Published online Jul 14, 2011. doi: 10.3748/wjg.v17.i26.3126
Revised: March 5, 2011
Accepted: March 12, 2011
Published online: July 14, 2011
AIM: To analyze the long-term prognosis in a cohort of western cirrhotic patients with single hepatocellular carcinoma treated with ethanol injection.
METHODS: One-hundred forty-eight patients with solitary hepatocellular carcinoma were enrolled. The tumor diameter was lower than 2 cm in 47 patients but larger in the remaining 101 patients. The impact of some pre-treatment clinical and laboratory parameters and of tumor recurrence on patients’ survival was assessed.
RESULTS: Among the pre-treatment parameters, only a tumor diameter of less than 2 cm was an independent prognostic factor of survival. The occurrence of new nodules in other liver segments and the neoplastic portal invasion were linked to a poorer prognosis at univariate analysis. Patients with a single hepatocellular carcinoma smaller than 2 cm showed a better 5-year cumulative survival (73.0% vs 47.9%) (P = 0.009), 3-year local recurrence rate (29.1% vs 51.5%) (P = 0.011), and 5-year distant intrahepatic recurrence rate (52.9% vs 62.8%) (P = 0.054) compared to patients with a larger tumor.
CONCLUSION: The 5-year survival rate of patients with single hepatocellular carcinoma < 2 cm undergoing ethanol injection is excellent and comparable to that achieved using radiofrequency ablation.
- Citation: Pompili M, Nicolardi E, Abbate V, Miele L, Riccardi L, Covino M, Matthaeis ND, Grieco A, Landolfi R, Rapaccini GL. Ethanol injection is highly effective for hepatocellular carcinoma smaller than 2 cm. World J Gastroenterol 2011; 17(26): 3126-3132
- URL: https://www.wjgnet.com/1007-9327/full/v17/i26/3126.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i26.3126
Hepatocellular carcinoma (HCC) is the sixth more common cancer worldwide, and is the leading cause of death among patients with cirrhosis in Europe and the USA[1-3]. Since the rate of tumors diagnosed early in a subclinical stage is still low, screening and surveillance strategies in cirrhotic patients based on an ultrasound (US) study of the liver and serum assay of α-fetoprotein (AFP) have been developed and applied in both the Eastern and Western world. Accordingly, a trend towards a progressive decrease of the mean diameter of HCC diagnosed by screening during the last few years has been demonstrated, and this seems to improve patients’ prognosis by increasing their access to curative treatments[4].
Surgical resection and liver transplantation provide the best effective cure in HCC. However, resection is usually offered to cirrhotics with a single lesion and preserved liver function without severe portal hypertension, while liver transplantation is an effective option for patients with tumors within the Milan criteria (one nodule < 5 cm or no more than 3 nodules < 3 cm), but can only be considered in a limited number of patients due to the problem of organ shortage[5]. US-guided percutaneous ablation is currently regarded as the first line approach in the treatment of early-stage HCC deemed unsuitable for surgery or liver transplantation. Percutaneous ethanol injection (PEI) was the first percutaneous treatment introduced in clinical practice; it has been widely used during the last 20 years with excellent results[6], and was recommended as the standard ablation therapy of HCC according to the European guidelines for the management of HCC published in 2001[7]. At the end of the nineties, radiofrequency ablation (RFA) became available and progressively replaced PEI[8,9]. Accordingly, a question about the usefulness of PEI in the treatment of HCC has been recently raised. In agreement with Forner et al[10], we think that PEI is still useful for the treatment of lesions located at risky sites for RFA, for residual areas of viable tumors after RFA, and as a bridge treatment in HCC patients listed for liver transplantation. Furthermore, according to the present guidelines for the management of HCC[11], the efficacy of PEI is probably similar to that of RFA in patients with compensated cirrhosis and single tumors smaller than 2 cm in which 5-year survival has been shown to be higher than 70% in eastern series[9,12,13].
The aim of this study was to assess the factors affecting long term prognosis in a single-centre cohort of western cirrhotic patients with single HCC treated with PEI, focusing on the subgroup of patients with small tumors smaller than 2 cm.
Two hundred-eighteen cirrhotic patients with single HCC treated with PEI in our centre during the period 1991-2008 were evaluated. In all patients cirrhosis was confirmed by histological and/or clinical findings (blood chemistry, US, and/or endoscopic signs of liver cirrhosis and/or portal hypertension). Most patients were diagnosed during a 6 mo-interval screening program for early diagnosis of HCC based upon ultrasound study of the liver and serum assay of AFP. After detection of the suspicious HCC nodule, all patients underwent characterization of the lesion using computed tomography (CT), magnetic resonance imaging (MRI), contrast enhanced ultrasound (CEUS), and/or fine-needle biopsy, in accordance to the current diagnostic guidelines; for this reason, most of the lesions diagnosed before 2001 were further evaluated using cyto-histological assessment, while lesions larger than 2 cm detected after 2001 were mainly diagnosed using two imaging studies showing coincident typical dynamic findings after contrast enhancement; a cytological and/or histological evaluation using fine-needle biopsy was reserved for lesions showing non-typical features on dynamic imaging study[7]. After 2005 this policy was extended to lesions less than 2 cm in size[11].
Patients were usually staged before treatment using CT scan. MRI was reserved for patients with contraindications to the administration of iodinated contrast media.
Among the 218 patients included in the study group, 48 were excluded because PEI was associated with other locoregional therapies as initial treatment, 19 because complete necrosis was not achieved after treatment completion, and 3 because of insufficient follow-up data. Hence, the study refers to 148 patients with a minimum post-treatment follow-up period of 6 mo. One hundred-three patients were treated during the period 1991-2000 while the remaining 45 patients underwent PEI during the period 2001-2008. All patients gave informed consent to all diagnostic investigations and therapeutic procedures and the study protocol conforms to the ethical guidelines of the of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee. For the whole population, the last follow-up data were recorded on February 2010.
The patients were 109 men and 39 women (mean age 67 ± 8 years, range 34-87 years). The cirrhosis etiology was post-hepatic C in 107 cases, post-hepatic B in 18 cases, post-hepatic C and B in 7 cases, alcoholic in 10 cases, and cryptogenic in 6 cases. According to the Child-Pugh scoring system[14], 130 patients were scored Class A, and 18 Class B. Fifteen patients had mild ascites, while 6 patients showed partial non-neoplastic thrombosis of the portal system according to dynamic imaging studies[15]. The mean HCC diameter was 2.8 cm (range 1.1-5.8 cm). Forty-seven patients (31.8%) had a single tumor < 2 cm, 57 (38.5%) between 2 and 3 cm, and 44 (27.0%) > 3 cm. In only 3 cases the tumor diameter exceeded 5 cm. Overall, a cytological and/or histological diagnosis of HCC was available in 63/148 HCCs (well differentiated in 52 cases, moderately differentiated in 10 cases, and poorly differentiated in 1 case).
All patients were excluded from resective surgery due to one or more of the following reasons: severe portal hypertension, impaired liver function, refusal of surgery, presence of severe comorbidities increasing the surgical risk, and severely impaired clotting parameters. Four patients were waiting for liver transplantation and PEI was used as a bridging treatment; all these patients were transplanted and the follow-up period ended at the moment of transplantation. For patients diagnosed after 2000, when RFA became available in our centre, the main reasons for choosing PEI for treatment were the following: impaired clotting parameters preventing the use of large bore needles, location of the tumor in a dangerous position for RFA (e.g. near the gall bladder, the glissonian capsule, or an intestinal loop adjacent to the liver edge), location of the tumor near a large vessel lowering the level of heating needed to induce tumor necrosis, and patient choice.
Patients were usually treated by multisession US-guided PEI on an out-patient basis. After local antisepsis and intradermal local anaesthesia at the site of the needle insertion, sterile 95% ethanol (Salf, Bergamo, Italy) was injected into the lesion using either a 20-cm-long Chiba needle with an inner calibre of 20 Gauge (Ekoject, Hospital Service, Italy) or a PEI dedicated 20-cm long multi-hole 21-Gauge needle (Peit Needle, Hospital Service, Italy) at a dosage of 1-8 mL per session. The Chiba needles were preferentially employed to treat small tumors while the multi-hole needles were usually reserved for lesions larger than 2-3 cm. Treatment was performed once or twice per week and the total amount of ethanol injected was calculated according to the numerical expression V=(4/3) π (r+0.5)3, where V (in mL) is the volume of ethanol and r (in cm) is the radius of the lesion increased by 0.5 cm based on the concept that the volume of ethanol injected must overcome the theoretical volume of the lesion[16]. The number of PEI procedures needed to achieve tumor ablation was 6 on average (range 1-11) in the whole population and 2 (range 1-4) in the subgroup of patients with HCC of up to 2 cm. The amount of ethanol injected per session ranged between 2 and 9 mL, with no more than 15 min needed for each PEI session. The effectiveness of PEI was assessed by CT scan with contrast enhancement performed within one month after treatment completion. Complete necrosis was considered achieved when the lesion appeared as a non perfused area during the arterial phase of the study. In the case of intolerance to iodinated contrast media, MRI with gadolinium contrast enhancement was used. During the last 7 years, we added CEUS in the post-PEI assessment of the treated lesion; our policy was to perform CEUS about one month after PEI; in case of presence of residual arterial enhancement, suggesting the presence of a viable tumor, the lesion was immediately re-treated without further imaging evaluation while in the case of absence of contrast enhancement in the arterial phase, suggesting complete necrosis, we used CT or MRI as a confirmatory test[17].
After completion of treatment, the patients were followed by serum assay of AFP and US liver study performed every 3 mo. Local recurrence of the treated lesion was suspected on the basis of size increase and/or US pattern change; in this case, CEUS, CT scan or MRI were performed to assess the presence of arterial enhancement areas suggesting recurrent disease. Furthermore, each new lesion visualized by US in a liver segment different from that of the first neoplasm was characterized using CEUS, CT scan, MRI, and/or US-guided liver biopsy, following the current guidelines for HCC diagnosis[7,11].
The following clinical and biochemical parameters assessed before PEI were analyzed to examine their value as predictive factors for survival (Table 1): age, gender, hepatitis B surface antigen (HBsAg), hepatitis C virus (HCV) antibody, Child-Pugh class, aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, prothrombin time ratio, serum bilirubin, serum albumin, platelet count, presence of ascites, presence of portal thrombosis, serum AFP and HCC size ≤ 2 cm. Finally, the impact on survival of local recurrence (HCC recurrence in the same liver segment), distant recurrence (HCC recurrence in a liver segment different from that of the first neoplasm), portal invasion (imaging detection or cytological diagnosis of neoplastic thrombosis of the portal tree), and disease free survival (DFS, defined as the interval in months between last PEI session until local HCC recurrence and/or appearance of new HCC lesions within or outside the liver) were also evaluated.
| Variables | Median (Interquartile range) | P value at Log-Rank1 | Cox regression2 |
| Pre-PEI | |||
| Age (yr) | 67 (61-72) | 0.640 | - |
| Male gender | 109 (73.6) | 0.640 | - |
| HCV Pos | 114 (77.0) | 0.615 | - |
| HBsAg Pos. | 25 (16.9) | 0.624 | - |
| Child B | 18 | 0.329 | - |
| AST (IU/L) | 66.5 (44.5-89.5) | 0.794 | - |
| ALT (IU/L) | 64 (41.5-106.5) | 0.877 | - |
| TAP | 79 (69-86) | 0.248 | - |
| Tot. Bilirubin | 1.0 (0.80-1.45) | 0.438 | - |
| Albumin | 3.6 (3.3-3.9) | 0.116 | - |
| PLT | 100 (76.5-139.5) | 0.342 | - |
| AFP | 11.1 (6-31) | 0.330 | - |
| Ascites | 15 (10.1) | 0.108 | - |
| Portal thrombosis | 6 (4.1) | 0.133 | - |
| Size < 2 cm | 47 (31.8) | 0.009 | 0.421 (0.216-0.821), P = 0.011 |
| Post-PEI | |||
| Portal invasion | 14 (9.5) | 0.003 | - |
| Local recurrence | 56 (37.8) | 0.210 | - |
| Distance recurrence | 61 (41.2) | 0.003 | - |
| Global recurrence | 86 (58.1) | 0.180 | - |
Overall survival was defined as the interval in months between the first PEI session until death or the last recorded follow-up. Cumulative survival and recurrence curves were obtained using the Kaplan-Meier curves. For each variable taken into account, the differences between curves were assessed using the post hoc log-rank test. For age, ALT level, AST level, prothrombin time ratio, bilirubin level, albumin level, platelet count, and AFP, the patients were separated into groups: those ≤ the median and those > the median. For the purpose of the study, patients were split into two subgroups according to a HCC size smaller or larger than 2 cm. The parameters significant at univariate analysis were tested using the Cox’s proportional hazard model. The post treatment parameters of neoplastic portal invasion, local recurrence, distant recurrence, and DFS were only tested by univariate analysis. Correlation was analyzed with the Spearman rank test. A P value of ≤ 0.05 was considered significant. All statistical analyses were performed using the SPSSTM 13.0 software package.
The mean follow-up period was 42 mo (range 8-166 mo), and was longer than 60 mo in 34 patients. In no case was the PEI procedure associated with mortality or complications requiring emergency treatment. Seeding of HCC to the abdominal wall was not recorded.
The 1-, 3-, and 5-year survival rate of the entire cohort was 95.8%, 73.4%, and 55.9%, respectively, and the estimated median survival rate was 78 mo [CI 95% 54.38-101.62] (Table 2). During the follow-up, 4 patients underwent OLT and 51 (34.5%) died. The causes of death were: liver failure due to progression of the neoplasm involving more than 50% of the liver parenchyma with or without invasion of the main intrahepatic vessels in 35 cases (68.6%), liver failure unrelated to tumor progression in 7 cases (13.7%), bleeding from esophageal or gastric varices in 2 cases (2.9%), and other extrahepatic diseases in 3 cases (sepsis, myocardial infarction, pulmonary embolism, 5.9%). The cause of death was unknown in 4 cases (7.8%).
| Months | Overall survival | Disease free survival | ||
| % | n | % | n | |
| 0 | 148 | 148 | ||
| 6 | 100 | 147 | 89.2 | 132 |
| 12 | 95.8 | 135 | 71.0 | 99 |
| 24 | 86.7 | 99 | 48.5 | 51 |
| 36 | 73.4 | 66 | 33.5 | 27 |
| 48 | 64.1 | 42 | 32.1 | 23 |
| 60 | 55.9 | 33 | 24.1 | 9 |
| Median (95% CI) | 78 (54.38-101.62) | 24 (16.90-31.09) | ||
Fifty-six patients showed local HCC recurrences, 61 distant recurrences, and 31 both local and distant recurrences. The median time elapsed between the PEI completion and the first detection of local or distant recurrence were 12 mo (CI 95% 7.0-16.5), and 21 mo (CI 95% 14.0-32.0), respectively. No cases of local recurrence were recorded after 36 mo from PEI completion. The overall DFS rate was 71.0%, 33.5%, and 24.1% at 1, 3, and 5 years, respectively (Table 2). Local recurrences were treated with PEI, RFA or transarterial chemoembolization (TACE) in 41 cases (73.2%). The recurrent lesions in segments different from that of the first neoplasm were treated with PEI, RFA, TACE or systemic therapy including tamoxifen, octreotide, or sorafenib in 34 cases (55.7%). In 15 cases of local recurrence and in 27 cases of distant recurrence, no treatment was applied due to one or more of the following reasons: end stage liver failure, multifocal or infiltrative pattern of recurrence with or without vascular invasion, and concomitant diagnosis of extrahepatic spreading.
Of the whole population, HCC size < 2 cm was the only pre-PEI parameter significantly linked to survival (P = 0.009). This parameter resulted to be an independent predictor of survival after multivariate analysis using the Cox regression model [HR 0.421 (0.216-0.821); P = 0.011]. Among the post-treatment parameters, only distant recurrence and portal invasion were significantly linked to survival (P = 0.003 for both parameters) (Table 1).
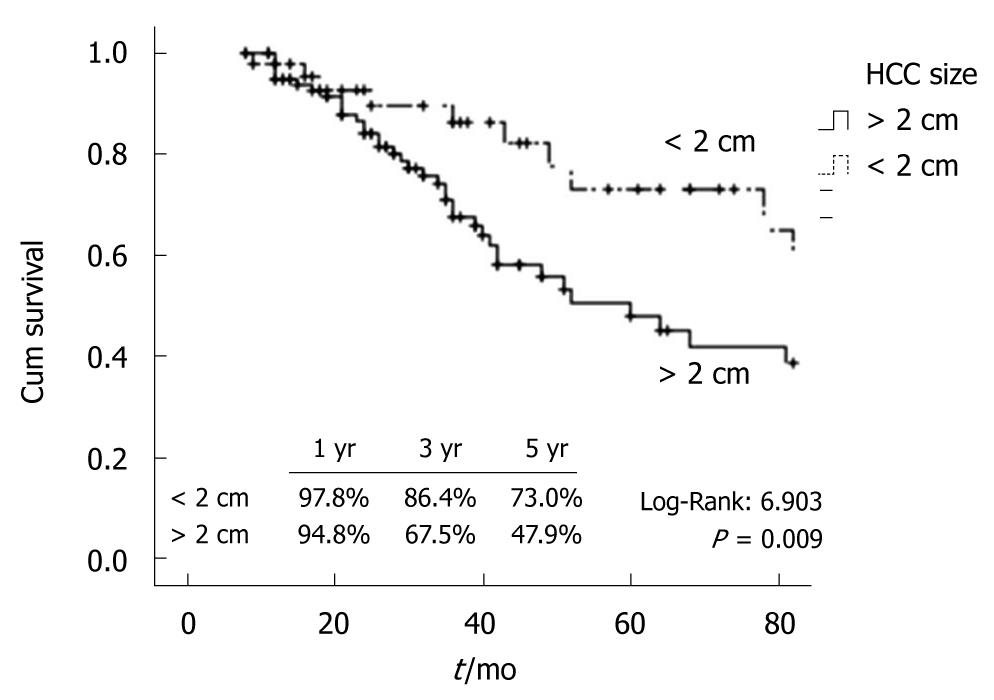
For further analysis, the 47 patients with a single HCC smaller than 2 cm were compared to the 108 patients with tumors larger than 2 cm. The clinical (age, sex, Child-Pugh class, ascites, portal thrombosis) and laboratory (HBsAg positive, anti-HCV positive, ALT, AST, prothrombin time ratio, bilirubin, albumin, platelet count, AFP) features were not significantly different between groups. As expected, the 1-, 3-, and 5- year survival rate of the patients with HCC < 2 cm (97.8%, 86.3%, and 73.0%) was significantly better than that of patients with larger tumors (94.8%, 67.5%, and 47.9%) (P = 0.009) (Figure 1). The estimated median survival of patients with HCC < 2 cm was longer than that of patients with HCC > 2 cm [93 mo (CI 95% 43.9-142.1) vs 60 mo (CI 95% 41.9-79.4)]. Furthermore, the cumulative 1-, 2- and 3-year local recurrence (13.2%, 21.6%, and 29.1% vs 28.2%, 43.7%, and 51.5%) of the patients with HCC < 2 cm was significantly lower than that of patients with larger tumors (log rank χ2 0.825, P = 0.011). Likewise, the cumulative distant recurrence at 1-, 3-, and 5-years in patients with HCC < 2 cm was lower (2.3%, 27.2%, and 52.9% vs 11.0%, 49.6%, 62.2%); this difference demonstrated a clear trend towards statistical significance (log rank χ2 5.338, P = 0.054). In order to analyze the possible influence of local on distant recurrence, we investigated the correlation between these 2 events. Interestingly, 62/92 (67.4%) of patients without local did not experience distant recurrence, while 31/56 (55.4%) of subjects with local developed distant recurrence of HCC. On this ground, we found a positive correlation at Spearman-rho test (Coefficient: 0.224; P = 0.006) and calculated that subjects with local recurrence had an increased risk to develop distant recurrence with an OR of 1.698 (95% CI 1.165-2.473).
PEI has been the first ablation technique extensively used for the treatment of HCC and is usually indicated for lesions smaller than 3 cm in diameter since complete tumor necrosis may be achieved in 90%-100% of tumors smaller than 2 cm, 70%-80% of lesions between 2 and 3 cm, and about 50% of lesions between 3 and 5 cm[18]. Furthermore, PEI has been recently shown to achieve complete necrosis of the treated tumors at explant analysis in 38/59 patients (64.3%) undergoing liver transplantation[19]. However, PEI has been largely replaced by RFA during the last years, and this is due to the best predictability of the necrotic effect in all tumor sizes and to the best effectiveness in tumors larger than 2 cm[11]. According to some recent randomized trials involving tumors with a maximal size of 3 or 4 cm, RFA is more efficacious than PEI in terms of initial complete tumor necrosis rate (93%-100% vs 66%-100%), 3-year survival rate (63%-81% vs 48%-67%) and local tumor recurrence (8%-14% vs 22%-34%)[20-25]. Furthermore, a few recent meta-analytic studies support the superiority of RFA versus PEI in terms of patient survival and local disease recurrence[26-28]. However, when the analysis is restricted to lesions smaller than 2 cm, the superiority of RFA is questionable: a recent meta-analysis about the clinical outcomes of RFA, PEI and percutaneous acetic acid injection for HCC shows that for lesions smaller than 2 cm there is no significant difference between RFA and PEI for the proportion of patient mortality and for local recurrence[28].
Our long term cohort study confirms that PEI is still an effective treatment for compensated cirrhotics with a single HCC < 2 cm since the 5-year survival rate was as high as 73.0% and a tumor diameter up to this size was the only pre-treatment parameter independently linked to survival. This datum is comparable to that shown in eastern single center series. In a group of 270 cirrhotics with HCC treated by PEI, Ebara et al[13] described a subgroup of 96 Child-Pugh Class A patients with solitary HCC smaller than 2 cm with a 5-year survival of 78.3%. Omata et al[9] reported a survival rate of 70% in a series of 144 patients with a single HCC < 2 cm undergoing PEI. Less favourable results were shown by Arii et al[29] in a retrospective multicenter Japanese survey, showing a 54% survival rate in 767 patients with Stage I HCC < 2 cm submitted to PEI.
Available data from the literature do not provide unequivocally a survival benefit of RFA over PEI in these patients. A 5-year survival of 83.8% was reported by Tateishi et al[30] in a cohort of 87 patients with HCC lower than 2 cm treated by RFA. A similar rate of 5-year survival (83.8%) has been recently reported for Child-Pugh Class A patients with a single lesion of up to 2 cm treated with RFA and registered by the Liver Cancer Study Group of Japan[31]. However, in the recent western series by Livraghi et al[32] involving 218 patients with a single HCC < 2 cm undergoing ablation with RFA, the 5-year survival rate was 55% for the whole population and 68% for the subgroup of potentially operable patients.
In our cohort of patients, intrahepatic HCC progression was the cause of death in more than two thirds of the cases, and the 5-year survival of patients with a single nodule < 2 cm was significantly better than that of patients with HCC > 2 cm (73.0% vs 47.9%). This is not surprising, considering that in tumors > 2 cm the success of ethanol in achieving necrosis of the entire mass is limited by intra-tumoral septa, and that in most of these tumors PEI does not induce a peritumoral necrosis, preventing the persistence of peripheral minute neoplastic foci or extra-tumoral satellites[10]. Accordingly, in our group of patients, the cumulative local recurrence rate of tumors smaller than 2 cm was significantly lower than that of patients with larger HCC. The reappearance of a viable tumor in a lesion assessed as completely necrotic shortly after ablation implies, in most cases, the need for additional locoregional therapy and is negatively linked to survival. In a large study by Sala et al[33], among Child-Pugh Class A patients, the 5-year survival rate of patients achieving a sustained complete response at the end of follow-up was significantly higher than that of patients without a sustained complete response due to initial treatment failure, late local recurrence, or appearance of new HCC nodules outside the segment of the first neoplasm. Similarly, in our study, distant intrahepatic recurrence was linked to survival and occurred significantly more frequently in patients with tumors larger than 2 cm. This may be in part related to the insufficient local disease control obtained by PEI in tumors more than 2 cm large. Indeed, the reappearance of viable tumor tissue may promote the HCC spreading within the liver through microvessel invasion and satellitosis. The observation of a significantly increased risk of new HCC lesions within the liver in patients with local recurrence in this series reinforces this hypothesis.
In conclusion, our study shows that a tumor diameter of up to 2 cm is an independent predictor of survival in cirrhotics with single HCC treated by PEI. In this subgroup of patients, we observed a 5-year survival rate as high as 73.0%, significantly better than that observed in patients with solitary larger tumors. This datum supports the hypothesis that in compensated cirrhotic patients with small HCC up to 2 cm submitted to ablation the long term prognosis is excellent independently from the application of PEI or RFA as therapeutic procedure[10]. Although PEI could be less effective than RFA in providing complete necrosis of the tumor even in this subset of patients, this is counterbalanced by the universal feasibility of PEI in tumors located at risky sites for RFA, or in which a thermal ablation may be less efficient due to the proximity of large vessels. For these reasons, a large prospective randomized study, designed to assess the overall survival as primary end point according to the principles of the intention-to-treat analysis, taking into account the site of the tumor in the liver, and including a rigorous cost-effectiveness evaluation , is still needed.
Small hepatocellular carcinomas of up to 2 cm are increasingly being recognized due to the diffusion of the screening program for early diagnosis of this tumor in liver cirrhosis. The ideal treatment of such small lesions is a controversial issue.
Prospective randomized studies comparing the available treatments for small hepatocellular carcinoma are lacking and should be planned.
This study shows that the survival rate of compensated cirrhotic patients with solitary hepatocellular carcinoma of up to 2 cm treated with percutaneous ethanol injection is higher than 70%. Furthermore, in the series of 148 patients with a single tumor up to 5.8 cm in size treated with this technique, a tumor diameter equal or lower than 2 cm was the only factor significantly linked to survival.
In authors’ opinion, percutaneous ethanol injection is still a valuable treatment of small hepatocellular carcinoma of up to 2 cm in size emerging in liver cirrhosis. In this subset of patients, the demonstration of a better therapeutic performance of radiofrequency ablation in terms of overall survival and cost-effectiveness is still lacking.
Percutaneous ethanol injection is a well standardized technique of chemical ablation of hepatocellular carcinoma. When introduced within the neoplasm through a fine-needle, ethanol shows a direct cytotoxic effect and produces coagulative necrosis, followed by fibrosis. In addition, due to the lesive effect of ethanol on the endothelial cells, tumor ischemic necrosis is elicited by thrombosis of the small tumor feeding vessels. The effectiveness of this technique has been questioned after the introduction in clinical practice of the thermal ablation techniques of liver tumors.
This is an interesting series showing a continued role for PEI for single small HCC. However, my guess is that RFA has replaced PEI in most centers related to the number of procedures to achieve benefit. There are not many reports from western countries, making this report of interest and worthy of consideration for publication.
Peer reviewer: Emmet B Keeffe, MD, Professor, Chief of Hepatology, Medical Director, Liver Transplant Program, Program Director, Gastroenterology Fellowship, Stanford University Medical Center, 750 Welch Road, Suite 210, Palo Alto, CA 94304, United States
S- Editor Tian L L- Editor Rutherford A E- Editor Ma WH
| 1. | Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30. |
| 2. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. |
| 3. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. |
| 4. | Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005-1014. |
| 6. | Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S, Torzilli G. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101-108. |
| 7. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. |
| 8. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661. |
| 9. | Omata M, Tateishi R, Yoshida H, Shiina S. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: Ethanol injection therapy and radiofrequency ablation. Gastroenterology. 2004;127:S159-S166. |
| 10. | Forner A, Bruix J. Ablation for hepatocellular carcinoma: Is there need to have a winning technique? J Hepatol. 2010;52:310-312. |
| 11. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. |
| 12. | Sakamoto M, Hirohashi S. Natural history and prognosis of adenomatous hyperplasia and early hepatocellular carcinoma: multi-institutional analysis of 53 nodules followed up for more than 6 months and 141 patients with single early hepatocellular carcinoma treated by surgical resection or percutaneous ethanol injection. Jpn J Clin Oncol. 1998;28:604-608. |
| 13. | Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, Kondo F, Saisho H. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458-464. |
| 14. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. |
| 15. | Piscaglia F, Gianstefani A, Ravaioli M, Golfieri R, Cappelli A, Giampalma E, Sagrini E, Imbriaco G, Pinna AD, Bolondi L. Criteria for diagnosing benign portal vein thrombosis in the assessment of patients with cirrhosis and hepatocellular carcinoma for liver transplantation. Liver Transpl. 2010;16:658-667. |
| 16. | Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, Hamada E, Takahashi M, Shiratori Y, Terano A. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;160:1023-1028. |
| 17. | Pompili M, Riccardi L, Covino M, Barbaro B, Di Stasi C, Orefice R, Gasbarrini G, Rapaccini GL. Contrast-enhanced gray-scale harmonic ultrasound in the efficacy assessment of ablation treatments for hepatocellular carcinoma. Liver Int. 2005;25:954-961. |
| 18. | Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;31:461-476. |
| 19. | Branco F, Brú C, Vilana R, Bianchi L, Alves de Mattos A. Percutaneous ethanol injection before liver transplantation in the hepatocellular carcinoma. Ann Hepatol. 2009;8:220-227. |
| 20. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. |
| 21. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004;127:1714-1723. |
| 22. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151-1156. |
| 23. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. |
| 24. | Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727-735. |
| 25. | Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453-459. |
| 26. | Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514-524. |
| 27. | Bouza C, López-Cuadrado T, Alcázar R, Saz-Parkinson Z, Amate JM. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009;9:31. |
| 28. | Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgrò G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol. 2010;52:380-388. |
| 29. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. |
| 30. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. |
| 31. | Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78 Suppl 1:113-124. |
| 32. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. |
| 33. | Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, Brú C, Bruix J. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352-1360. |