Published online Jul 14, 2011. doi: 10.3748/wjg.v17.i26.3165
Revised: March 5, 2011
Accepted: March 12, 2011
Published online: July 14, 2011
Alopecia areata is a disease of the hair follicles, with strong evidence supporting autoimmune etiology. Alopecia areata is frequently associated with immune-mediated diseases with skin manifestations such as psoriasis and lichen planus, or without skin manifestations such as autoimmune thyroiditis and idiopathic thrombocytopenic purpura. Helicobacter pylori (H. pylori) infection is present in around 50% of the world’s population and has been associated with a variety of immune-mediated extra-digestive disorders including autoimmune thyroiditis, idiopathic thrombocytopenic purpura, and psoriasis. A case of a 43-year old man with an 8-mo history of alopecia areata of the scalp and beard is presented. The patient was being treated by a dermatologist and had psychiatric support, without any improvement. He had a history of dyspepsia and the urea breath test confirmed H. pylori infection. The patient went into remission from alopecia areata after H. pylori eradication. If such an association is confirmed by epidemiological studies designed for this purpose, new therapeutic options could be available for these patients, especially in areas where infection with H. pylori is highly prevalent.
-
Citation: Campuzano-Maya G. Cure of alopecia areata after eradication of
Helicobacter pylori : A new association? World J Gastroenterol 2011; 17(26): 3165-3170 - URL: https://www.wjgnet.com/1007-9327/full/v17/i26/3165.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i26.3165
Alopecia areata is a disease of the hair follicles, with strong evidence supporting an autoimmune origin[1], although the exact pathogenesis of the disease is not clear. Alopecia areata has a frequency ranging from 0.7% to 3.8% in patients attending dermatology clinics, affects both sexes[2], and a familial occurrence is often reported[3,4]. The pattern of hair loss can vary and can affect any part of the body. Alopecia areata frequently occurs in association with other autoimmune diseases, including autoimmune thyroiditis[5], psoriasis[6-8] and Sjögren syndrome[9], among others.
Helicobacter pylori (H. pylori) is a microaerophilic Gram-negative bacterium that colonizes the gastric mucosa[10] and is present in around 50% of the world’s population[11], with varying prevalence rates between 7% in the Czech Republic and 87% in a South African population[12]. In the case of Medellín, Colombia, prevalence of H. pylori infection in children under 12 years is 60.9%[13] and in adults, it is 77.2%[14]. H. pylori infection has been associated with the pathogenesis of gastric disorders such as gastritis, duodenal and gastric ulcers, gastric cancer, mucosa-associated lymphoid tissue lymphoma[10], and a variety of extra-digestive disorders, many of them clearly identified as immune-mediated[15], such as idiopathic thrombocytopenic purpura[16,17], autoimmune thyroiditis[18,19], Sjögren’s syndrome[20,21], rosacea[22] and psoriasis[23,24].
A case of a 43-year-old man with patchy alopecia areata and H. pylori infection is presented. The patient had hair regrowth after bacterial eradication.
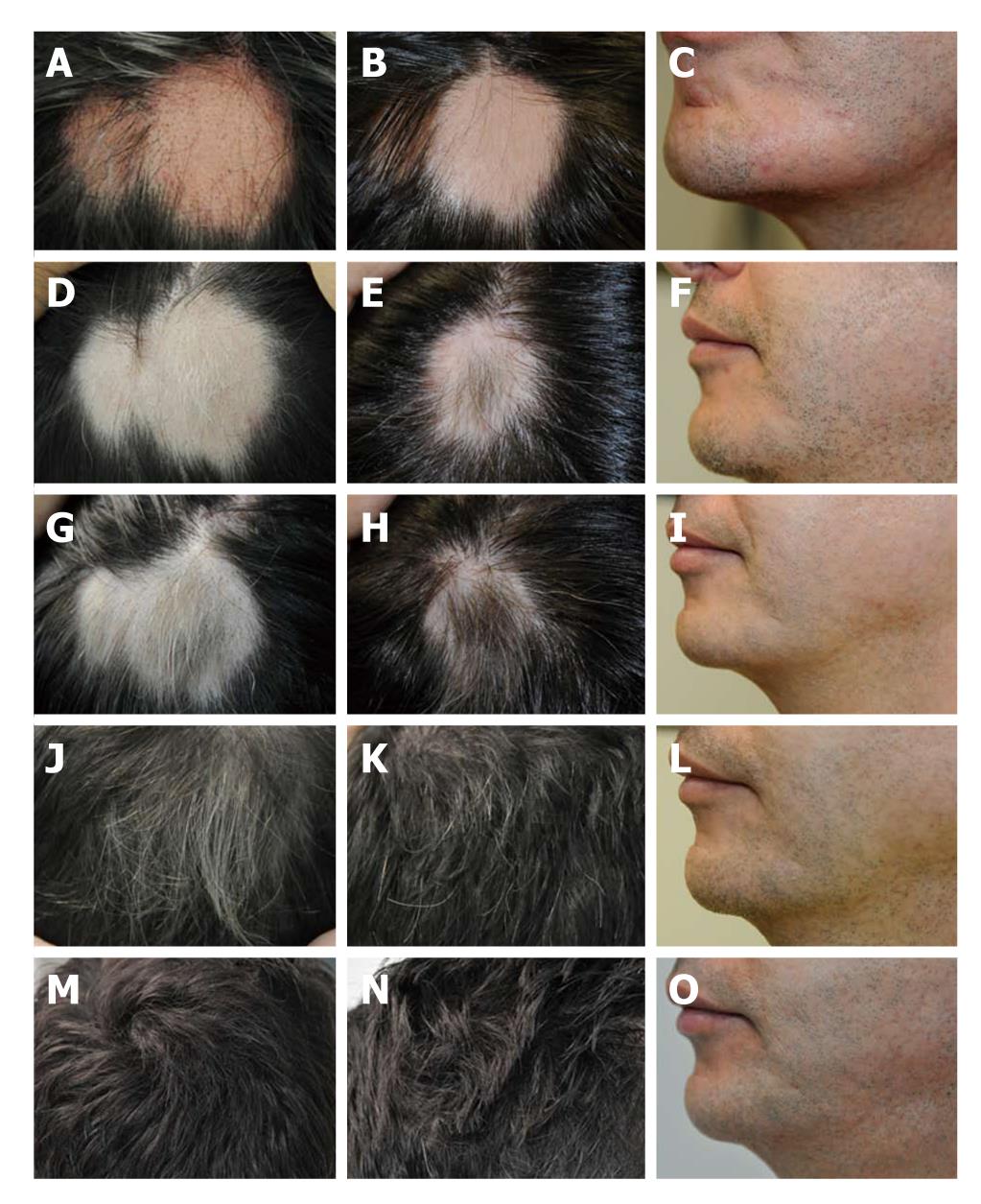
A 43-year-old man presented with an 8-mo history of patchy hair loss in the scalp and beard (Figure 1A-C). He had consulted a dermatologist who prescribed 0.25% desoximetasone and 5% minoxidil, according to the guidelines for the management of alopecia[25], and had psychiatric support with escitalopram 5 mg/d, without any response other than progression of the condition.
The patient had a history of dyspepsia, therefore, he underwent analysis to determine H. pylori status. Urea breath test (13C-UBT) (6.95 δ13CO2; negative, < 1)[26], and H. pylori IgG antibodies (IgG index: 52.4; negative, < 9) were positive. Subsequent laboratory evaluation included normal values of ultrasensitive thyroid stimulating hormone, free thyroxine and free tri-iodothyronine; and negative antinuclear, antithyroid peroxidase and intrinsic factor antibodies. The patient was prescribed first line H. pylori eradication with proton pump inhibitor (omeprazole) 20 mg twice daily, amoxicillin 1000 mg twice daily, and clarithromycin 500 mg twice daily for 14 d, according to recommendations from the Maastricht III Consensus Report[27], and was followed photographically every 2 wk. He was instructed not to take or apply any medications for alopecia areata. H. pylori eradication was confirmed 6 wk after treatment with a negative result of the 13C-UBT (0.81 δ13CO2).
Figure 1 shows the photographic sequence of the lesions before and after H. pylori eradication. From week 4, there was evidence of hair regrowth in the scalp and beard (Figure 1D-F). To date, the patient continues in complete remission from alopecia areata, as shown in Figure 1M-O.
H. pylori infection has been associated with numerous immune and non-immune disorders including dermatological conditions, such as chronic urticaria[28-30], rosacea[22,28,31-39], psoriasis[23,24], Schönlein-Henoch purpura[40-46], Behçet’s disease[47,48], prurigo nodularis[49], chronic cutaneous pruritus[50], progressive systemic sclerosis[51-54], Sjögren’s syndrome[20,21,55-57], and Sweet’s syndrome[58]; many of them improving or going into remission after eradication of H. pylori infection[24,30,49,59-61]. Several mechanisms have been suggested to mediate the systemic effects of H. pylori infection, including the development of antigen-antibody complexes and cross-reactive antibodies (by molecular mimicry)[61-63], where antibodies developed against H. pylori cross-react with autoantigens to cause tissue damage, as has been reported in atrophic gastritis[62,64], chronic gastritis[65-67], chronic idiopathic thrombocytopenic purpura[16,17,68-70], Hashimoto’s thyroiditis[19], atherosclerosis[71], arterial hypertension[72], unstable angina pectoris[73], ischemic heart disease[74,75], Alzheimer’s disease[76], systemic sclerosis[77,78], central serous chorioretinopathy[79], iron deficiency[80,81], autoimmune pancreatitis[82-86], and chronic urticaria[87].
Alopecia areata has been described to be of autoimmune origin[88], with the presence of inflammatory cells around and within the human hair follicles. Alopecia areata has been associated with other autoimmune disorders including thyroid disease[89-93], psoriasis[6,7], and celiac disease[94-97]; conditions that have also been associated with H. pylori infection.
In the literature, there is ample evidence to suggest an association between H. pylori and alopecia areata that could explain the cure in this patient after eradication of infection. There is concurrent alopecia areata with immune diseases that are also concurrent with H. pylori infection. There are three different scenarios: immune-mediated skin diseases associated with H. pylori infection and alopecia areata, including psoriasis[6,7,23,24,98-103] and lichen planus[101,104-109]; immune-mediated non-skin conditions associated with H. pylori infection and alopecia areata, including autoimmune thyroiditis[18,19,110-115], celiac disease[94-97,116-118], idiopathic thrombocytopenic purpura[119,120], and autoimmune pancreatitis[82,84,85,121-124]; and laboratory findings that show the immunological nature of the conditions that are found in H. pylori-infected patients as well as in alopecia areata patients, including parietal cell antibodies[117,125-127] and thyroid antibodies[90,128].
After reviewing the medical literature, an association between H. pylori infection and alopecia areata has not been clearly demonstrated; only three reports have explored such association and had different results[129-131]. Abdel Hafez et al[131] have compared 31 patients with alopecia areata with 24 healthy controls and have found no significant difference in the H. pylori status, as determined by an antigen stool test. Rigopoulos et al[130] have compared H. pylori seroprevalence in 30 patients with alopecia areata and 30 healthy controls, and found no significant difference between the groups, whereas Tosti et al[129] have found, in a group of 68 patients with alopecia areata, that the seroprevalence of H. pylori infection was higher than in matched controls. It is of note that the presence of IgG antibodies against H. pylori does not confirm current infection and is only an indicator of previous exposure to the bacterium[132]. However, none of the studies tried to eradicate the infection and evaluate posterior hair regrowth.
Here, I have described the case of one patient who had patchy hair loss of the scalp and beard. The patient’s condition started to improve within 4 wk of completing H. pylori eradication (Figure 1D-F). By week 16 (Figure 1J-L), the patient had completely reversed the hair loss, and by week 44 (Figure 1M-O), he remained H. pylori-negative and completely cured of alopecia areata. Although prior studies have only reported the prevalence of H. pylori infection in alopecia areata patients, to the best of my knowledge, this is the first documented case of reversed hair loss after H. pylori eradication.
There have been a few early studies in which antibiotic treatment was used in an attempt to cure alopecia areata, but in no case was there information on whether the patients were infected with H. pylori. Dapsone was used unsuccessfully[133,134]. There was one case of a 13-year-old girl with multiple autoimmune diseases who was successfully treated for alopecia areata with co-trimoxazole, a drug with antibiotic properties and immunomodulatory effects that could have been responsible for hair regrowth. Finally, there was one case in the literature describing the occurrence of alopecia areata after antibiotic treatment with rifampicin[135]. However, further case-control studies could be useful to rule out this possibility completely.
Hence, a common denominator in various autoimmune diseases is H. pylori infection; therefore, H. pylori status could be determined in several autoimmune conditions, and if positive, eradication treatment could follow as an initial step. More studies are needed to clarify the reality of the proposed association.
Peer reviewers: Eyvind J Paulssen, MD, PhD, Department of Gastroenterology, University Hospital of North Norway, PO Box 83, Tromsø, N-9038, Norway; Zeinab Nabil Ahmed, Professor of Microbiology, Microbiology and Immunology Department, Faculty of Medicine (for girls), Al-Azhar University, Nasr City, 1047, Cairo, Egypt
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Rosenstein ED, Warshauer BL. Alopecia areata and autoimmunity. J Am Acad Dermatol. 2010;62:1065. |
| 2. | Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern and profile of alopecia areata in Singapore-a study of 219 Asians. Int J Dermatol. 2002;41:748-753. |
| 3. | Treem WR, Veligati LN, Rotter JI, Targan SR, Hyams JS. Ulcerative colitis and total alopecia in a mother and her son. Gastroenterology. 1993;104:1187-1191. |
| 4. | Goh C, Finkel M, Christos PJ, Sinha AA. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055-1060. |
| 5. | Seyrafi H, Akhiani M, Abbasi H, Mirpour S, Gholamrezanezhad A. Evaluation of the profile of alopecia areata and the prevalence of thyroid function test abnormalities and serum autoantibodies in Iranian patients. BMC Dermatol. 2005;5:11. |
| 7. | Ganor S. Diseases sometimes associated with psoriasis. II. Alopecia areata. Dermatologica. 1977;154:338-341. |
| 8. | Shuster S. Psoriatic alopecia. Arch Dermatol. 1990;126:397. |
| 9. | Sato M, Saga K, Takahashi H. Postmenopausal frontal fibrosing alopecia in a Japanese woman with Sjögren’s syndrome. J Dermatol. 2008;35:729-731. |
| 10. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. |
| 11. | Correa P, Piazuelo MB. Natural history of Helicobacter pylori infection. Dig Liver Dis. 2008;40:490-496. |
| 12. | Ford AC, Axon AT. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2010;15 Suppl 1:1-6. |
| 13. | Duque JJ. Helicobacter pylori en la mucosa gastrica de cadaveres de ninos. Iatreia. 1999;12:135-138. |
| 14. | Campuzano-Maya G, Hoyos-Castaño D, Calvo-Betancur VD, Suárez-Ramírez OA, Lizcano-Cardona D, Rojas-Arbeláez CA. [Prevalence of Helicobacter pylori infection in physicians in Medellín, Colombia]. Acta Gastroenterol Latinoam. 2007;37:99-103. |
| 15. | Figura N, Franceschi F, Santucci A, Bernardini G, Gasbarrini G, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2010;15 Suppl 1:60-68. |
| 16. | Campuzano-Maya G. Proof of an association between Helicobacter pylori and idiopathic thrombocytopenic purpura in Latin America. Helicobacter. 2007;12:265-273. |
| 17. | Stasi R, Sarpatwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, Provan D, Newland A, Amadori S, Bussel JB. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113:1231-1240. |
| 18. | de Luis DA, Varela C, de La Calle H, Cantón R, de Argila CM, San Roman AL, Boixeda D. Helicobacter pylori infection is markedly increased in patients with autoimmune atrophic thyroiditis. J Clin Gastroenterol. 1998;26:259-263. |
| 19. | Franceschi F, Satta MA, Mentella MC, Penland R, Candelli M, Grillo RL, Leo D, Fini L, Nista EC, Cazzato IA. Helicobacter pylori infection in patients with Hashimoto’s thyroiditis. Helicobacter. 2004;9:369. |
| 20. | Aragona P, Magazzù G, Macchia G, Bartolone S, Di Pasquale G, Vitali C, Ferreri G. Presence of antibodies against Helicobacter pylori and its heat-shock protein 60 in the serum of patients with Sjögren’s syndrome. J Rheumatol. 1999;26:1306-1311. |
| 21. | Sorrentino D, Faller G, DeVita S, Avellini C, Labombarda A, Ferraccioli G, Kahlow-Toussaint S. Helicobacter pylori associated antigastric autoantibodies: role in Sjögren’s syndrome gastritis. Helicobacter. 2004;9:46-53. |
| 22. | Rebora A, Drago F, Picciotto A. Helicobacter pylori in patients with rosacea. Am J Gastroenterol. 1994;89:1603-1604. |
| 23. | Ali M, Whitehead M. Clearance of chronic psoriasis after eradication therapy for Helicobacter pylori infection. J Eur Acad Dermatol Venereol. 2008;22:753-754. |
| 24. | Martin Hübner A, Tenbaum SP. Complete remission of palmoplantar psoriasis through Helicobacter pylori eradication: a case report. Clin Exp Dermatol. 2008;33:339-340. |
| 25. | MacDonald Hull SP, Wood ML, Hutchinson PE, Sladden M, Messenger AG. Guidelines for the management of alopecia areata. Br J Dermatol. 2003;149:692-699. |
| 26. | Campuzano-Maya G. An optimized 13C-urea breath test for the diagnosis of H pylori infection. World J Gastroenterol. 2007;13:5454-5464. |
| 27. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. |
| 28. | Mini R, Figura N, D’Ambrosio C, Braconi D, Bernardini G, Di Simplicio F, Lenzi C, Nuti R, Trabalzini L, Martelli P. Helicobacter pylori immunoproteomes in case reports of rosacea and chronic urticaria. Proteomics. 2005;5:777-787. |
| 29. | Galadari IH, Sheriff MO. The role of Helicobacter pylori in urticaria and atopic dermatitis. Skinmed. 2006;5:172-176. |
| 30. | Abdou AG, Elshayeb EI, Farag AG, Elnaidany NF. Helicobacter pylori infection in patients with chronic urticaria: correlation with pathologic findings in gastric biopsies. Int J Dermatol. 2009;48:464-469. |
| 31. | Utas S, Ozbakir O, Turasan A, Utaş C. Helicobacter pylori eradication treatment reduces the severity of rosacea. J Am Acad Dermatol. 1999;40:433-435. |
| 32. | Szlachcic A, Sliwowski Z, Karczewska E, Bielański W, Pytko-Polonczyk J, Konturek SJ. Helicobacter pylori and its eradication in rosacea. J Physiol Pharmacol. 1999;50:777-786. |
| 33. | Mayr-Kanhäuser S, Kränke B, Kaddu S, Müllegger RR. Resolution of granulomatous rosacea after eradication of Helicobacter pylori with clarithromycin, metronidazole and pantoprazole. Eur J Gastroenterol Hepatol. 2001;13:1379-1383. |
| 34. | Szlachcic A. The link between Helicobacter pylori infection and rosacea. J Eur Acad Dermatol Venereol. 2002;16:328-333. |
| 35. | Diaz C, O’Callaghan CJ, Khan A, Ilchyshyn A. Rosacea: a cutaneous marker of Helicobacter pylori infection? Results of a pilot study. Acta Derm Venereol. 2003;83:282-286. |
| 36. | Argenziano G, Donnarumma G, Iovene MR, Arnese P, Baldassarre MA, Baroni A. Incidence of anti-Helicobacter pylori and anti-CagA antibodies in rosacea patients. Int J Dermatol. 2003;42:601-604. |
| 37. | Zandi S, Shamsadini S, Zahedi MJ, Hyatbaksh M. Helicobacter pylori and rosacea. East Mediterr Health J. 2003;9:167-171. |
| 38. | Boixeda de Miquel D, Vázquez Romero M, Vázquez Sequeiros E, Foruny Olcina JR, Boixeda de Miquel P, López San Román A, Alemán Villanueva S, Martín de Argila de Prados C. Effect of Helicobacter pylori eradication therapy in rosacea patients. Rev Esp Enferm Dig. 2006;98:501-509. |
| 39. | Daković Z, Vesić S, Vuković J, Milenković S, Janković-Terzić K, Dukić S, Pavlović MD. Ocular rosacea and treatment of symptomatic Helicobacter pylori infection: a case series. Acta Dermatovenerol Alp Panonica Adriat. 2007;16:83-86. |
| 40. | Reinauer S, Megahed M, Goerz G, Ruzicka T, Borchard F, Susanto F, Reinauer H. Schönlein-Henoch purpura associated with gastric Helicobacter pylori infection. J Am Acad Dermatol. 1995;33:876-879. |
| 41. | Machet L, Vaillant L, Machet MC, Büchler M, Lorette G. Schönlein-Henoch purpura associated with gastric Helicobacter pylori infection. Dermatology. 1997;194:86. |
| 42. | Mozrzymas R, d’Amore ES, Montini G, Guariso G. Schönlein-Henoch vasculitis and chronic Helicobacter pylori associated gastritis and duodenal ulcer: a case report. Pediatr Med Chir. 1997;19:467-468. |
| 43. | Cecchi R, Torelli E. Schönlein-Henoch purpura in association with duodenal ulcer and gastric Helicobacter pylori infection. J Dermatol. 1998;25:482-484. |
| 44. | Fu KI, Yagi S, Mashimo Y, Sugitani K, Imamaki K, Yanagisawa M, Maekawa S, Morimoto Y, Fujimori T. Regression of Helicobacter pylori-negative duodenal ulcers complicated by Schonlein-Henoch purpura with H. pylori eradication therapy: the first report. Dig Dis Sci. 2005;50:381-384. |
| 45. | Grivceva-Panovska V, Grivceva Stardelova K, Serafimoski V. Henoch-Schönlein purpura in an adult patient: extragastric, cutaneous manifestation of helicobacter pylori infection. Prilozi. 2008;29:291-301. |
| 46. | Hoshino C. Adult onset Schönlein-Henoch purpura associated with Helicobacter pylori infection. Intern Med. 2009;48:847-851. |
| 47. | Avci O, Ellidokuz E, Simşek I, Büyükgebiz B, Güneş AT. Helicobacter pylori and Behçet's disease. Dermatology. 1999;199:140-143. |
| 48. | Imamura Y, Kurokawa MS, Yoshikawa H, Nara K, Takada E, Masuda C, Tsukikawa S, Ozaki S, Matsuda T, Suzuki N. Involvement of Th1 cells and heat shock protein 60 in the pathogenesis of intestinal Behcet’s disease. Clin Exp Immunol. 2005;139:371-378. |
| 49. | Neri S, Ierna D, D’Amico RA, Giarratano G, Leotta C. Helicobacter pylori and prurigo nodularis. Hepatogastroenterology. 1999;46:2269-2272. |
| 50. | Kandyil R, Satya NS, Swerlick RA. Chronic pruritus associated with Helicobacter pylori. J Cutan Med Surg. 2002;6:103-108. |
| 51. | Reinauer S, Goerz G, Ruzicka T, Susanto F, Humfeld S, Reinauer H. Helicobacter pylori in patients with systemic sclerosis: detection with the 13C-urea breath test and eradication. Acta Derm Venereol. 1994;74:361-363. |
| 52. | Yazawa N, Fujimoto M, Kikuchi K, Kubo M, Ihn H, Sato S, Tamaki T, Tamaki K. High seroprevalence of Helicobacter pylori infection in patients with systemic sclerosis: association with esophageal involvement. J Rheumatol. 1998;25:650-653. |
| 53. | Danese S, Zoli A, Cremonini F, Gasbarrini A. High prevalence of Helicobacter pylori type I virulent strains in patients with systemic sclerosis. J Rheumatol. 2000;27:1568-1569. |
| 54. | Farina G, Rosato E, Francia C, Proietti M, Donato G, Ammendolea C, Pisarri S, Salsano F. High incidence of Helicobacter pylori infection in patients with systemic sclerosis: association with Sicca Syndrome. Int J Immunopathol Pharmacol. 2001;14:81-85. |
| 55. | De Vita S, Ferraccioli G, Avellini C, Sorrentino D, Dolcetti R, Di Loreto C, Bartoli E, Boiocchi M, Beltrami CA. Widespread clonal B-cell disorder in Sjögren’s syndrome predisposing to Helicobacter pylori-related gastric lymphoma. Gastroenterology. 1996;110:1969-1974. |
| 56. | Nishimura M, Miyajima S, Okada N. Salivary gland MALT lymphoma associated with Helicobacter pylori infection in a patient with Sjögren’s Syndrome. J Dermatol. 2000;27:450-452. |
| 57. | Theander E, Nilsson I, Manthorpe R, Jacobsson LT, Wadström T. Seroprevalence of Helicobacter pylori in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2001;19:633-638. |
| 58. | Kürkçüoğlu N, Aksoy F. Sweet's syndrome associated with Helicobacter pylori infection. J Am Acad Dermatol. 1997;37:123-124. |
| 59. | Di Campli C, Gasbarrini A, Nucera E, Franceschi F, Ojetti V, Sanz Torre E, Schiavino D, Pola P, Patriarca G, Gasbarrini G. Beneficial effects of Helicobacter pylori eradication on idiopathic chronic urticaria. Dig Dis Sci. 1998;43:1226-1229. |
| 60. | Wedi B, Kapp A. Helicobacter pylori infection in skin diseases: a critical appraisal. Am J Clin Dermatol. 2002;3:273-282. |
| 61. | Hernando-harder AC, Booken N, Goerdt S, Singer MV, Harder H. Helicobacter pylori infection and dermatologic diseases. Eur J Dermatol. 2009;19:431-444. |
| 62. | Negrini R, Savio A, Poiesi C, Appelmelk BJ, Buffoli F, Paterlini A, Cesari P, Graffeo M, Vaira D, Franzin G. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996;111:655-665. |
| 63. | Gasbarrini A, Franceschi F, Armuzzi A, Ojetti V, Candelli M, Torre ES, De Lorenzo A, Anti M, Pretolani S, Gasbarrini G. Extradigestive manifestations of Helicobacter pylori gastric infection. Gut. 1999;45 Suppl 1:I9-I12. |
| 64. | D’Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004;10:316-323. |
| 65. | Negrini R, Savio A, Appelmelk BJ. Autoantibodies to gastric mucosa in Helicobacter pylori infection. Helicobacter. 1997;2 Suppl 1:S13-S16. |
| 66. | Bodger K, Crabtree JE. Helicobacter pylori and gastric inflammation. Br Med Bull. 1998;54:139-150. |
| 67. | Amedei A, Bergman MP, Appelmelk BJ, Azzurri A, Benagiano M, Tamburini C, van der Zee R, Telford JL, Vandenbroucke-Grauls CM, D’Elios MM. Molecular mimicry between Helicobacter pylori antigens and H+, K+ -adenosine triphosphatase in human gastric autoimmunity. J Exp Med. 2003;198:1147-1156. |
| 68. | Takahashi T, Yujiri T, Shinohara K, Inoue Y, Sato Y, Fujii Y, Okubo M, Zaitsu Y, Ariyoshi K, Nakamura Y. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124:91-96. |
| 69. | Jackson S, Beck PL, Pineo GF, Poon MC. Helicobacter pylori eradication: novel therapy for immune thrombocytopenic purpura? A review of the literature. Am J Hematol. 2005;78:142-150. |
| 70. | Stasi R, Provan D. Helicobacter pylori and Chronic ITP. Hematology Am Soc Hematol Educ Program. 2008;206-211. |
| 71. | Lamb DJ, El-Sankary W, Ferns GA. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis. 2003;167:177-185. |
| 72. | Migneco A, Ojetti V, Specchia L, Franceschi F, Candelli M, Mettimano M, Montebelli R, Savi L, Gasbarrini G. Eradication of Helicobacter pylori infection improves blood pressure values in patients affected by hypertension. Helicobacter. 2003;8:585-589. |
| 73. | Rechciński T, Kasprzak JD, Chmiela M, Krzemińska-Pakuła M, Rudnicka W. Patients with unstable angina pectoris present increased humoral response against Helicobacter pylori in comparison with patients with aggravated dyspepsia. Acta Microbiol Pol. 2002;51:339-344. |
| 74. | Franceschi F, Leo D, Fini L, Santoliquido A, Flore R, Tondi P, Roccarina D, Nista EC, Cazzato AI, Lupascu A. Helicobacter pylori infection and ischaemic heart disease: an overview of the general literature. Dig Liver Dis. 2005;37:301-308. |
| 75. | Manolakis A, Kapsoritakis AN, Potamianos SP. A review of the postulated mechanisms concerning the association of Helicobacter pylori with ischemic heart disease. Helicobacter. 2007;12:287-297. |
| 76. | Kountouras J, Gavalas E, Zavos C, Stergiopoulos C, Chatzopoulos D, Kapetanakis N, Gisakis D. Alzheimer’s disease and Helicobacter pylori infection: Defective immune regulation and apoptosis as proposed common links. Med Hypotheses. 2007;68:378-388. |
| 77. | Randone SB, Guiducci S, Cerinic MM. Systemic sclerosis and infections. Autoimmun Rev. 2008;8:36-40. |
| 78. | Radic M, Kaliterna DM, Radic J. Helicobacter pylori infection and systemic sclerosis-is there a link? Joint Bone Spine. 2010;Epub ahead of print. |
| 79. | Giusti C. Association of Helicobacter pylori with central serous chorioretinopathy: hypotheses regarding pathogenesis. Med Hypotheses. 2004;63:524-527. |
| 80. | Hershko C, Ronson A. Iron deficiency, Helicobacter infection and gastritis. Acta Haematol. 2009;122:97-102. |
| 81. | Hershko C, Skikne B. Pathogenesis and management of iron deficiency anemia: emerging role of celiac disease, helicobacter pylori, and autoimmune gastritis. Semin Hematol. 2009;46:339-350. |
| 82. | Kountouras J, Zavos C, Chatzopoulos D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J Cell Mol Med. 2005;9:196-207. |
| 83. | Bhatia M. Molecular mimicry in autoimmune pancreatitis: an interesting idea. J Cell Mol Med. 2005;9:745. |
| 84. | Kountouras J, Zavos C, Gavalas E, Tzilves D. Challenge in the pathogenesis of autoimmune pancreatitis: potential role of helicobacter pylori infection via molecular mimicry. Gastroenterology. 2007;133:368-369. |
| 85. | Okazaki K, Uchida K, Fukui T. Recent advances in autoimmune pancreatitis: concept, diagnosis, and pathogenesis. J Gastroenterol. 2008;43:409-418. |
| 86. | Jesnowski R, Isaksson B, Möhrcke C, Bertsch C, Bulajic M, Schneider-Brachert W, Klöppel G, Lowenfels AB, Maisonneuve P, Löhr JM. Helicobacter pylori in autoimmune pancreatitis and pancreatic carcinoma. Pancreatology. 2010;10:462-466. |
| 87. | Greaves MW. Pathophysiology of chronic urticaria. Int Arch Allergy Immunol. 2002;127:3-9. |
| 88. | Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177-188, quiz 189-190. |
| 89. | Cunliffe WJ, Hall R, Stevenson CJ, Weightman D. Alopecia areata, thyroid disease and autoimmunity. Br J Dermatol. 1969;81:877-881. |
| 90. | Pasaoglu H, Soyuer U, Astaal M. Thyroid antibodies in alopecia totalis. Cent Afr J Med. 1991;37:337-339. |
| 91. | Nanda A, Alsaleh QA, Al-Hasawi F, Al-Muzairai I. Thyroid function, autoantibodies, and HLA tissue typing in children with alopecia areata. Pediatr Dermatol. 2002;19:486-491. |
| 92. | Kurtev A, Iliev E. Thyroid autoimmunity in children and adolescents with alopecia areata. Int J Dermatol. 2005;44:457-461. |
| 93. | Kasumagić-Halilović E. Thyroid autoimmunity in patients with alopecia areata. Acta Dermatovenerol Croat. 2008;16:123-125. |
| 94. | Corazza GR, Andreani ML, Venturo N, Bernardi M, Tosti A, Gasbarrini G. Celiac disease and alopecia areata: report of a new association. Gastroenterology. 1995;109:1333-1337. |
| 95. | Zampetti M, Filippetti R. Alopecia areata and celiac disease. G Ital Dermatol Venereol. 2008;143:168. |
| 96. | Aydogdu S, Cakir M, Yuksekkaya HA, Tumgor G, Baran M, Arikan C, Yagci RV. Helicobacter pylori infection in children with celiac disease. Scand J Gastroenterol. 2008;43:1088-1093. |
| 97. | Fayed SB, Aref MI, Fathy HM, Abd El Dayem SM, Emara NA, Maklof A, Shafik A. Prevalence of celiac disease, Helicobacter pylori and gastroesophageal reflux in patients with refractory iron deficiency anemia. J Trop Pediatr. 2008;54:43-53. |
| 98. | Vosmík F, Hausner P. Immunological aspects of psoriasis and alopecia areata. Acta Univ Carol Med (Praha). 1985;31:57-72. |
| 99. | Appell ML, Sherertz EF. A kindred with alopecia, keratosis, pilaris, cataracts, and psoriasis. J Am Acad Dermatol. 1987;16:89-95. |
| 100. | Halasz CL. Helicobacter pylori antibodies in patients with psoriasis. Arch Dermatol. 1996;132:95-96. |
| 101. | Daudén E, Vázquez-Carrasco MA, Peñas PF, Pajares JM, García-Díez A. Association of Helicobacter pylori infection with psoriasis and lichen planus: prevalence and effect of eradication therapy. Arch Dermatol. 2000;136:1275-1276. |
| 102. | Qayoom S, Ahmad QM. Psoriasis and Helicobacter pylori. Indian J Dermatol Venereol Leprol. 2003;69:133-134. |
| 103. | Daudén E, Cabrera MM, Oñate MJ, Pajares JM, García-Díez A. CagA seropositivity in Helicobacter pylori positive patients with psoriasis. J Eur Acad Dermatol Venereol. 2004;18:116-117. |
| 104. | Tan RS. Ulcerative colitis, myasthenia gravis, atypical lichen planus, alopecia areata, vitiligo. Proc R Soc Med. 1974;67:195-196. |
| 105. | Epidemiological evidence of the association between lichen planus and two immune-related diseases. Alopecia areata and ulcerative colitis. Gruppo Italiano Studi Epidemiologici in Dermatologia. Arch Dermatol. 1991;127:688-691. |
| 106. | Kanwar AJ, Ghosh S, Thami GP, Kaur S. Twenty-nail dystrophy due to lichen planus in a patient with alopecia areata. Clin Exp Dermatol. 1993;18:293-294. |
| 107. | Dhar S, Dhar S. Colocalization of alopecia areata and lichen planus. Pediatr Dermatol. 1996;13:258-259. |
| 108. | Brenner W, Diem E, Gschnait F. Coincidence of vitiligo, alopecia areata, onychodystrophy, localized scleroderma and lichen planus. Dermatologica. 1979;159:356-360. |
| 109. | Kar BR, Ebenezer G, Job CK. Colocalisation of alopecia areata and lichen planus. Indian J Dermatol Venereol Leprol. 2004;70:242-243. |
| 110. | Tomasi PA, Dore MP, Fanciulli G, Sanciu F, Realdi G, Delitala G. Is there anything to the reported association between Helicobacter pylori infection and autoimmune thyroiditis? Dig Dis Sci. 2005;50:385-388. |
| 111. | Sterzl I, Hrda P, Potuznikova B, Matucha P, Hana V, Zamrazil V. Autoimmune thyroiditis and Helicobacter pylori--is there a connection? Neuro Endocrinol Lett. 2006;27 Suppl 1:41-45. |
| 112. | Hart ZH, Hoffman W, Winbaum E. Polyneuropathy, alopecia areata, and chronic lymphocytic thyroiditis. Neurology. 1979;29:106-108. |
| 113. | Cowan CL, Grimes PE, Chakrabarti S, Minus HR, Kenney JA. Retinitis pigmentosa associated with hearing loss, thyroid disease, vitiligo, and alopecia areata: retinitis pigmentosa and vitiligo. Retina. 1982;2:84-88. |
| 114. | Alviggi C, Carrieri PB, Pivonello R, Scarano V, Pezzella M, De Placido G, Colao A, Matarese G. Association of pelvic endometriosis with alopecia universalis, autoimmune thyroiditis and multiple sclerosis. J Endocrinol Invest. 2006;29:182-189. |
| 115. | Sheehan MT, Islam R. Silent thyroiditis, isolated corticotropin deficiency, and alopecia universalis in a patient with ulcerative colitis and elevated levels of plasma factor VIII: an unusual case of autoimmune polyglandular syndrome type 3. Endocr Pract. 2009;15:138-142. |
| 116. | Konturek PC, Karczewska E, Dieterich W, Hahn EG, Schuppan D. Increased prevalence of Helicobacter pylori infection in patients with celiac disease. Am J Gastroenterol. 2000;95:3682-3683. |
| 117. | Hershko C, Hoffbrand AV, Keret D, Souroujon M, Maschler I, Monselise Y, Lahad A. Role of autoimmune gastritis, Helicobacter pylori and celiac disease in refractory or unexplained iron deficiency anemia. Haematologica. 2005;90:585-595. |
| 118. | Villanacci V, Bassotti G, Liserre B, Lanzini A, Lanzarotto F, Genta RM. Helicobacter pylori infection in patients with celiac disease. Am J Gastroenterol. 2006;101:1880-1885. |
| 119. | Lamminger C, Näher H. [Alopecia areata universalis in Werlhof disease]. Hautarzt. 1990;41:324-325. |
| 120. | Levin RM, Travis SF, Heymann WR. Simultaneous onset of alopecia areata and idiopathic thrombocytopenic purpura: A potential association? Pediatr Dermatol. 1999;16:31-34. |
| 121. | Manes G, Dominguez-Muñoz JE, Hackelsberger A, Leodolter A, Rössner A, Malfertheiner P. Prevalence of Helicobacter pylori infection and gastric mucosal abnormalities in chronic pancreatitis. Am J Gastroenterol. 1998;93:1097-1100. |
| 122. | Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J Cell Mol Med. 2005;9:741-744. |
| 123. | Kountouras J, Zavos C, Chatzopoulos D. Autoimmune pancreatitis, Helicobacter pylori infection, and apoptosis: a proposed relationship. Pancreas. 2005;30:192-193. |
| 124. | Chang MC, Chang YT, Wei SC, Kuo CH, Liang PC, Wong JM. Autoimmune pancreatitis associated with high prevalence of gastric ulcer independent of Helicobacter pylori infection status. Pancreas. 2009;38:442-446. |
| 125. | Sterzl I, Hrdá P, Matucha P, Cerovská J, Zamrazil V. Anti-Helicobacter Pylori, anti-thyroid peroxidase, anti-thyroglobulin and anti-gastric parietal cells antibodies in Czech population. Physiol Res. 2008;57 Suppl 1:S135-S141. |
| 126. | Kumar B, Sharma VK, Sehgal S. Antismooth muscle and antiparietal cell antibodies in Indians with alopecia areata. Int J Dermatol. 1995;34:542-545. |
| 127. | Tzellos TG, Tahmatzidis DK, Lallas A, Apostolidou K, Goulis DG. Pernicious anemia in a patient with Type 1 diabetes mellitus and alopecia areata universalis. J Diabetes Complications. 2009;23:434-437. |
| 128. | Dore MP, Fastame L, Tocco A, Negrini R, Delitala G, Realdi G. Immunity markers in patients with Helicobacter pylori infection: effect of eradication. Helicobacter. 2005;10:391-397. |
| 129. | Tosti A, Pretolani S, Figura N, Polini M, Cameli N, Cariani G, Miglio F, Bonvicini F, Baldini L, Gnucci E. Helicobacter pylori and skin diseases. Gastroenterol Int. 1997;10 Suppl 1:37-39. |
| 130. | Rigopoulos D, Katsambas A, Karalexis A, Papatheodorou G, Rokkas T. No increased prevalence of Helicobacter pylori in patients with alopecia areata. J Am Acad Dermatol. 2002;46:141. |
| 131. | Abdel Hafez HZ, Mahran AM, Hofny EM, Attallah DA, Sayed DS, Rashed H. Alopecia areata is not associated with Helicobacter pylori. Indian J Dermatol. 2009;54:17-19. |
| 132. | McNulty C, Teare L, Owen R, Tompkins D, Hawtin P, McColl K. Test and treat for dyspepsia-but which test? BMJ. 2005;330:105-106. |
| 133. | Friedmann PS. Unsuccessful treatment of alopecia areata with dapsone. Br J Dermatol. 1981;104:597-598. |
| 134. | van Baar HM, van der Vleuten CJ, van de Kerkhof PC. Dapsone versus topical immunotherapy in alopecia areata. Br J Dermatol. 1995;133:270-274. |
| 135. | McMillen R, Duvic M. Alopecia areata occurring in sisters after administration of rifampicin. J Am Acad Dermatol. 2001;44:142-143. |