 | 0 |
| 12 | 71.24 | 28.76 | 0 |
| 13 | 71.24 | 28.76 | 0 |
Since liver transplantation becomes the most promising treatment for advanced hepatic diseases in terminal stage, surgical shunting has been much less practiced in treating cirrhotic patients with portal hypertension. However, not all cirrhotic patients, especially those with better liver function and variceal hemorrhage, have chance to receive liver transplantation in China. Therefore, surgical shunting is still the main treatment for controlling variceal hemorrhage. The present study was conducted to investigate the long-term effectiveness of small-diameter proximal splenorenal venous shunt for patients with portal hypertensive bleeding.
The main etiology of hepatic cirrhosis is hepatitis B in China. Splenomegaly and hypersplenism exist in almost all patients with portal hypertension simultaneously. Splenectomy and proximal splenorenal venous shunt became the first modus operandi in our study. In addition, a high incidence of rebleeding and encephalopathy was observed in patients with Child C variceal hemorrhage who received small-diameter portacaval shunt[3]. There are also higher operative mortality and the lower long-term survival rate observed in patients with Child C variceal hemorrhage after partial portacaval shunting than those with Child A and B variceal hemorrhage[14]. So it has been suggested that the indication for partial portacaval shunting is Child A and some selected Child B variceal hemorrhage patients and the anticipated outcome of shunting is at least equivalent to that of liver transplantation in these patients[14]. Thus only patients with Child A or B variceal hemorrhage were enrolled for shunting in our center and selective operation was adopted instead of urgent shunting.
It was confirmed that small-diameter (mostly 8-10 mm) proximal splenorenal venous shunt could effectively prevent variceal rehemorrhage in our study. Rebleeding rate in the peri-operative period (6.02%) was reasonable considering that the main cause of bleeding was the thrombosis. When compared with the results from other types of small-diameter portacaval shunt, the long-term rebleeding rate was relatively low (4.04%). In H-graft portacaval shunting reported by Rosemurgy et al[15]. Collins et al[14], late rehemorrhage was 5.4% and 8%, respectively. In small-stoma (10-12 mm) side-to-side portacaval shunting by Johansen et al[16], 4 patients (8%) had rebleeding. The most important reason for the successful prevention of rehemorrhage is to maintain a relatively hypertensive portal system (a higher FPP or portacaval pressure gradient). It was suggested that high portal pressures may reduce absorption of potential neurotoxins from the gut[17]. We aimed to obtain 2.64 kPa (26.94 cmH2O) of FPP after shunting. The FPP before and after shunting showed that this requirement is essential. The mean FPP after shunting was slightly higher than target value (29.18 cm vs 26.94 cm) during the operation. The data by Sarfeh et al[18] showed that portal venous pressure would decrease after shunting. Although there is lack of direct information concerning long-term portal hemodynamics of shunting, our clinical observation suggested that higher FPP might have maintained in most cases.
As demonstrated by Bismuth et al[19], the small diameter side-to-side portacaval shunting was aimed to prevent hemorrhage by reducing variceal pressure while keeping considerable hepatic portal flow. However, the progressive enlargement of the stoma after shunting will eventually keep the total shunting of blood away from the liver[2], which will consequently increase the incidence of chronic encephalopathy. When compared with the results in a side-to-side portacaval anastomosis with small stomas, a low incidence of encephalopathy was observed in our study. There are two potential reasons to explain the difference. First, one important intervention was that continuous suture using nonabsorbent stitches was adopted in our study instead of using interrupted suture introduced by Capussotti et al[20] and Bismuth et al[19]. Although the underlying change of anastomosis after shunting was not fully understood, we could hypothesize that the stoma might keep its original size for a long time after operation. The second important factor was that FPP reached a higher level after shunting. Prevention of encephalopathy depends on the preservation of splanchnic venous pressure[17,21] and/or to preservation of nutrient hepatic blood flow[22]. However, it has been shown that long-term outcome after small-diameter H-graft portacaval shunt was not determined by direction or reversal of portal vein blood flow[23]. Johansen et al[16] suggested that portal vein pressure should not be reduced to normal range after portacaval shunting in order to prevent rehemorrhage and that maintenance of a higher portal vein pressure could keep a prograde portal flow.
The long-term survival rate was considerable in our cohort of patients. The 5-year and 10-year survival rates were 82% and 71% in patients with Child A and B variceal hemorrhage. After small-diameter H-graft portacaval shunting, a 7-year survival of 54% of Child A and B risk was reported by Collins et al[14], and 5/10-year survival of 67%/33% in Child A and 49%/16% in Child B reported by Rosemurgy et al[24]. Our long-term survival was higher than that of the two studies. Two reasons might exist: first, primary hepatic disease was different. Most of our patients suffered from hepatitis B compared with the patients with alcoholic cirrhosis in the Europe and United States. Second, some of the reported cases were emergent operations which might affect the long-term prognosis. In our study, all of the shunting was performed as selective operations. Progressive hepatic dysfunction was a major risk for late deaths. Eight of 13 patients died of hepatic dysfunction in this study. And there was no difference between Child A and Child B or between lower MELD scores in long-term survival. These results also suggested that there was a higher survival in patients with better liver function despite of pre-shunting or post-shunting. Although there are various prognostic factors such as age, occurrence of hepatocellular carcinoma, cardiovascular diseases, etc., liver function is still a determinant factor of survival on the whole.
As a nonsurgical intervention, transjugular intrahepatic portosystemic shunt (TIPS) has been used to treat the complications of portal hypertension. TIPS is accepted widely because of its high rate of decompressing the portal circulation, no requirement for anesthesia, very low procedure-related mortality and suitableness for severe cirrhotic patients. For control of esophageal and gastric variceal bleeding, TIPS has an excellent hemostatic effect (95%) with a low rebleeding rate (< 20%)[25]. However, encephalopathy and stent dysfunction are two major drawbacks[25]. Comparing with TIPS, small-diameter proximal splenorenal venous shunt can also acquire a high rate of bleeding control and low occurrence of encephalopathy. However, for patients with poor hepatic function, TIPS is more advantageous than partial portacaval venous shunt. As TIPS may not be the mainstream therapy in China, small-diameter proximal splenorenal venous shunting remains the first choice for the treatment of portal hypertension.
In conclusion, small-diameter proximal splenorenal venous shunting can be performed successfully in patients with better liver function. After shunting, the incidence of variceal rehemorrhage can be controlled effectively and the incidence of encephalopathy is reduced significantly as well.
ACKNOWLEDGMENTS
The authors thank Dr. Yan-Yan Song for her help with statistical analysis and Dr. Lu Hui for his help in the revision of the manuscript.
Total portacaval shunt provides an excellent effect of the treatment for hemorrhage due to esophageal and/or gastric varices but with a deleterious effect on liver function leading to a high encephalopathy rate. Partial portacaval shunt could effectively decompress portal vein while maintaining prograde portal flow to the liver to diminish postoperative encephalopathy.
This retrospective investigation analyzed the long-term outcome of proximal splenorenal venous shunting with small stoma. Variceal rehemorrhage could be prevented by this operation.
For patients with variceal hemorrhage, the suitable shunting diameter of anastomosis could be calculated according to the hemodynamic data of portal venous system by Duplex Doppler ultrasonography before the operation of proximal splenorenal venous shunt.
Small-diameter proximal splenorenal venous shunt could be used to control the occurrence of variceal rehemorrhage effectively while the incidence of encephalopathy can be reduced significantly.
Small-diameter proximal splenorenal venous shunt: this is a surgical procedure in which the proximal splenic vein is attached to the left renal vein with small-diameter anastomosis; Esophageal variceal hemorrhage: bleeding from esophageal varices due to portal hypertension.
This is an interesting paper with some restrictions. The period from 1996 to 2009 is very long and such studies have problems with changes in general management of these patients and the outcome. The authors did not mention the role of transjugular intrahepatic portasystemic shunting as an important part in the management of portal hypertension.
Peer reviewer: Dr. Oliver Mann, MD, Senior Attending Physician and Deputy Director, Department of General, Visceral and Thoracic Surgery, University of Hamburg, Martini Str. 52, D-20246 Hamburg, Germany
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
| 1. | Sarfeh IJ, Rypins EB, Mason GR. A systematic appraisal of portacaval H-graft diameters. Clinical and hemodynamic perspectives. Ann Surg. 1986;204:356-363. [PubMed] |
| 2. | Adam R, Diamond T, Bismuth H. Partial portacaval shunt: renaissance of an old concept. Surgery. 1992;111:610-616. [PubMed] |
| 3. | Darling RC, Shah DM, Chang BB, Thompson PN, Leather RP. Long-term follow-up of poor-risk patients undergoing small-diameter portacaval shunts. Am J Surg. 1992;164:225-27; discussion 225-27;. [PubMed] |
| 4. | Rosemurgy AS, McAllister EW, Kearney RE. Prospective study of a prosthetic H-graft portacaval shunt. Am J Surg. 1991;161:159-63; discussion 163-4. [PubMed] |
| 5. | Sarfeh IJ, Rypins EB, Conroy RM, Mason GR. Portacaval H-graft: relationships of shunt diameter, portal flow patterns and encephalopathy. Ann Surg. 1983;197:422-426. [PubMed] |
| 6. | Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5:419-424. [PubMed] |
| 7. | Vinel JP, Cassigneul J, Levade M, Voigt JJ, Pascal JP. Assessment of short-term prognosis after variceal bleeding in patients with alcoholic cirrhosis by early measurement of portohepatic gradient. Hepatology. 1986;6:116-117. [PubMed] |
| 8. | Yang WP, Li HW, Cai WY, et al. Investigation into the prediction of proper diameter for portal-systemic shunt by percutaneous transhepatic portagraphy. Zhong Hua Xiao Hua Za Zhi. 1991;11:151-153. |
| 9. | ROUSSELOT LM, MORENO AH, PANKE WF. Studies on portal hypertension. IV. The clinical and physiopathologic significance of self-established (nonsurgical) portal systemic venous shunts. Ann Surg. 1959;150:384-412. [PubMed] |
| 10. | Burcharth F, Sørensen TI, Andersen B. Findings in percutaneous transhepatic portography and variceal bleeding in cirrhosis. Surg Gynecol Obstet. 1980;150:887-890. [PubMed] |
| 11. | Cai WY, Yang WP, Chen H, et al. Prediction of the optimum diameter for portasystemic shunt by preoperative Duplex ultrasonography. Wai Ke Li Lun Yu Shi Jian. 1999;4:81-83. |
| 12. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 13. | Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trial. Am J Dig Dis. 1978;23:398-406. [PubMed] |
| 14. | Collins JC, Ong MJ, Rypins EB, Sarfeh IJ. Partial portacaval shunt for variceal hemorrhage: longitudinal analysis of effectiveness. Arch Surg. 1998;133:590-52; discussion 590-52;. [PubMed] |
| 15. | Rosemurgy AS, Serafini FM, Zervos EE, Goode SE. Small-diameter prosthetic H-graft portacaval shunt: definitive therapy for variceal bleeding. J Gastrointest Surg. 1998;2:585-591. [PubMed] |
| 16. | Johansen K. Partial portal decompression for variceal hemorrhage. Am J Surg. 1989;157:479-482. [PubMed] |
| 17. | Rikkers LF. Portal hemodynamics, intestinal absorption, and postshunt encephalopathy. Surgery. 1983;94:126-133. [PubMed] |
| 18. | Sarfeh IJ, Rypins EB. Partial versus total portacaval shunt in alcoholic cirrhosis. Results of a prospective, randomized clinical trial. Ann Surg. 1994;219:353-361. [PubMed] |
| 19. | Bismuth H, Franco D, Hepp J. Portal-systemic shunt in hepatic cirrhosis: does the type of shunt decisively influence the clinical result? Ann Surg. 1974;179:209-218. [PubMed] |
| 20. | Capussotti L, Vergara V, Polastri R, Bouzari H, Galatola G. Liver function and encephalopathy after partial vs direct side-to-side portacaval shunt: a prospective randomized clinical trial. Surgery. 2000;127:614-621. [PubMed] |
| 21. | Johansen K. Prospective comparison of partial versus total portal decompression for bleeding esophageal varices. Surg Gynecol Obstet. 1992;175:528-534. [PubMed] |
| 22. | Rypins EB, Milne N, Sarfeh IJ. Analysis of nutrient hepatic blood flow after 8-mm versus 16-mm portacaval H-grafts in a prospective randomized trial. Am J Surg. 1995;169:197-200; discussion 200-1. [PubMed] |
| 23. | Rosemurgy AS, McAllister EW, Goode SE. Direction or reversal of preshunt portal blood flow as determinants of outcome up to 1 year after small-diameter prosthetic H-graft portacaval shunt. J Surg Res. 1995;58:432-434. [PubMed] |
| 24. | Rosemurgy A, Thometz D, Clark W, Villadolid D, Carey E, Pinkas D, Rakita S, Zervos E. Survival and variceal rehemorrhage after shunting support small-diameter prosthetic H-graft portacaval shunt. J Gastrointest Surg. 2007;11:325-332. [PubMed] |
| 25. | Colombato L. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension. J Clin Gastroenterol. 2007;41 Suppl 3:S344-S351. [PubMed] |