Published online Jul 21, 2011. doi: 10.3748/wjg.v17.i27.3220
Revised: December 5, 2010
Accepted: December 12, 2010
Published online: July 21, 2011
AIM: To investigate the expression levels of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), vascular endothelial growth factor receptor-3 (VEGFR-3) and CD44 genes and the relationship between their levels and clinicopathological parameters in gastric cancer.
METHODS: Tissue samples were obtained from 33 patients (8 females) with gastric cancer. mRNA levels of LYVE-1, VEGFR-3 and CD44 in normal and tumor tissues were quantitatively measured using real time polymerase chain reaction. The results were correlated with lymph node metastasis, histological type and differentiation of the tumor, T-stage, and presence of vascular, perineural and lymphatic invasions. The distribution of molecules in the tissue was evaluated using immunohistochemistry.
RESULTS: LYVE-1, CD44 and VEGFR-3 gene expression levels were significantly higher in gastric cancer than in normal tissue. While there was no correlation between gene expressions and clinicopathologic features such as histologic type, differentiation and stage, gene expression levels were found to be increased in conjunction with positive lymph node/total lymph node ratio and the presence of perineural invasion. A significant correlation was also found between LYVE-1 and CD44 over-expressions and perineural invasion and lymph node positivity in gastric cancers. When the distribution of LYVE-1 antibody-stained lymphatic vessels in tissue was evaluated, lymphatic vessels were located intra-tumorally in 13% and peri-tumorally in 27% of the patients. Moreover, lymph node metastases were also positive in all patients with LYVE-1-staining.
CONCLUSION: LYVE-1, VEGFR-3 and CD44 all play an important role in lymphangiogenesis, invasion and metastasis. LYVE-1 is a perfectly reliable lymphatic vessel marker and useful for immunohistochemistry.
- Citation: Ozmen F, Ozmen MM, Ozdemir E, Moran M, Seçkin S, Guc D, Karaagaoglu E, Kansu E. Relationship between LYVE-1, VEGFR-3 and CD44 gene expressions and lymphatic metastasis in gastric cancer. World J Gastroenterol 2011; 17(27): 3220-3228
- URL: https://www.wjgnet.com/1007-9327/full/v17/i27/3220.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i27.3220
Gastric cancer is the second most common cause of cancer deaths worldwide[1]. As the stage of disease has definitive influence on survival, earlier diagnosis is highly critical. Lymphatic spread of gastric cancer cells to regional lymph nodes is one of the earliest events associated with distant metastasis and poor prognosis[2]. Depth of invasion, lymph node metastases and presence of distant metastases have all been found to be essential prognostic factors[3]. Vascular endothelial growth factor receptor-3 (VEGFR-3) is a tyrosine kinase receptor and is expressed in lymphatic endothelial cells. Increased VEGFR-3 expression correlates with regional lymph node metastasis in colorectal cancers[4-6]. In particular, VEGF-D and VEGFR-3 were recently reported to be independent prognostic markers in gastric cancer, and VEGF-D was found to be correlated with lymphatic metastasis[7]. Many factors have recently been proposed as markers for lymphatic endothelium. Lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) is the lymphatic vessel endothelial hyaluronic acid (HA) receptor, located in lymph nodes and in the luminal and abluminal surfaces of lymphatic vessels. LYVE-1 is very effective in the passage of lymphocytes and tumor cells into the lymphatics[8,9]. CD44 is another cell surface receptor for HA, expressed by lymphocytes, macrophages and tumor cells. CD44 is also responsible for the passage of lymphocytes and tumor cells into the lymphatics, similar to LYVE-1[10]. LYVE-1 has 43% similarity with CD44 but its specificity to HA is higher than that of CD44[11]. Quantification of the lymphatic vessel density (LVD) in the tumor might also be important for the evaluation of lymphangiogenesis and lymphatic metastasis[11].
The present study aimed to investigate the expression levels of LYVE-1, VEGFR-3 and CD44 genes in human tissues with or without tumor using real-time polymerase chain reaction (RT-PCR), and to evaluate the relationship between these expression levels and clinicopathological parameters such as tumor type, stage, differentiation, and the presence of lymph node metastasis, vascular invasion and neural/perineural invasion in gastric cancer. The LYVE-1, CD44 and VEGFR-3 protein expressions in tissues were also demonstrated using immunohistochemical and Western blotting (WB) analysis.
All tissue samples used in this study were obtained from patients who underwent radical surgery for gastric cancer in the 7th Surgical Clinic of the Ankara Numune Teaching and Research Hospital. All molecular studies were carried out in the research laboratories of Hacettepe University Institute of Oncology and Department of Pathology, Ankara Numune Hospital.
The study protocol was approved by the local ethics committee of Hacettepe University (04/12-19), in accordance with the Declaration of Helsinki, and all participants provided written informed consent.
Thirty-three patients [8 females; median age (range) 58 years (42-78)] with gastric cancer who underwent radical gastrectomy and D2α lymph node dissection were included in the study. All patients provided written informed consent for the procedure and to participate in the study. During and after surgery, paired tissue samples (tumoral and normal tissues) from all patients were obtained according to the protocol. During surgical removal of each tumor, an adjacent section of normal tissue was also removed following pathological confirmation that it was free from tumor deposits. Samples were then transferred to the laboratory and kept at either -20°C (tissues in RNA-Later) or -80°C (tissues in liquid nitrogen), for further testing.
During the operations, lymph nodes were sampled according to the Japanese Research Society of Gastric Cancer classification[12]. Macroscopically involved lymph nodes or lymph nodes of more than 1 cm in size were sampled. The same procedure was applied for the normal-appearing lymph nodes. After laparotomy, in parallel with the dissection protocol, paraaortic lymph nodes were sampled first, followed by perivascular (celiac trunk) and perigastric lymph nodes sampling. After completion of surgery and total or subtotal removal of the stomach, samples were obtained from the tumor and normal tissues. Routine pathological examination of surgical specimens was carried out to determine the lymph node status and for accurate staging.
Total RNA was extracted from normal/tumoral tissues (200 mg) and normal/tumoral lymph node specimens (100 mg) using an RNeasy Midi Kit (Qiagen, CA, USA) according to the manufacturer’s instructions. Tissue lysis was performed with Mini Bead Beater-8 (Biospec, OK, USA). Concentration and purity of RNAs were determined on the NanoDrop 1000 spectrophotometer (Thermo, DE, USA). All samples were run on denaturing agarose gel. cDNA was subsequently synthesized using the RevertAid First Strand cDNA Synthesis Kit (Fermentas, MD, USA) with 800 ng of total RNA isolated for each sample.
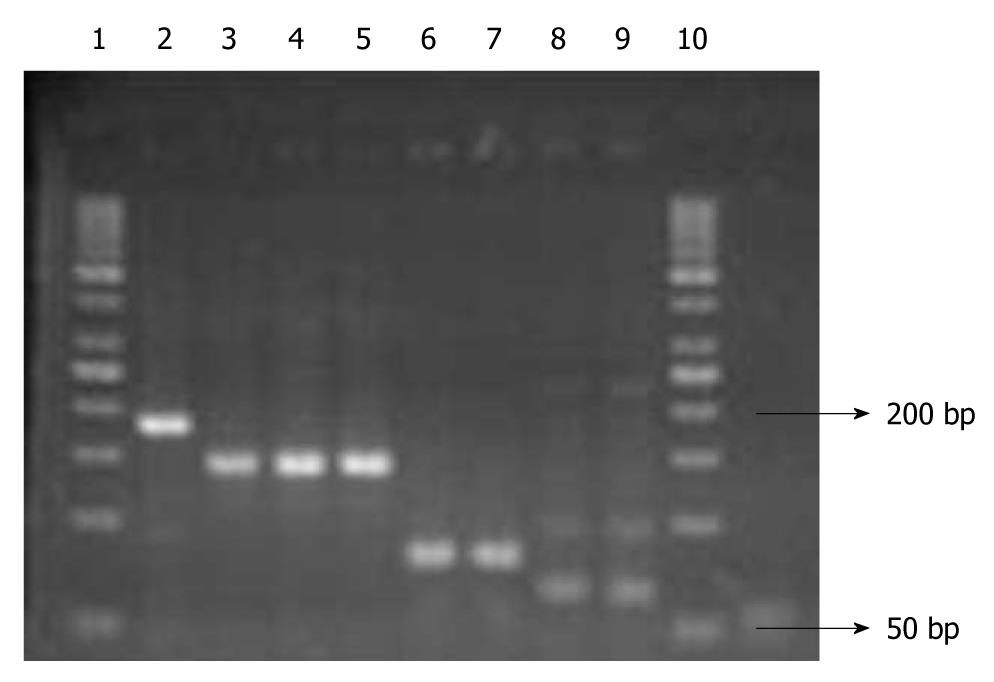
Before RT-PCR, traditional PCR was performed in order to test the primers of LYVE-1, VEGFR-3, CD44 and GAPDH genes and the annealing degrees of the primers, as well as to optimize the levels of MgCl2. The primers for the genes are given in Table 1. To ensure that the correct products were obtained, amplified products were separated by 3% agarose gel electrophoresis (Figure 1).
| Genes | Primer sequences (5'-3') | Chromosome | PCR products (bp) |
| LYVE-1 | F: CCAGTGAGCCGACAGTTTGCAG | ||
| R: CAGGTATTGTAGAGTAAGGGGATGCC | 11p15 | 184 | |
| VEGFR-3 | F: ACGGCCTGGTGAGTGGC | ||
| R: CGTTTGACTCCTCCGTGATG | 5q35.3 | 63 | |
| CD44 | F: GCAACTCCTAGTAGTACAACGGAAGA | ||
| R: CGATATCCCTCATGCCATCTGA | 11p13 | 80 | |
| GAPDH | F: GGCTGAGAACGGGAAGCTTGTCAT | ||
| R: CAGCCTTCTCCATGGTGGTGAAGA | 12p13 | 143 |
The quantitative RT-PCR (qRT-PCR) assays were performed with the Corbet 6000 (Rotor-Gene, CA, USA) instrument using Lightcycler-DNA master SYBR Green I (Roche Diagnostics, Mannheim, Germany), and the expression levels of lymphangiogenic genes were quantified. The RT-PCR reactions were set up in a volume of 20 μL, containing 5 μL of sample cDNA, 1 × SYBR Green I dye, 1.5 mmol/L MgCl2, and 5 pmol of LYVE-1, VEGFR-3, CD44 and GAPDH specific primers. The cycling conditions were as follows: 95°C for 30 s, 60°C for 30 s, 81°C for 5 s and 72°C for 30 s for 40 cycles, with initial melting at 95°C for 5 min.
Relative expression levels were calculated using the PCR threshold cycle number (CT) for each tissue and control sample using the formula 2-(ΔCTsample - ΔCTcontrol)[13,14]. ΔCT represents the difference in CT values between the target and GAPDH transcripts. RT-PCR was performed in duplicate for each sample and average CT values were calculated.
Consecutive 4-μm-thick sections were cut from each paraffin sample. Sections were immunolabeled for LYVE-1, VEGFR-3 and CD44.
The stains were carried out using the Bond Max (Leica Microsystems - Biosystems Division, Wetzlar, Germany) with the Polymer Refine Detection Kit (Vision Biosystem, MA, USA). Immunohistochemical stains were performed with rabbit polyclonal antibodies LYVE-1 (Abcam, MA, USA) (1:100), VEGFR-3 (Gene-Tex, CA, USA) and CD44 (Gene-Tex, CA, USA).
LVD was determined from the counts of LYVE-1-positive vessels. Vessel density was assessed by light microscopy of the intratumoral region containing the greatest number of capillaries and small venules. Highly vascular areas were identified by scanning tumor sections at low power (× 40 and × 100). After the six areas of greatest neovascularization were identified, a vessel count was performed at X200, and the mean count of six fields was calculated. In slides immunolabeled for LVYE-1 and VEGFR-3, only vessels with typical morphology (including a lumen) were counted as lymphatic vessels[15]. CD44-positive cells were determined in tumor and normal tissue sections and compared.
Data from patients and laboratory studies were stored and evaluated using SPSS 15.00 for Windows (SPSS, Chicago, IL, USA). All descriptive data were expressed as median (range). The relationships between these and expression levels of LYVE-1, VEGFR-3 and CD44 were evaluated using the Fisher Exact Probability test. As the distribution of genes was not normal, the nonparametric tests were used for evaluation. The comparison between clinicopathological characteristics and gene expression levels was performed using Mann-Whitney U and Kruskal-Wallis tests. The correlation between clinicopathological features and gene expression levels was evaluated using logistic regression analysis and Spearman’s correlation coefficients. P-values less than 0.05 were considered significant.
All information on patients with gastric cancer, including age, sex, Lauren histological tumor type, degree of differentiation, T-stage, final stage, number of total lymph nodes (TLN) removed, number of positive lymph nodes (PLN), the PLN/TLN ratio, presence of vascular, neural and perineural invasion, and relative expression levels of LYVE-1, VEGFR-3 and CD44 calculated using qRT-PCR and the 2-ΔΔCT method[13,14], was stored and evaluated using SPSS 15.00 for Windows.
LYVE-1, VEGFR-3 and CD44 gene expression levels in relation to clinicopathological features are shown in Table 2.
| Features | n | LYVE-1 | P | CD44 | P | VEGFR-3 | P |
| Lauren type | |||||||
| Intestinal | 15 | 0.76 (0.04-2.66) | NS | 1.14 (0.17-2.80) | NS | 1.20 (0.02-3.70) | NS |
| Diffuse | 18 | 0.81 (0.09-3.12) | 1.14 (0.28-2.51) | 0.87 (0.26-1.83) | |||
| Differentiation | |||||||
| Well | 14 | 0.80 (0.04-2.66) | NS | 1.14 (0.17-2.80) | NS | 1.23 (0.02-3.70) | NS |
| Poor | 19 | 0.78 (0.09-3.12) | 1.14 (0.28-2.51) | 0.86 (0.26-1.83) | |||
| T-stage | |||||||
| T2 | 2 | 0.54 (0.15-0.93) | NS | 0.39 (0.17-0.60) | NS | 0.6 | NS |
| T3 | 17 | 0.69 (0.04-2.66) | 1.17 (0.28-2.80) | 0.99 (0.02-2.94) | |||
| T4 | 14 | 1.0 (0.10-3.12) | 1.24 (0.47-2.14) | 1.0 (0.26-3.70) | |||
| Vascular invasion | |||||||
| Negative | 15 | 0.82 (0.15-1.68) | NS | 1.80 (0.60-2.80) | NS | 1.06 (0.60-1.57) | NS |
| Positive | 18 | 0.96 (0.10-3.12) | 1.07 (0.28-2.14) | 1.13 (0.26-3.70) | |||
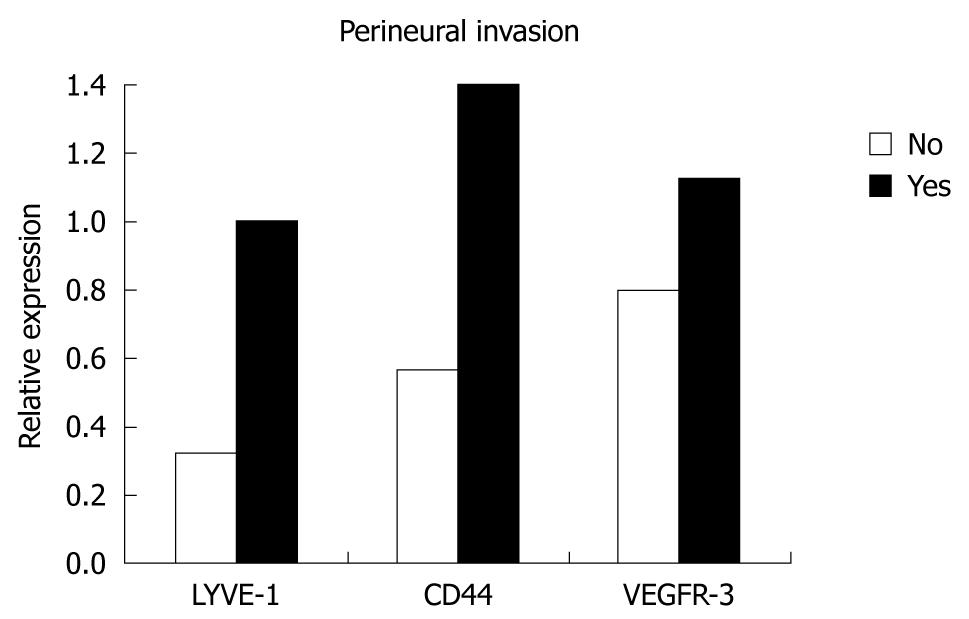
| Perineural invasion | |||||||
| Negative | 10 | 0.32 (0.10-0.93) | 0.020 | 0.57 (0.17-0.76) | 0.020 | 0.8 (0.26-2.21) | NS |
| Positive | 23 | 1.0 (0.04-3.12) | 1.40 (0.31-2.80) | 1.13 (0.02-3.70) | |||
| Neural invasion | |||||||
| Negative | 15 | 0.78 (0.04-3.12) | NS | 0.95 (0.17-2.80) | NS | 0.93 (0.02-2.94) | NS |
| Positive | 18 | 0.84 (0.21-2.22) | 1.48 (0.46-2.51) | 1.24 (0.40-3.70) | |||
| Lymph nodes | |||||||
| Negative | 5 | 0.66 (0.15-1.68) | NS | 0.74 (0.17-1.61) | NS | 0.86 (0.30-2.21) | NS |
| Positive | 28 | 0.82 (0.04-3.12) | 1.22 (0.28-2.80) | 1.06 (0.02-3.70) | |||
| PLN/TLN ratio | |||||||
| ≤ 0.4 | 25 | 0.53 (0.04-1.68) | NS | 1.06 (0.17-2.80) | NS | 0.83 (0.02-2.21) | NS |
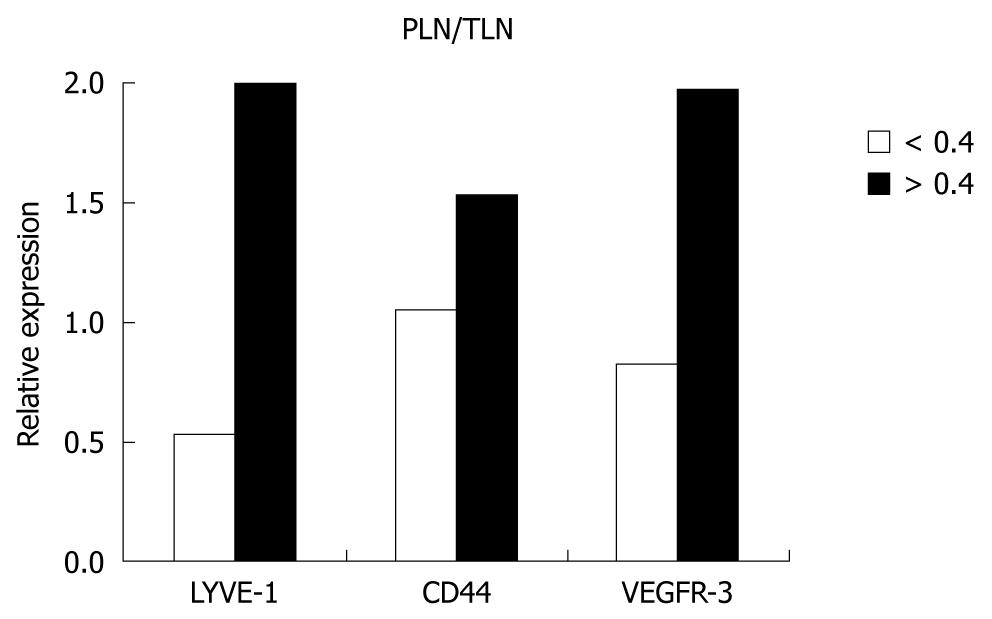
| > 0.4 | 8 | 2.0 (0.69-3.12) | 0.003 | 1.53 (0.47-2.40) | NS | 1.97 (0.28-3.70) | 0.050 |
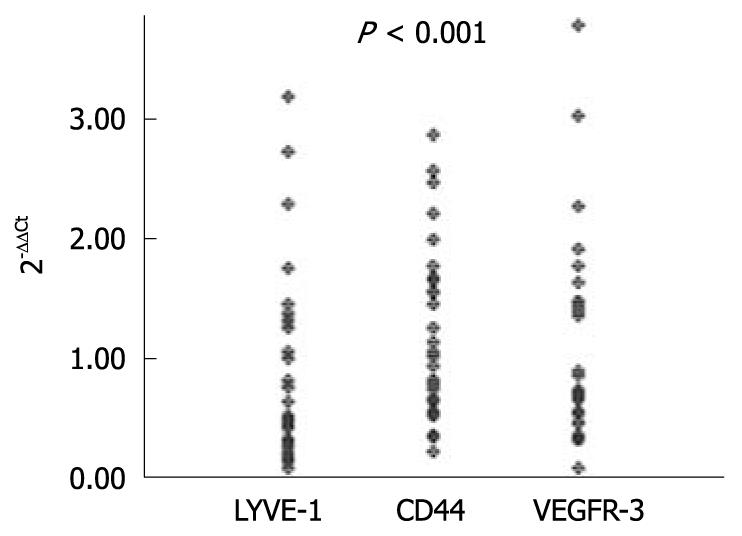
Expression levels of LYVE-1, VEGFR-3 and CD44 genes were significantly higher in tumoral tissues than in the normal tissues in gastric cancer patients, which was found to be independent of the age and gender of the patients (P < 0.001) (Figure 2). There was a linear correlation between the expression levels of LYVE-1 and CD44 molecules, and the same correlation was also found between their over-expression levels (Spearman’s rank correlation, P = 0.025 and P = 0.033).
The expression levels of the genes were higher in patients with lymph node metastases than patients without metastases, however, the difference did not reach the level of significance. When the PLN/TLN ratio was investigated, the expression levels of LYVE-1, VEGFR-3 and CD44 genes were significantly less in patients with a ratio of ≤ 0.20 than in patients with a PLN/TLN ratio of ≥ 0.4 (P = 0.001). Both expression levels and over-expression of the genes were increased with increased PLN/TLN ratio (Spearman’s rank correlations; P = 0.01 for LYVE-1, P = 0.036 for CD44 and P = 0.016 for VEGFR-3). The value of significance was most apparent when the PLN/TLN ratio was taken as ≥ 0.4 or < 0.4 (Figure 3). There was also a significant correlation between the PLN/TLN ratio, tumor volume and number of PLN (P < 0.001). When the expression levels of the genes in the lymph nodes of the stations were evaluated, it was found that their levels increased in parallel with expression levels in the tumor, but the difference was not found to be statistically significant.
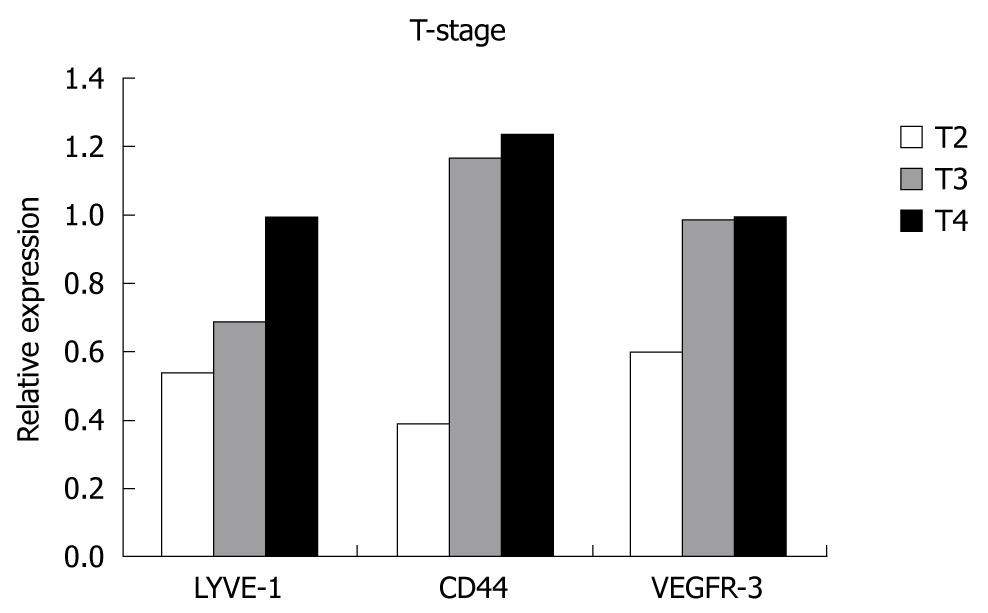
When the relationship between the T-stage of the tumor and relative expression levels of the genes was evaluated, we found that expression levels increased with increased T-stage, however, this increase was not statistically significant. Although the number of patients in earlier stages was too small, expression levels were much higher in T4-stage than T2, but the difference did not reach statistical significance, and no correlation was found between the T-stage and over-expressions (Figure 4).
On the other hand, there was a significant correlation between the final stage and T-stage, presence of lymph node metastasis, number of PLNs, and the PLN/TLN ratio.
There was also no correlation between over-expressions and neural invasion, whereas relative expression levels of LYVE-1, VEGFR-3 and CD44 were significantly increased with the presence of perineural invasion and reached statistical significance for LYVE-1 and CD44 (P < 0.001). All three genes were found to be over-expressed with perineural invasion and a statistically significant correlation was found between them (Figure 5).
Although the relative expression levels of all three genes were increased with the presence of vascular invasion, the difference was not statistically significant.
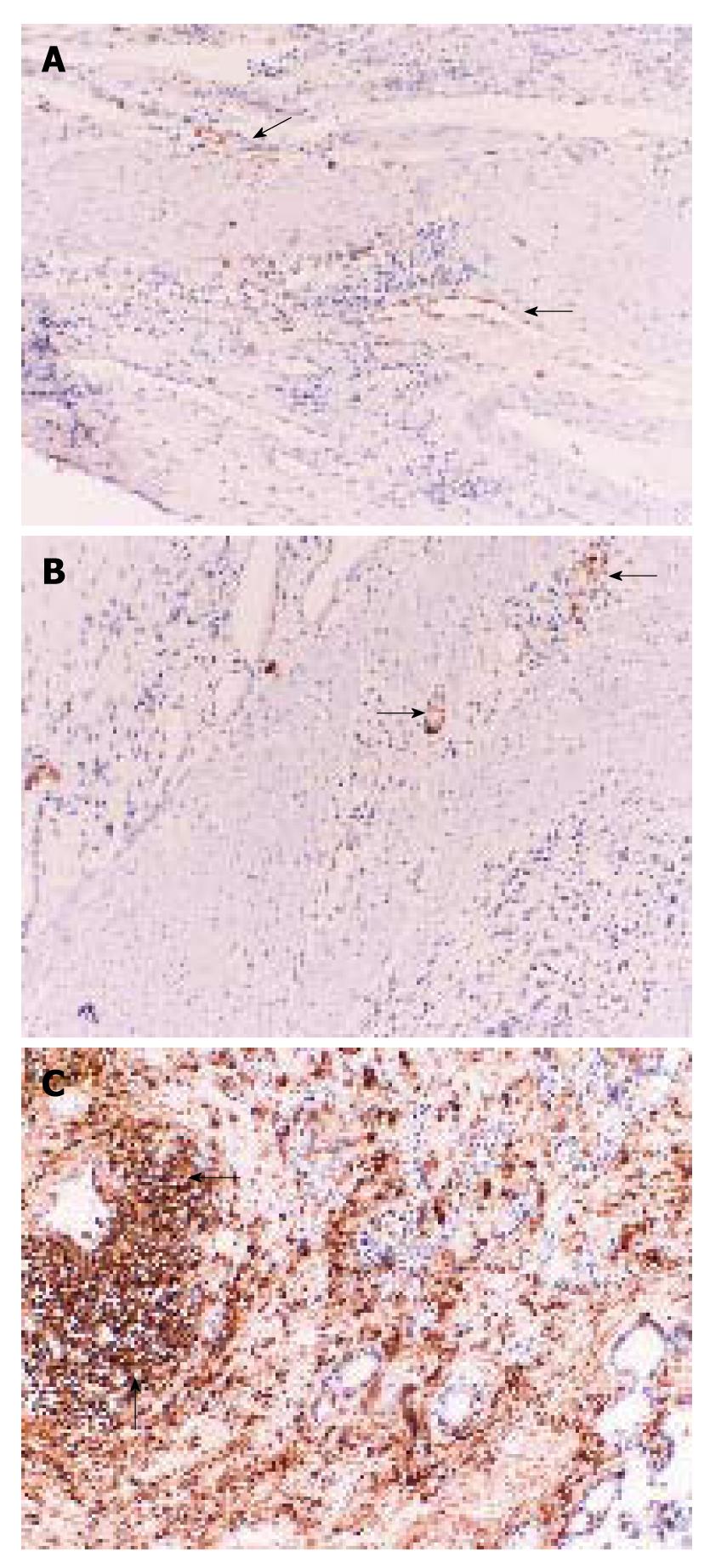
The expressions of LYVE-1, VGFR-3 and CD44 were all shown in tumor tissues using immunohistochemistry (Figure 6A-C). When the LVD at the central zone and periphery of the tumor was measured, 13% of patients had intratumoral staining and 27% had peritumoral staining for LYVE-1. Although the peritumoral distribution of LYVE-1+ LVD was higher, the difference was not statistically significant (χ2 test, 2.773, Df = 1, P = 0.09). Lymph node metastasis was positive in all patients with LYVE-1-stained lymphatic vessels, whether the LVD was peritumoral or intratumoral. However, we failed to find any correlation between LVD and lymph node metastasis (Spearman’s rank correlation coefficient, 0.26, P = 0.92).
The present study was designed to investigate whether LYVE-1, which is a specific molecule for lymphatics, as well as CD44 and VEGFR-3 might be used as markers for lymph node metastases of gastric tumors. We chose gastric cancer since it is well known to exhibit early metastasis to lymph nodes.
We evaluated the expressions of LYVE-1, VEGFR-3 and CD44 in normal gastric tissues, tumoral gastric tissues and lymph nodes with or without metastases using RT-PCR. The levels were compared to those in normal tissues.
Using qRT-PCR, we were able to show significantly higher relative expression levels of all three genes in tumoral tissue than in the normal tissue counterparts. The expression levels of LYVE-1, VEGFR-3 and CD44 were higher in patients with lymph node metastasis than in those without metastasis, however, the difference was not statistically significant. On the other hand, when we measured the correlation between the PLN/TLN ratio and relative expression levels of LYVE-1 in tumors, levels were significantly higher in patients with a PLN/TLN ratio ≥ 0.4. There was no significant correlation for VEGFR-3 or CD44. Over-expressions of LYVE-1, VEGFR-3 and CD44 were all significantly increased with the increased number of involved lymph nodes.
The lymphatic metastatic process of tumor cells follows a series of complex biologic reactions. In the initial phase, tumor cells first invade the stroma and penetrate into lymphatic vessels and reach the lymph nodes via lymphatic flow. Lymphangiogenesis plays a very important role in this stage. The secretion of growth factors such as VEGF-C, platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) from tumor cells increases the expression of VEGFR-3, which triggers lymphangiogenesis followed by the development of new lymphatic capillaries and increases the number of LYVE-1-secreting cells[16]. The increase in VEGFR-3 and LYVE-1 expressions is highly critical because it reflects lymphangiogenesis, indicating how tumor cells might easily reach the lymphatic system[17]. Molecules such as LYVE-1 and VEGFR-3 provide information on the behavior of the tumor, as the increase in these molecules reflects the increase in the number of lymphatic endothelial cells.
LYVE-1, VEGFR-3 and CD44 expression and over-expression levels were increased with the presence of neural and perineural invasion; on the other hand, VEGFR-3 and LYVE-1 were both increased with vascular invasion. However, the increase in LYVE-1 and CD44 was only significant in the presence of perineural invasion, which might be explained partly by the size of the study, heterogeneity of the patients and variation in tumor behavior.
The present study also investigated the correlation between increased expression levels of the genes and poor prognostic factors. It was found that expression levels did not change with Lauren type or with the degree of differentiation. LYVE-1, VEGFR-3 and CD44 expression levels were increased in conjunction with T stage.
We evaluated the gene expression levels in metastatic lymph nodes and found that the expression levels of LYVE-1, VEGFR-3 and CD44 all increased in parallel with the expression levels in tumor tissue, which was most apparent in LYVE-1 levels. When the relationships between the gene expression levels were analyzed, it was found that CD44 levels increased in parallel with LYVE-1 expression in tumors.
Hyaluronic acid (HA) is an extracellular matrix glycosaminoglycan found in tissues and body fluids, and plays a role in inflammation, leukocyte extravasation, wound healing, and metastasis of tumor cells[18]. The CD44 molecule is one of the most well-defined HA cell surface receptors with a very important role in HA homeostasis and is expressed on the surface of endothelial, mesenchymal and lymphoid cells. However, CD44 is functionally silent in resting leukocytes and is activated only to bind HA[19].
Lymphatic vessel endothelial HA receptor (LYVE-1) is a receptor for hyaluronan and is expressed by the endothelial cells of the lymphatic vessels. LYVE-1 is located both intra- and extra-luminally in these vessels; therefore, it is thought that LYVE-1 plays an important role in the transportation of both lymphocytes and other similar cells into the lymphatic system. Lymphocytes are routed into the lymphatic flow and caught by lymph nodes with the help of CD44 cells integrated with HA and with LYVE-1. As it is specific to the lymphatic endothelium, LYVE-1 might be a useful marker in identifying tissue lymphatics.
Vascular endothelial growth factor receptor-3 (VEGFR-3) is another lymphatic endothelial cell receptor that has an important role in lymphangiogenesis. Increased expression of VEGF-C during growth of the tumor increases the development of new lymphatic vessels so that VEGF-C may be considered as a marker of lymph node metastasis. VEGFR-3 might also be a marker for lymphangiogenesis; however, it appears to be inadequate for suggesting lymph node metastasis. Despite the definitions of LYVE-1, VEGFR-3 and CD44 and rapidly growing information on tumor biology, the molecular mechanisms regarding lymphatic invasion and the metastatic process to lymph nodes remain unclear.
Lymphatic vessels act not only in metastasis but also in the invasion of cancer cells. The molecules that are released from lymphatic endothelial cells such as matrix metalloproteinases and urokinase plasminogen activators increase invasion by helping tumor cell movement in adjacent tissues[20]. VEGF-C stimulates the secretion of CCL1 chemokine from endothelial cells, which interacts with CCR8 expressed by tumor cells and moves tumor cells towards lymphatic vessels[21]. This interaction is one of the most important steps in metastasis. All these molecules are secreted by both the endothelium of new lymphatic capillaries and that of other lymphatics already present around the tumor[22,23]. In addition to lymphatic markers such as LYVE-1 and VEGFR-3, we also used lymphatic involvement and perineural, neural and vascular invasion in order to better explain the process of tumor invasion and metastasis. Information gathered from this study supports this relationship.
CD44, which is secreted by tumor cells, binds HA, and during the transport of HA, it also intervenes in the transportation of tumor cells into the lymphatic flow[24]. Furthermore, over-secretion of CD44 by tumor cells might cause tumor cells to be perceived as lymphocytes by the immune system, which might trigger the occurrence of immune escape[25]. As we have shown, increased secretion of CD44 mRNA in gastric cancer might be an important indicator of the biologic process explained above.
We are not aware of any previous study investigating LYVE-1 mRNA levels in gastric cancer. In two previous studies, VEGFR-3 expressions were shown to be increased in tumor tissues and with the presence of lymph node involvement using RT-PCR[26,27]. It was recently shown that there was a correlation between increased VEGFR-3 levels and metastasis[11,28]. It has also been reported that increased expression levels of VEGFR-3 might be a prognostic indicator, especially in patients with advanced gastric cancer[7,29].
To our knowledge, there has only been one published study investigating the quantitatively measured relative expression levels of the CD44 gene in human gastric cancer[30]. Most of the previous studies on CD44 were based on immunohistochemical analysis and analyzed the correlation between poor prognosis and presence of CD44[31-33].
We found that 40% of cases (13/33) had lymphatic vessels in tumor tissue; 27% were located peritumorally and 13% were located intratumorally. However, we failed to find any correlation between LVD and lymph node metastasis, possibly due to the small study size.
There is still controversy regarding whether the intratumoral or the peritumoral lymphatics are functional, and which should be used for evaluation[34,35]. Although our study population was too small to comment, peritumoral lymphatics with wide lumens seem to be more functional and effective for the occurrence of metastasis as compared to the intratumoral lymphatics with either narrow or obstructed lumen. This hypothesis appears to be in accordance with previous findings obtained from animal studies[17,36,37]. It is also known that the lymphatic vessels are located at the submucosa and extend through the muscularis propria. When tumor cells reach this anatomic location, they can easily move to the lymphatic vessels, thereby increasing metastatic potential[17].
The question of whether the tumor uses new lymphatic capillaries occurring at its center due to lymphangiogenesis or lymphatic vessels already located peritumorally remains unanswered[36]. We believe that peritumoral lymphatics have a very important role in the metastatic process.
The present study also shows that immunohistochemical staining using LYVE-1 antibodies is more sensitive than staining using VEGFR-3 antibodies, which is in parallel with previous findings by other researchers[38]. VEGFR-3 antibodies stain venous capillaries as well as lymphatic vessels, which makes differentiation difficult. The presence of VEGFR-3 proteins in both normal and tumor tissues was shown by Western blotting, which correlated with the findings of Yonemura et al[26].
A previous study in patients with colorectal cancers revealed that the expression levels of VEGFR-3 are increased, whereas LYVE-1 levels were decreased in tumor tissue[39]. LYVE-1 levels were found to be increased in another study using immunohistochemistry, but the authors failed to show any increase in quantitatively measured relative expression levels[40]. Yuanming et al[28] investigated the expression levels of LYVE-1 and VEGFR-3 in gastroenteric tumors and evaluated their relationships with lymphatic metastasis and tumor progression. They concluded that VEGFR-3 expression was increased when lymph node metastasis was present, but they failed to show any correlation with LYVE-1. By using LYVE-1 antibody and immunohistochemistry, they found that there was a correlation between LVD and lymph node metastasis[28]. Although we were unable to show this correlation using immunohistochemistry, we were able to determine that relative expression levels of LYVE-1 increased with lymph node involvement in parallel with PLN/TLN ratios. Therefore, the findings of Chen et al[41] using immunohistochemistry support our study based on quantitative measurements. In the literature, there are only a few immunohistochemical studies on the quantitative evaluation of CD44 in gastric cancer and they did not reach a definitive conclusion[41,42].
In conclusion, we found that the expression levels of LYVE-1, CD44 and VEGFR-3 genes in gastric tumors were significantly increased when compared to levels in normal tissue. We failed to show any correlation between these expression levels and clinicopathological features such as histological type, differentiation and stage, however, a significant correlation was found between relative expression levels of LYVE-1 and CD44 genes and perineural invasion and lymphatic involvement, which supports the hypothesis that those genes play an important role during the process of lymphangiogenesis, invasion and lymph node metastasis in gastric cancer.
Although it is not possible to make definitive comments based on our findings regarding the relationships between clinicopathological features and peritumoral/intratumoral LVD and their effects on prognosis, LYVE-1 appears to be an excellent lymphatic vessel marker and LVD can be evaluated using immunohistochemical techniques. We believe that increased expression levels of these genes in biopsy specimens can be used as a predictor of metastasis, which might further facilitate disease staging, treatment planning and prognosis estimation.
Lymph node metastasis is one of the most important prognostic factors in gastric cancer. Quantification of the lymphatic vessel density in the tumor may be important for the evaluation of lymphangiogenesis and lymphatic metastasis. Lymphatic metastasis involves a series of complex reactions. Tumor cells first invade the stroma, penetrate the lymphatic vessels and reach the lymph nodes via lymphatic flow. Lymphangiogenesis is involved in this stage. The secretion of growth factors from the tumor cells increases the expression of vascular endothelial growth factor receptor-3 (VEGFR-3) which triggers lymphangiogenesis followed by the development of new lymphatic capillaries and an increase in the number of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) secreting cells. The increase in VEGFR-3 and LYVE-1 expressions is very important as it reflects lymphangiogenesis which allows tumor cells to reach the lymphatic system easily. Molecules such as LYVE-1 and VEGFR-3 provide information on the behaviour of the tumor, as an increase in these molecules reflects the increased number of lymphatic endothelial cells. Furthermore, over-secretion of CD44 by tumor cells might cause tumor cells to be perceived as lymphocytes by the immune system which might trigger the occurrence of immune escape. The present study aimed to investigate the expression levels of LYVE-1, VEGFR-3, CD44 genes and the relationship between these levels and clinicopathological parameters in gastric cancer.
To briefly introduce important areas in the research field related to this article.
Using quantitative real-time polymerase chain reaction, the authors were able to show significantly higher relative expression levels of all three genes in tumoral tissue than in the normal tissue counterparts. They failed to show any correlation between expression levels and clinicopathological features such as histological type, differentiation and stage, however, a significant correlation was found between relative expression levels of LYVE-1 and CD44 genes and perineural invasion and lymphatic involvement, which supports the hypothesis that these genes play an important role during the process of lymphangiogenesis, invasion and lymph node metastasis in gastric cancer.
Increased expression levels of these genes in biopsy specimens can be used as a predictor of metastasis, which might further facilitate disease staging, treatment planning and prognosis estimation.
This paper investigates the importance of LYVE1, VEGFR-3 and CD44 expression levels in gastric cancer and its relation to the metastasis.
Peer reviewer: Boris Kirshtein, MD, Department of Surgery "A" , Soroka Medical Center, Ben Gurion University of the Negev, POB 151, Beer Sheva, 84101, Israel
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
| 1. | Pisani P, Parkin DM, Bray F, Ferlay J. Erratum: Estimates of the worldwide mortality from 25 cancers in 1990. Int. J. Cancer, 83, 18-29 (1999). Int J Cancer. 1999;83:870-873. |
| 2. | Adachi Y, Shiraishi N, Suematsu T, Shiromizu A, Yamaguchi K, Kitano S. Most important lymph node information in gastric cancer: multivariate prognostic study. Ann Surg Oncol. 2000;7:503-507. |
| 3. | Ozmen MM, Ozmen F, Zulfikaroglu B. Lymph nodes in gastric cancer. J Surg Oncol. 2008;98:476-481. |
| 4. | Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186-191. |
| 5. | Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219-227. |
| 6. | Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387-395. |
| 7. | Jüttner S, Wissmann C, Jöns T, Vieth M, Hertel J, Gretschel S, Schlag PM, Kemmner W, Höcker M. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24:228-240. |
| 8. | Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526-538. |
| 9. | Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317-321. |
| 10. | Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789-801. |
| 11. | Kitadai Y, Kodama M, Cho S, Kuroda T, Ochiumi T, Kimura S, Tanaka S, Matsumura S, Yasui W, Chayama K. Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer. 2005;115:388-392. |
| 12. | Sue-Ling HM. Detection and treatment of early gastric cancer in the West. Gastric Cancer. 1998;1:8-9. |
| 13. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. |
| 14. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. |
| 15. | Kato T, Prevo R, Steers G, Roberts H, Leek RD, Kimura T, Kameoka S, Nishikawa T, Kobayashi M, Jackson DG. A quantitative analysis of lymphatic vessels in human breast cancer, based on LYVE-1 immunoreactivity. Br J Cancer. 2005;93:1168-1174. |
| 16. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. |
| 17. | Duff SE, Jeziorska M, Kumar S, Haboubi N, Sherlock D, O’Dwyer ST, Jayson GC. Lymphatic vessel density, microvessel density and lymphangiogenic growth factor expression in colorectal cancer. Colorectal Dis. 2007;9:793-800. |
| 18. | Weigel JA, Raymond RC, McGary C, Singh A, Weigel PH. A blocking antibody to the hyaluronan receptor for endocytosis (HARE) inhibits hyaluronan clearance by perfused liver. J Biol Chem. 2003;278:9808-9812. |
| 19. | Adamia S, Maxwell CA, Pilarski LM. Hyaluronan and hyaluronan synthases: potential therapeutic targets in cancer. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:3-14. |
| 20. | Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Ylä-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593-4599. |
| 21. | Alitalo K, Mohla S, Ruoslahti E. Lymphangiogenesis and cancer: meeting report. Cancer Res. 2004;64:9225-9229. |
| 22. | Jackson DG. The lymphatics revisited: new perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc Med. 2003;13:1-7. |
| 23. | Martín-Villar E, Scholl FG, Gamallo C, Yurrita MM, Muñoz-Guerra M, Cruces J, Quintanilla M. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113:899-910. |
| 24. | Heldin P, Karousou E, Bernert B, Porsch H, Nishitsuka K, Skandalis SS. Importance of hyaluronan-CD44 interactions in inflammation and tumorigenesis. Connect Tissue Res. 2008;49:215-218. |
| 25. | Seiter S, Arch R, Reber S, Komitowski D, Hofmann M, Ponta H, Herrlich P, Matzku S, Zöller M. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993;177:443-455. |
| 26. | Yonemura Y, Fushida S, Bando E, Kinoshita K, Miwa K, Endo Y, Sugiyama K, Partanen T, Yamamoto H, Sasaki T. Lymphangiogenesis and the vascular endothelial growth factor receptor (VEGFR)-3 in gastric cancer. Eur J Cancer. 2001;37:918-923. |
| 27. | Liu XE, Sun XD, Wu JM. Expression and significance of VEGF-C and FLT-4 in gastric cancer. World J Gastroenterol. 2004;10:352-355. |
| 28. | Yuanming L, Feng G, Lei T, Ying W. Quantitative analysis of lymphangiogenic markers in human gastroenteric tumor. Arch Med Res. 2007;38:106-112. |
| 29. | Sung JY, Lee S, Kim YW, Park YK. Vascular endothelial growth factor receptor-3 is a favorable prognostic factor in advanced gastric carcinoma. Oncol Rep. 2008;19:939-944. |
| 30. | Lee JL, Wang MJ, Sudhir PR, Chen GD, Chi CW, Chen JY. Osteopontin promotes integrin activation through outside-in and inside-out mechanisms: OPN-CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 2007;67:2089-2097. |
| 31. | Ringel J, Jesnowski R, Schmidt C, Ringel J, Köhler HJ, Rychly J, Batra SK, Löhr M. CD44 in normal human pancreas and pancreatic carcinoma cell lines. Teratog Carcinog Mutagen. 2001;21:97-106. |
| 32. | Gotoda T, Matsumura Y, Kondo H, Saitoh D, Shimada Y, Kosuge T, Kanai Y, Kakizoe T. Expression of CD44 variants and its association with survival in pancreatic cancer. Jpn J Cancer Res. 1998;89:1033-1040. |
| 33. | Satoh K, Shimosegawa T, Koizumi M, Toyota T. Expression of CD44 in duct cell carcinomas and in intraductal neoplasms of the pancreas. Anticancer Res. 1997;17:215-219. |
| 34. | Van der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, Fox SB, Harris AL, Dirix LY, Vermeulen PB. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer. 2006;95:1611-1625. |
| 35. | Ji RC. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 2006;25:677-694. |
| 36. | Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883-1886. |
| 37. | Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672-682. |
| 38. | Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC, Detmar M. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951-1960. |
| 39. | Parr C, Jiang WG. Quantitative analysis of lymphangiogenic markers in human colorectal cancer. Int J Oncol. 2003;23:533-539. |
| 40. | Gao F, Lu YM, Cao ML, Liu YW, He YQ, Wang Y. Expression and quantification of LYVE-1 in human colorectal cancer. Clin Exp Med. 2006;6:65-71. |
| 41. | Chen XY, Wang ZC, Li H, Cheng XX, Sun Y, Wang XW, Wu ML, Liu J. Nuclear translocations of beta-catenin and TCF4 in gastric cancers correlate with lymph node metastasis but probably not with CD44 expression. Hum Pathol. 2005;36:1294-1301. |
| 42. | Gulmann C, Grace A, Leader M, Butler D, Patchett S, Kay E. CD44v6: a potential marker of malignant transformation in intestinal metaplasia of the stomach? An immunohistochemical study using tissue microarrays. Eur J Gastroenterol Hepatol. 2003;15:981-986. |