INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are the 2 main entities of inflammatory bowel disease (IBD). Whereas CD mostly involves the distal ileum and/or the colon, the inflammation in UC is restricted to the colon. Since both disease locations are characterized by a high concentration of intestinal bacteria (107-108 organisms/g luminal content in the distal ileum and 1011-1012 in the colon), it is not surprisingly that these luminal microbes are playing an important role in the development of IBD (see our review in the World Journal of Gastroenterology[1]). In healthy mucosa, these microbes are sufficiently controlled by an adequate secretion of antimicrobial peptides and by the mucus layer, acting as a physical and chemical barrier (Figure 1A). Even if the pathogenesis of both forms of IBD is still under investigation, the last few years have revealed evidence that the differentiation from the intestinal stem cell towards the Paneth cell in ileal CD and towards the goblet cell in UC may be disturbed. This may result in a defective antimicrobial and mucus barrier, which enables the intestinal bacteria to invade the mucosa and trigger the inflammation. In this review, we focus on the intestinal stem cells, their differentiation towards Paneth and goblet cells, and the defective antimicrobial shield in ileal CD and mucus layer in UC as a consequence of a stem cell differentiation defect.
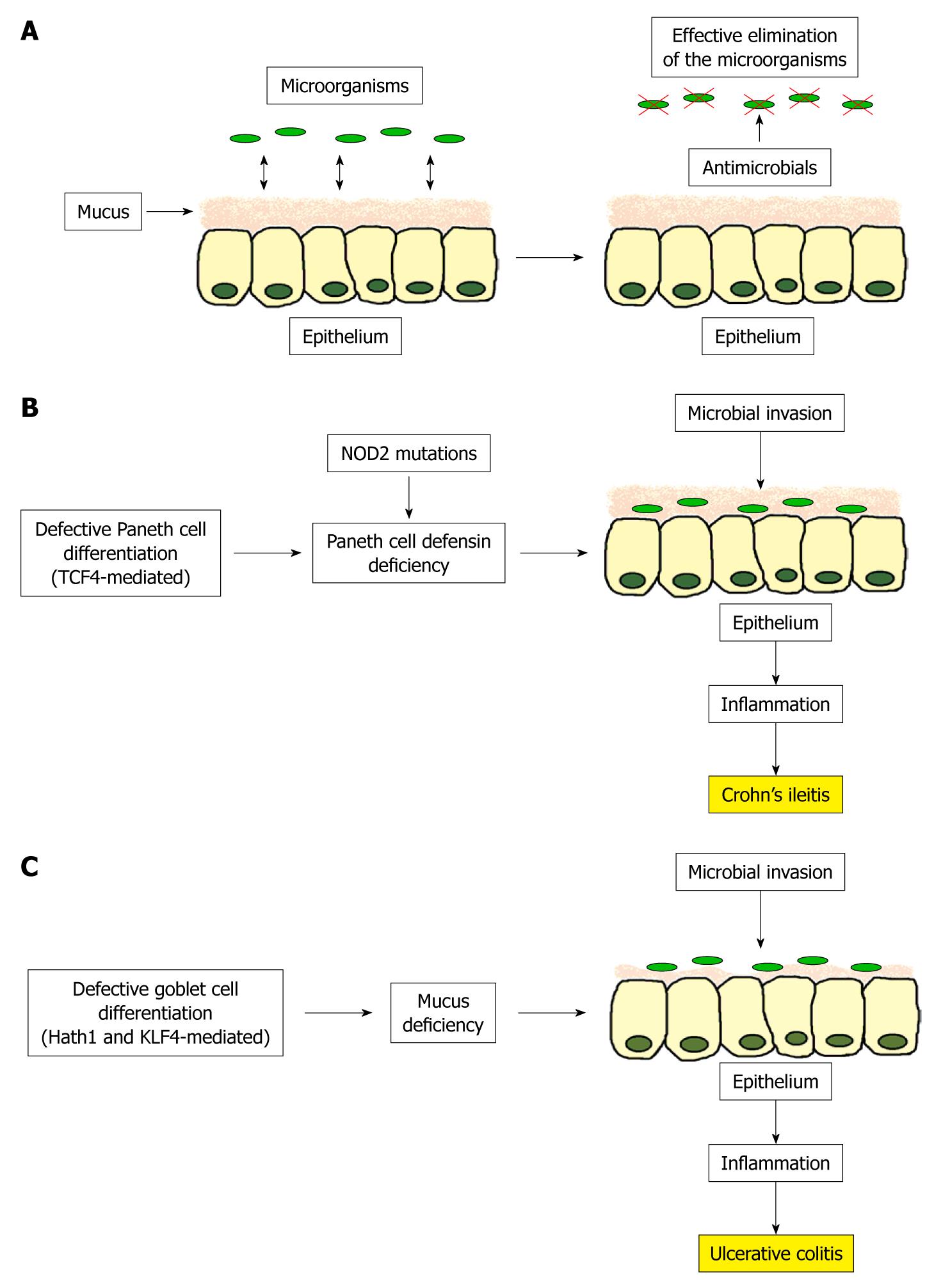
Figure 1 Proposed model for the pathogenesis of ileal Crohn’s disease and ulcerative colitis.
A: In the healthy ileal and colonic mucosa, luminal microbes are sufficiently controlled by an adequate secretion of antimicrobials peptides and a sufficient mucus layer; B: In ileal Crohn’s disease, defective Paneth cell differentiation mediated by a reduction of the Wnt transcription factor TCF4 leads to a decreased Paneth cell defensin secretion, especially in case of NOD2 mutations. This allows the luminal microbes to attach and invade in the mucosa causing inflammation; C: In ulcerative colitis, a defective goblet cell differentiation based on a missing induction of the transcription factors Hath1 and KLF4 results in mucus deficiency enabling the luminal microbes to invade the mucosa and trigger inflammation.
INTESTINAL STEM CELLS AND THEIR MARKERS
The whole intestinal tract is a rapidly self-renewing tissue maintained by a population of intestinal stem cells. For many decades, scientists tried to identify these relatively undifferentiated enigmatic cells - unfortunately with limited success due to a lack of good cellular markers. Nevertheless, about 30 years ago, 2 models were established concerning intestinal stem cell location in the human gut, which are still debated. The first hypothesis is called the “+4 position model”. It assumes the intestinal stem cells to be located above the Paneth cells at position +4 relative to the crypt base[2]. The second hypothesis, also called the “stem cell zone model”, proposed that the crypt base columnar cells, a cell population at the bottom of the crypt, represent the intestinal stem cells[2]. Both models suggest that about 6 intestinal stem cells are found in each crypt[2], surrounded by epithelial and mesenchymal cells regulating stem cell behavior[3].
The intestinal stem cells differentiate into 4 epithelial cell types[3]. The most abundant cells in the epithelium are the columnar cells. Their main function is the absorption of nutrients by apical microvilli. Goblet cells produce and secrete mucins in order to form a protective luminal mucus layer. Neuroendocrine cells release hormones in an endocrine and paracrine fashion. Finally, the Paneth cells, located in the crypt base of the small intestine and ascending colon secrete defensins and other antimicrobial peptides to keep the crypts sterile[4].
In 2007, Hans Clevers and his group found the Wnt target gene leucine-rich-repeat-containing G-protein-coupled receptor 5 (Lgr5) to be expressed in cells at the crypt base[5]. Furthermore, irreversible labeling of these cells revealed, that all 4 epithelial cell types (columnar cells, goblet cells, Paneth cells and neuroendocrine cells) arise from these cells. Since LGR5-positive cells are located at the crypt base, are pluripotent and also self-renewing, they concluded that LGR5 is a good marker for the intestinal stem cells[5]. In 2009, the same group found olfactomedin 4 (also known as OLFM4, hGC1 or GW112), a protein with unknown function, to be a robust and specific marker for these LGR5 stem cells in the human intestine[6].
Intestinal stem cells are regulated by several cell signaling pathways, including the Wnt and Notch pathway[7,8]. Defects in these pathways are known to be related to the development of intestinal cancer[3,8,9]. The role of stem cell differentiation towards Paneth cells in ileal CD and towards goblet cells in UC is a new and promising field of science which could contribute to a better understanding of these 2 diseases[10,11].
DEFECTIVE PANETH CELL DIFFERENTIATION IN ILEAL CD IS RELATED TO α-DEFENSIN DEFIENCY
Paneth cells were first described more than 100 years ago as granular cells at the base of the intestinal crypts of Lieberkühn[12]. About 5-12 Paneth cells can be found in each small intestinal crypt and, in contrast to columnar cells, goblet cells and neuroendocrine cells, they do not migrate upwards in the crypt but remain beneath or between the stem cells in the crypt base[13]. Their numerous apical cytoplasmic granula are filled with antimicrobial peptides, especially the α-defensins HD5 and HD6, but also other antimicrobials such as lysozyme and phospholipase A2[14]. These Paneth cell defensins, which are active against a large number of microbes, including Gram-positive and Gram-negative bacteria, fungi and viruses[15], can be released into the crypt lumen in order to regulate the microbial density and therefore protect the intestinal epithelium against bacterial invasion and inflammation.
Paneth cells derive from intestinal stem cells under the control of the Wnt signaling pathway. In particular, the Wnt transcription factor TCF4 is essential in Paneth cell differentiation, as shown in the embryonic mouse intestine[16]. After the activation of Wnt signaling, an intracellular β-catenin-TCF4-complex is formed and translocates into the nucleus, where the compound acts as a transcription factor controlling the expression of several downstream target genes, such as Paneth cell defensins. This was shown in TCF4 null mice, where TCF4 regulates the expression of the cryptids (the mouse homologs to the human α-defensins)[16].
This Paneth cell differentiation factor TCF4 was found to be reduced in ileal CD as compared to colonic CD and colonic UC[10]. Interestingly, this observation was independent of the degree of inflammation and independent of the NOD2 genotype[10]. In human samples HD5 and HD6 expression correlated well with TCF4, thus it seems that not only in animal models, but also in humans, Paneth cell defensins are regulated by TCF4[10]. The functional relevance of this TCF4 decrease was shown in TCF4 knockout mice: the reduced TCF4 expression apparently resulted in a decreased HD5 and HD6 expression[10]. Moreover, we found a high affinity binding site in the HD5 and HD6 promotor for TCF4, and notably, we detected genetic variants in the putative promoter region of the Wnt transcription factor TCF4 (TCF7L2) to be associated with ileal CD[17]. This genetic finding emphasizes the primary role of the TCF4 defect in CD.
This also explains in part why in ileal CD, but not in colonic CD or UC, and also not in pouchitis samples, we had found a decreased expression of the α-defensins HD5 and HD6[14,18]. The other Paneth cell products were unchanged or even increased as compared with control samples[14]. This observation was independent of the degree of inflammation[14,18], and even more pronounced in patients with NOD2 mutations[14,18], which are clearly associated with ileal CD[19,20]. This is interesting, since
Kobayashi et al[21] found NOD2-deficient mice to be susceptible to bacterial infection and, in addition, NOD2 seems to be essential for the expression of cryptdins. Elphick et al[22] confirmed this low defensin formation in ileal CD especially in those with a NOD2 mutation. Another study showed the same low defensin synthesis in CD but linked it to inflammation and loss of Paneth cells[23]. In contrast to this observation, Kelly et al[24] and also our group found an unaltered number of Paneth cells in ileal CD[14]. We were also able to demonstrate a reduced antibacterial activity in mucosal extracts from patients with ileal CD suggesting that the missing expression of Paneth cell defensins in humans leads to a defective antimicrobial shield[14]. Since transgenic mice with human HD5 expression had an abnormal composition of the luminal microbial flora, it seems plausible that Paneth cell defensins can influence the makeup of the bacterial flora[14].
Taken together, the defective differentiation from the intestinal stem cell towards the Paneth cell is mediated by a diminished expression of the Wnt signaling transcription factor TCF4 resulting in a specific deficiency of the Paneth cell defensins in humans, especially in case of NOD2 mutations. This leads to a dysfunction of the mucosal barrier, which enables the luminal microbes to invade the mucosa and cause inflammation (Figure 1B). Besides the genetic link of TCF4 to ileal CD, other molecules, such as NOD2, ATG16L1, XBP1, KCNN4 and HD5 are genetically defective in ileal CD. As all these molecules are necessary for Paneth cell function, it seems to be plausible that the decrease in Paneth cell α-defensins is a primary (genetic) factor in disease pathogenesis[17,25].
IMPAIRED GOBLET CELL DIFFERENTIATION IN UC IS LINKED TO A DEFECTIVE MUCUS LAYER
Goblet cells are glandular epithelial cells scattered among the absorptive cells in the epithelium. The cytoplasm of these cells is filled with granula containing mucins, which are secreted into the intestinal lumen. These mucins form the mucus layer, acting as a barrier between the luminal contents and the epithelial surface[26].
Goblet cells derive from intestinal stem and progenitor cells located in the lower part of the crypt. Their differentiation is controlled by several transcription factors, especially by the basic helix-loop-helix transcription factor Hath1, the zinc-finger transcription factor KLF4 and the Notch target gene Hes1. For example, Math1 null mice (Math1 is the mouse homologe of human Hath1) are not able to develop goblet cells, whereas the columnar cells are intact[27]. In accordance to Hath1, goblet cell differentiation was also defective in KLF4 null mice as shown by a decrease of about 90% of colonic goblet cells in these mice[28]. Hath1 seems to be involved in an early stage of goblet cell differentiation, KLF4 appears to play a crucial role especially in the terminal stage of goblet cell differentiation[28]. In contrast, the genetic knockout of Hes1 gives rise to an increased number of goblet cells accompanied by a reduced number of nonsecretory cells[27,29,30], suggesting that Hes1 is a antagonist of Hath1[31]. In particular, the balance between Hath1 and Hes1 seems to be essential in controlling goblet cell differentiation in the epithelium, at least in the small intestine[31].
In 2009, we investigated these 3 crucial goblet cell differentiation factors in IBD. We found a significant induction of the goblet cell differentiation factors Hath1 and KLF4 in noninflamed vs inflamed CD, but not in UC[11]. Particularly in inflamed CD, the expression of Hath1 and KLF4 was about twice as high as that in inflamed UC samples[11]. For Hath1, these mRNA data were confirmed on the protein level by immunohistochemistry and Western blotting. Interestingly, this attenuated induction of Hath1 and KLF4 in UC was independent of the degree of inflammation and seems not to result from a downregulation by increased Notch activity[11]. Hes1 expression was also augmented in inflamed CD as compared to inflamed UC, but without reaching significance[11].
In contrast to CD, the expression of the colonic antimicrobials is increased in inflamed UC suggesting that the antimicrobial shield is intact in these patients[32-34]. Nevertheless, the colonic mucus layer, which cover the whole gastrointestinal tract and act as a physical and chemical barrier against the luminal microbes, is altered and even deficient in UC[35-37]. The thickness of the mucus layer in the healthy colon was measured between 100 and 300 μm, increasing from the ascending colon to the rectum[36,38,39], whereas in UC, this mucus layer is thinner, more variable and in part denuded[35,36].
Since the “raison d’être” of goblet cells is to secrete mucus, it is not surprising that a decrease of the mature goblet cells, located in the upper part of the crypts, was found in UC compared with controls[11]. Moreover, in our samples the expression of the main colonic mucins (Muc1, 2 and 4) tended to increase in inflamed IBD, whereas this induction was clearly less pronounced in inflamed UC compared with inflamed CD[11]. Since Hath1 and KLF4 were also highly correlated with these mucins, it is possible that the defective mucus layer in UC is related to these 2 differentiation factors[11].
Overall, the defective differentiation from intestinal stem cells towards goblet cells, which is mediated by the 2 crucial transcription factors Hath1 and KLF4, may lead to goblet cell depletion and deficient mucin induction in active UC. This could explain the defective mucus barrier in UC, resulting in a collapse of the physical barrier, which enables the luminal microbes to invade the mucosa and trigger inflammation (Figure 1C). The role of mucins in host defense was shown by MUC2-deficient mice which spontaneously develop colitis[40]. As Swidsinski et al[41] found high concentrations of mucosal bacteria in patients with IBD, and as kationic defensins bind to the negatively charged mucins[42], we hypothesize that in UC the mucosa is not capable of holding back the fecal bacteria based on a defective mucus layer which is unable to bind the adequate amounts of secreted defensins.
CONCLUSION
The pathogenesis of IBD is certainly complex and still under investigation. Nevertheless, ileal CD and colonic UC are associated with defects in stem cell differentiation either to protective Paneth cells or goblet cells. Diminished Paneth cell defensins result in a defective antimicrobial barrier in ileal CD, whereas in UC, an insufficient induction of goblet cell mucins can lead to a disturbed mucosal barrier. In both cases, luminal microbes are allowed to invade the mucosa and cause inflammation. New therapeutic strategies should focus on a stimulation of the antimicrobial shield in ileal CD and stabilization of the mucus layer in UC.
Peer reviewers: Jürgen Büning, MD, Internal Medicine I, Department of Gastroenterology, University Hospital of Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany; Daniel S Straus, PhD, Professor, Biomedical Sciences Division, University of California, Riverside, CA 9252, United States; Ki-Baik Hahm, MD, PhD, Professor, Gachon Graduate School of Medicine, Department of Gastroenterology, Lee Gil Ya Cancer and Diabetes Institute, Lab of Translational Medicine, 7-45 Songdo-dong, Yeonsu-gu, Incheon, 406-840, South Korea
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM