Published online Jul 7, 2011. doi: 10.3748/wjg.v17.i25.3012
Revised: March 1, 2011
Accepted: March 8, 2011
Published online: July 7, 2011
AIM: To determine in obese children with nonalcoholic fatty liver disease (NAFLD) the accuracy of magnetic resonance imaging (MRI) in assessing liver fat concentration.
METHODS: A case-control study was performed. Cases were 25 obese children with biopsy-proven NAFLD. Controls were 25 obese children matched for age and gender, without NAFLD at ultrasonography and with normal levels of aminotransferases and insulin. Hepatic fat fraction (HFF) by MRI was obtained using a modification of the Dixon method.
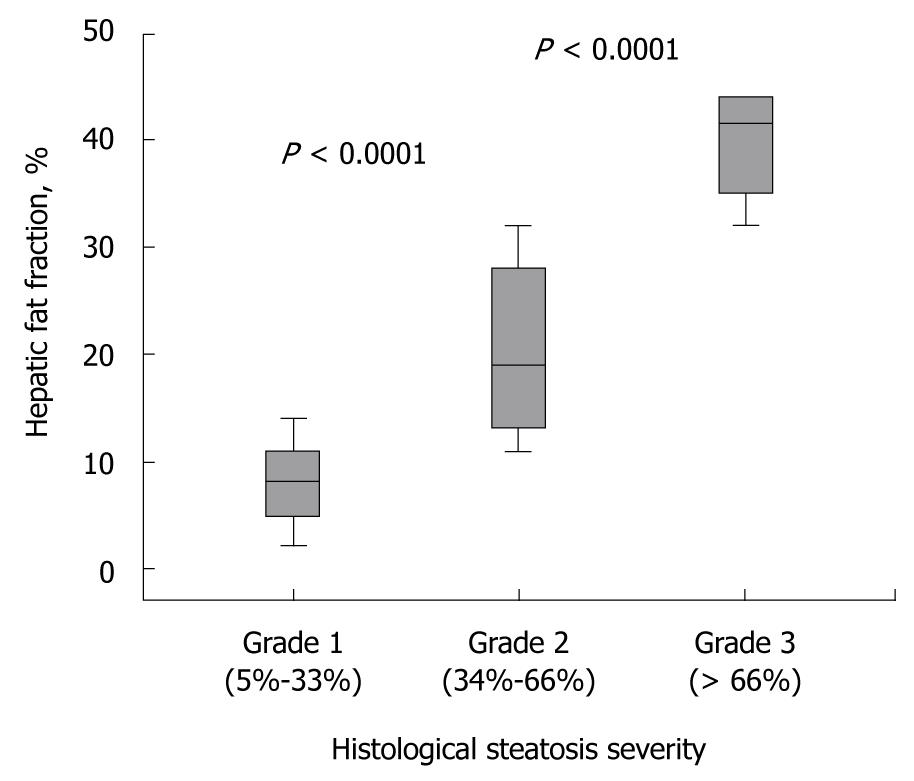
RESULTS: HFF ranged from 2% to 44% [mean, 19.0% (95% CI, 15.1-27.4)] in children with NAFLD, while in the controls this value ranged from 0.08% to 4.69% [2.0% (1.3-2.5), P < 0.0001]. HFF was highly correlated with histological steatosis (r = 0.883, P < 0.0001) in the NAFLD children. According to the histological grade of steatosis, the mean HFF was 8.7% (95% CI, 6.0-11.6) for mild, 21.6% (15.3-27.0) for moderate, and 39.7% (34.4-45.0) for severe fatty liver infiltration. With a cutoff of 4.85%, HFF had a sensitivity of 95.8% for the diagnosis of histological steatosis ≥ 5%. All control children had HFF lower than 4.85%; thus, the specificity was 100%. After 12 mo, children with weight loss displayed a significant decrease in HFF.
CONCLUSION: MRI is an accurate methodology for liver fat quantification in pediatric NAFLD.
- Citation: Pacifico L, Martino MD, Catalano C, Panebianco V, Bezzi M, Anania C, Chiesa C. T1-weighted dual-echo MRI for fat quantification in pediatric nonalcoholic fatty liver disease. World J Gastroenterol 2011; 17(25): 3012-3019
- URL: https://www.wjgnet.com/1007-9327/full/v17/i25/3012.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i25.3012
Over the last two decades, the rise in the prevalence rates of overweight and obesity probably explains the emergence of nonalcoholic fatty liver disease (NAFLD) as the leading cause of liver disease in the pediatric population worldwide[1,2]. NAFLD comprises a disease spectrum ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), progressive to cirrhosis. It is a likely common cause of cryptogenic cirrhosis[3]. There is currently no specific biochemical or serological test for fatty liver and the diagnosis can be established accurately only by liver biopsy. The invasive nature of liver biopsy means that it cannot be used to screen large numbers of subjects at risk, or be performed repeatedly to measure fat changes following treatment. Therefore, availability of an accurate non-invasive tool to assess the presence and severity of liver fat will have important clinical implications in children.
To date, several imaging techniques are used to detect hepatic steatosis: ultrasonography (US), computed tomography (CT), proton magnetic resonance spectroscopy (MRS), and magnetic resonance imaging (MRI)[4-7]. Ultrasonography is a low-cost, widely used technique for the qualitative assessment of steatosis. However, it cannot provide reliable quantitative data, and its sensitivity is reduced in morbidly obese subjects and in those with small amounts of fatty liver infiltration. CT is accurate in the semiquantitative diagnosis of macrovesicular steatosis of 30% or greater; in addition, its use for monitoring treatment response is somewhat limited due to exposure to ionizing radiation. MRS is currently considered the most accurate non-invasive technique for detecting fat quantities as low as 0.5%. However, MRS demonstrates some limitations in that it is too time consuming for routine clinical practice, and requires a skilled operator to correctly perform the examination, process the data, and interpret the results. Because of these limitations, MRS still lacks general availability in current clinical practice for assessment and monitoring of hepatic steatosis. Unlike MRS, MRI is easy to perform and interpret, and, therefore, may be more suitable for widespread use. In adult patients several investigations have demonstrated a good correlation between the severity of hepatic steatosis on MRI and liver biopsy[8-11]. However, to the best of our knowledge, no studies to date have validated MRI with liver histology in the pediatric population. Thus, the purpose of the present study was to determine in a cohort of obese children with biopsy-proven NAFLD the accuracy of MRI for the detection and quantitative assessment of liver steatosis, and to correlate results with clinical, metabolic and histologic findings. We also sought to assess the usefulness of MRI for the evaluation of liver fat changes after a 1-year lifestyle intervention.
Twenty-five obese children and adolescents, 16 males and 9 females, aged 7-16 years, with suspected NAFLD (“cases”) were recruited for study participation at the Department of Pediatrics, Sapienza University of Rome. Controls were 25 obese children matched for age, gender and pubertal stage, without ultrasound evidence of fatty liver and with normal levels of aminotransferases, as well as of insulin. All participants were of Caucasian ethnicity. The study was approved by the Institutional Review Board, and written consent was obtained from the parents or guardians of the children.
NAFLD was suspected if the patients had elevated serum alanine aminotransferase (ALT) either persistently or intermittently, associated with diffusely hyperechogenic liver at ultrasound examination, and hyperinsulinism. Secondary causes of steatosis, including alcohol consumption, total parenteral nutrition, and the use of hepatotoxic medications, were excluded in all cases. In all patients, hepatic virus infections (hepatitis A-E and G, cytomegalovirus, and Epstein-Barr virus), autoimmune hepatitis, metabolic liver disease, α-1-antitrypsin deficiency, cystic fibrosis, Wilson’s disease, and celiac disease were ruled out with appropriate tests. The final diagnosis of NAFLD was reached by liver biopsy.
The clinical indication for biopsy was either to assess the presence of NASH and degree of fibrosis or other likely independent or competing liver diseases. Percutaneous needle liver biopsy was performed with an 18-gauge needle, under general anaesthesia and ultrasound guidance. In all patients, in order to obtain an adequate sample, biopsy specimens were obtained twice at two different sites in the right hepatic lobe. Liver specimens that were at least 1.5 cm in length and contained at least 10-11 complete portal tracts were considered adequate for histological assessment. Sections were stained with hematoxylin-eosin, periodic acid Schiff, periodic acid Schiff-digested, iron stain, and Masson trichrome reagents. Biopsy specimens were evaluated for the following, using the NASH Clinical Research Network criteria[12]: steatosis [grade 0 (< 5% macrovesicular fat), grade 1 (mild = 5%-33%), grade 2 (moderate = 34%-66%), and grade 3 (severe ≥ 66%)], portal inflammation (0-2), lobular inflammation (0-3), ballooning degeneration (0-2), and fibrosis (stage 0 to 4).
All study participants underwent physical examination including measurements of weight, standing height, body mass index (BMI), waist circumference (WC), determination of the stage of puberty, as well as systolic blood pressure (BP) and diastolic BP as previously reported in detail[13]. The degree of obesity was quantified by Cole’s least mean square method, which normalizes the skewed distribution of BMI and expresses BMI as an SD score[14]. Blood samples were taken from each subject, after an overnight fast, for estimation of glucose, insulin, total and high density lipoprotein (HDL) cholesterol, triglycerides, ALT, aspartate aminotransferase (AST), and γ-glutamyl transferase (GGT).
Insulin concentrations were measured on a COBAS 6000 immunometric analyzer (Roche Diagnostics) by an electrochemiluminescent method. The remaining analytes were measured on a COBAS INTEGRA 800 analyzer (Roche Diagnostics). Insulin resistance (IR) was determined by a homeostasis model assessment of insulin resistance (HOMA-IR). Scores were calculated as the product of the fasting serum insulin level (μU/mL) and the fasting serum glucose level (mmol/L), divided by 22.5.
NAFLD patients underwent MRI before liver biopsy and within a short-time interval [mean (SD) 3.1 (2.1) d; range, 1-7]. In controls, MRI was performed within 1 wk of clinical, laboratory and sonographic assessment. Hepatic MRI was performed with a 1.5-T magnet (Magnetom Avanto, Siemens Medical Systems, Erlangen, Germany) equipped with a phased-array surface coil and a spine array coil. Image acquisition was performed in the axial plane during an end-expiratory breath-hold using a sensitivity encoding (SENSE) technique, to reduce the overall acquisition time to approximately 15 s. We used the two-point Dixon method as modified by Fishbein et al[15]. The method is based on phase-shift imaging in which hepatic fat fraction (HFF) is calculated from the signal difference between the vectors resulting from in-phase (IP) and out-of-phase (OP) signals.
The multi-breath-hold T1-weighted dual gradient-echo sequence parameters were as follows: repetition time of 174 ms, echo time of 2.1 ms for OP images and 4.9 ms for IP images; flip angle, 70°; section thickness, 5 mm; matrix size, 256 × 182; field of view, 35 cm × 40 cm. Pixel signal intensities (SI) from IP and OP images were obtained from selected regions of interest (ROIs). The SI values both in the liver and the spleen were recorded for the IP and OP images by means of 1 cm2 circular ROIs. At three different sections (above, at the level of, and below the transverse fissure of the liver), three different ROIs were drawn (2 in the right hepatic lobe; 1 in the left hepatic lobe), totaling nine ROIs. ROI selection within the liver parenchyma was performed taking care to avoid areas with vessels, motion artefacts, and partial volume effects. ROIs were placed at anatomically matched locations on paired images by using a co-registration tool available on the picture archiving and communication system workstation. The SI of the spleen was similarly measured and a mean SI was calculated from three ROIs selected at the corresponding liver levels. The standard deviation of the SI measurements within each ROI was kept to less than 10%. Liver fat was quantified as the percentage of relative SI loss of the liver on OP images, with the following formula: [(SIin-SIout)/2 × SIin] × 100 where SI is average liver signal intensity divided by the average spleen SI, SIin and SIout are signal intensity of IP and OP images, respectively. The SI of the spleen was used as a denominator in the formula to adjust for the lack of an objective SI scale at MRI[16,17]. MR imaging results were interpreted by an experienced radiologist who was blinded to clinical, laboratory, and histologic findings.
To assess reproducibility of MRI technique, measurements were performed again in 8 study subjects who agreed to a longer examination time. Standard deviations of the differences between measurements were less than 2% in HFF.
All 25 NAFLD children were offered the chance to take part in a 12-mo intervention program. This program consisted of physical exercise and nutrition education for the individual and his or her family. Diet was hypocaloric (25-30 calories/kg per day), consisting of carbohydrate (50%-60%), fat (23%-30%), and protein (15%-20%); fatty acid composition was two-thirds saturated, and one-third unsaturated; the ω6/ω3 ratio was 4:1 as recommended by the Italian Recommended Dietary Allowances. The diet regimen was prescribed with a recommendation to engage in a moderate daily exercise program (60 min/d at least 5 d a week).
Follow-up medical examinations (including assessment of changes in anthropometric characteristics) and laboratory measurements (including serum glucose, insulin, ALT, AST, GGT, total cholesterol, HDL cholesterol, and triglycerides) were performed at 6 and 12 mo of the intervention program. MRI was repeated at 12 mo. The NAFLD children were divided into those with and without substantial weight loss during the 1-year intervention. Substantial weight loss was defined as a decrease in the SDS-BMI ≥ 0.5. This division was used because in previous studies an improvement of cardiovascular risk factors and insulin resistance was only detectable if SDS-BMI decreased ≥ 0.5[18,19].
Statistical analyses were performed using the SPSS package. Data are expressed either as frequencies or as arithmetic means or geometric means with 95% confidence intervals (CI). The measured insulin, total cholesterol, HDL cholesterol, triglycerides and HOMA-IR values were distributed with a long tail to the right (positive skew), but their logarithms were approximately normally distributed. Thus, their mean values with 95% CI are reported as geometric means. Pearson correlations and linear regression analysis were used to analyse the relationship between HFF and the histological degree of steatosis as well as clinical variables. We also performed receiver operating characteristic (ROC) curve analysis to determine the best cut-off values for MRI to predict any grade, moderate, and severe hepatic steatosis. The area under the curve (AUC) was used to assess the accuracy of MRI. Values for sensitivity, specificity, and the optimum discriminative values were also obtained. We considered false-positive and false-negative results to be equally important, and thus values were chosen that maximized sensitivity plus specificity.
Pairwise comparisons were performed using paired t test or Wilcoxon’s rank sum test, as appropriate. A P value of less than 0.05 was considered to be statistically significant.
The clinical and laboratory characteristics of cases and controls are summarized in Table 1. Obese children with NAFLD had higher BMI, BMI-SDS, WC, systolic and diastolic BP, triglycerides, insulin, and HOMA-IR values than the control group. Furthermore, compared to controls, NAFLD children had significantly higher concentrations of ALT and AST, as well as of GGT. HFF ranged from 2% to 44% [mean, 19% (95% CI, 15.1 to 27.4)] in children with NAFLD, while in the control group this value ranged from 0.08% to 4.69% [2.0% (95% CI, 1.3 to 2.5), P < 0.0001].
| Characteristics | NAFLD (n = 25) | No NAFLD (n = 25) |
| BMI, kg/m2 | 28.4 (26.4-30.3)a | 25.6 (24.4-26.8) |
| BMI-Standard deviation score | 2.20 (2.02-2.30)a | 2.01 (1.92-2.17) |
| Waist circumference, cm | 96.9 (91.8-102.1)b | 85.2 (80.3-89.0) |
| Systolic BP, mmHg | 117 (112-122)b | 107 (105-109) |
| Diastolic BP, mmHg | 70 (67-74)b | 68 (65-70) |
| Aspartate aminotransferase, U/L | 45 (33-58)c | 24 (20-28) |
| Alanine aminotransferase, U/L | 73 (55-91)c | 21 (18-25) |
| γ-glutamyl transferase, U/L | 31 (23-39)c | 13 (12-14) |
| Total cholesterol, mg/dL | 162 (143-181) | 168 (146-190) |
| HDL cholesterol, mg/dL | 42 (38-49) | 40 (37-43) |
| Triglycerides, mg/dL | 161 (115-207)b | 112 (61-134) |
| Glucose, mmol/L | 4.89 (4.69-5.10) | 4.88 (4.77-5.02) |
| Insulin, μU/mL | 31.2 (21.9-40.6)a | 20.1 (16.2-24.1) |
| HOMA-IR values | 4.27 (3.40-5.10)a | 3.45 (2.97-4.01) |
| Hepatic fat fraction, % | 19.0 (15.1-27.4)c | 2.0 (1.3-2.5) |
All 25 cases fulfilled the histopathological requirements; that is, the length of liver specimens was on average 1.9 ± 0.2 cm, and included 14 ± 2 complete portal tracts. Macrovesicular steatosis was present in all cases and combined with microvesicular in 15% of cases. The amount of steatosis ranged from 10% to 95% with a mean of 42.6% (95% CI, 31.3 to 54.0) steatotic hepatocytes. The distribution of steatosis across the cohort was mild in 36%, moderate in 36%, and severe in 28% of subjects. Lobular inflammation was present in all patients, and it was of mild to moderate grade in 96% of patients. Sixty-four percent of children had ballooning of the hepatocytes that was in most cases of grade 1. Mild, more than mild, and no portal inflammation were found in 80%, 4%, and 16% of biopsies, respectively. Some degree of fibrosis was present in 72% of patients. Thirty-two percent showed stage 1 fibrosis, whereas 36% of biopsy specimens revealed stage 2 fibrosis (Table 2). Stage 3 fibrosis was present in 4%. No patient had hepatic iron deposit.
| Grade or stage | n | % | |
| Steatosis | 0 | - | - |
| 1 | 9 | 36 | |
| 2 | 9 | 36 | |
| 3 | 7 | 28 | |
| Total | 25 | 100 | |
| Lobular Inflammation | 0 | - | - |
| 1 | 15 | 60 | |
| 2 | 9 | 36 | |
| 3 | 1 | 4 | |
| Total | 25 | 100 | |
| Portal inflammation | 0 | 4 | 16 |
| 1 | 20 | 80 | |
| 2 | 1 | 4 | |
| Total | 25 | 100 | |
| Ballooning | 0 | 9 | 36 |
| 1 | 12 | 48 | |
| 2 | 4 | 16 | |
| Total | 25 | 100 | |
| Fibrosis | 0 | 7 | 28 |
| 1 | 8 | 32 | |
| 2 | 9 | 36 | |
| 3 | 1 | 4 | |
| 4 | - | - | |
| Total | 25 | 100 |
HFF was highly correlated with histological steatosis overall (r = 0.883; P < 0.0001). According to the histological grade of steatosis, the mean HFF was 8.7% (95% CI, 6.0 to 11.6) for mild, 21.6% (95% CI, 15.3 to 27.0) for moderate, and 39.7% (95% CI, 34.4 to 45.0) for severe fatty liver infiltration. MRI imaging could differentiate between mild and moderate steatosis (P < 0.001), and between moderate and severe steatosis (P < 0.001) (Figure 1). Linear regression analysis was performed to determine the influence of the stage of fibrosis as well as of the degree of inflammation on the relationship between MRI and histological assessment of steatosis. Fibrosis was found to have no statistically significant influence [unstandardized coefficient, 1.10 (95% CI, 2.25 to 4.45); P = 0.503] on the estimates of HFF. Similarly, inflammation had no impact on the accuracy of MRI for the assessment of steatosis.
The accuracy of MRI for the diagnosis of mild, moderate, and severe steatosis is shown in Table 3. At the diagnostic threshold of 4.85% for HFF, MRI had a 95.8% sensitivity for diagnosing “any” grade of steatosis. There was only one child with histological steatosis of grade 1 who had HFF of 2%. A threshold of 9.0% for HFF was the best cutoff for the diagnosis of moderate to severe steatosis (sensitivity, 100%). A cutoff value of 19.0% for HFF was indicative of severe steatosis (sensitivity, 100%). All control children had HFF lower than 4.85% (specificity, 100%).
| Steatotic hepatocytes | ≥ 5% | > 33% | > 66% |
| Cutoff | 4.85 | 9 | 19 |
| AUC | 0.98 (95% CI, 0.98-1.0) | 1 | 1 |
| Sensitivity (%) | 95.8 | 100 | 100 |
| Specificity (%) | 100 | 100 | 100 |
Among clinical and laboratory data, HFF was significantly associated with SDS-BMI (r = 0.486, P < 0.01), WC (r = 0.406, P < 0.01), triglycerides (r = 0.374, P < 0.05), insulin (r = 0.290, P < 0.05), and HOMA-IR values (r = 0.349, P < 0.05) in the whole study population. When the NAFLD group was analysed separately, HFF remained significantly associated with insulin (r = 0.425, P < 0.05), and HOMA-IR values (r = 0.506, P < 0.05).
After 12 mo, eleven NAFLD children demonstrated a substantial weight loss [mean SDS-BMI change: -0.88 (95% CI, -0.57 to -1.19)]. In these children, HFF [mean change: -13.1% (95% CI, -9 to -19); P < 0.05)], systolic BP [mean change: -8 mmHg (95% CI, -5 to -14); P < 0.05), ALT [mean change: -45 U/L (95% CI, - 37 to -70); P < 0.05), AST [mean change: -27 U/L (95% CI, -18 to -37); P < 0.05), triglycerides [mean change: -56 mg/dL (95% CI, -32 to -60); P < 0.05), and HOMA-IR values [mean change: -3.2 (95% CI, -2.0 to -5.8); P < 0.05)] decreased significantly. In contrast, in the 14 NAFLD children without substantial weight loss [mean SDS-BMI change: -0.10 (95% CI, -0.01 to -0.19), there was no significant change in HFF [mean change: -3.0% (95% CI, -0.3 to -5.0); P = 0.48] as well as in clinical and laboratory parameters.
Liver fat accumulation is becoming a common complication in pediatric obesity[1,2]. Biopsy remains the criterion standard to accurately determine, in a semiquantitative manner, the amount of fatty liver infiltration. Furthermore, liver biopsy is able to evaluate lesions associated with steatosis, such as fibrosis and inflammation, and thus, to evaluate the stage and grade of the disease. However, we cannot perform liver biopsy as a screening method to detect NAFLD in the general pediatric population. Therefore, a reliable, non-invasive method of screening NAFLD would represent a major advance in clinical hepatology.
Several non-invasive imaging techniques have been advocated as diagnostic tests. Standard MRI, MRS, and CT may not be feasible for children because of their long scan time, reliance on compliance of the patient, and ionizing radiation. Signal intensity loss on opposed-phase gradient-echo T1-weighted MR images frequently is regarded as an accurate method of detection and quantification of liver fat[8,20,21]. By using gradient-echo chemical shift imaging (Dixon method), MRI can be performed either as readily available T1-weighted dual echo, triple echo, multiecho, or multi interference. We chose to use the T1-weighted dual-echo MRI because of its simplicity.
In the adult population, a close relationship has been observed between the percentage of steatosis estimated by histology and dual-echo chemical shift imaging[8,11,21]. However, a major point to underline is that HFF appears to be influenced greatly by fat morphology[8]. Lipid is accumulated within the liver as a response to various disease states and may be deposited in a macrovesicular, microvesicular, or mixed steatosis pattern. In a group of 38 patients undergoing liver biopsy for a variety of liver diseases (including hepatitis C, NAFLD, methotrexate monitoring, chronic hepatitis of unknown etiology, cryptogenic cirrhosis, primary biliary cirrhosis, autoimmune hepatitis), Fishbein et al[8] showed that fast-MRI correlated better with macrovesicular steatosis (r = 0.92, P < 0.001) than with mixed steatosis (r = 0.60, P < 0.05). In NAFLD, a disorder associated with severe macrovesicular steatosis, fat fraction was higher than other liver disorders associated with lesser degrees of fatty infiltration, including hepatitis C. Similarly, in a very recent study including 46 patients undergoing liver resection, van Werven et al[21] showed that T1-weighted dual-echo MR imaging was strongly correlated with histopathologic steatosis assessment (r = 0.85, P < 0.001). In the 23 patients with macrovesicular steatosis greater than 5%, dual-echo MR imaging showed an even stronger correlation with histopathologic examination (r = 0.92, P < 0.001) than in the overall group.
In the pediatric population, the 2-point Dixon method as modified by Fishbein is an accepted technique for measuring hepatic fat content[22-24]. It can also be helpful in identifying fat regression or progression in children, and it has been found useful in differentiating increased liver echogenicity due to simple steatosis from that related to glycogen storage disease[25,26]. However, no previous studies in children have used the degree of hepatic steatosis at histologic analysis as the reference standard. In normal liver, lipid accounts for approximately 5% total wet weight[22]. Initial studies determining liver fat in children by fast-MRI defined as abnormal a threshold value for HFF greater than 2 SD above mean hepatic fat content of healthy adult volunteers[15] or lean, nondiabetic (mean age 21.6 ± 8.2 years) subjects[27]. Recent studies have validated the modified 2-point Dixon method against MRS in obese and lean adolescents who were at increased risk of having or developing hepatic steatosis, and found a very strong correlation between the two methods (r = 0.954, P < 0.001)[23,24]. In a cohort of 28 (mean age, 15.9 ± 5.3 years) obese and lean subjects, Kim et al[23] demonstrated that a 2-point Dixon HFF cutoff of 3.6% provided a good sensitivity (80%) and specificity (87%) compared to MRS reference. Our present results obtained in a homogeneous population indicate that the modified 2-point Dixon method may be a good alternative to biopsy for quantifying liver fat content in obese youngsters with NAFLD and for assessing the relation between HFF and metabolic outcomes in these patients. The clinical efficacy of fast-MRI has been previously demonstrated. Burgert et al[27] showed that obese children with a high HFF were significantly more insulin resistant, compared with those with a low HFF, and had higher triglycerides and lower adiponectin levels, even after adjustment for BMI-z scores, race/ethnicity, gender, and age. Furthermore, obese children with a high HFF had a significantly greater prevalence of the metabolic syndrome, after controlling for the above confounders. More recently, D’Adamo et al[28] suggested that the severity of fatty liver, as determined by the modified 2-point Dixon method, plays a central role in the insulin resistant state in obese adolescents, independently of visceral fat and intramyocellular lipid content. Using the disposition index, an estimate of β-cell function weighted by insulin sensitivity, the authors found this was reduced by 30% in children with fatty liver, thus increasing susceptibility to type 2 diabetes. We also previously showed that the increasing severity of MRI fat accumulation was strongly related to fasting hyperinsulinemia and insulin resistance after correction for confounding variables such as SD score-BMI, sex, age and pubertal status[29]. The present results obtained in obese youths with biopsy-proven NAFLD not only corroborate the clinical efficacy of the modified 2-point Dixon method, but also highlight the potential application of this method for tracking longitudinal changes in liver fat content in patients under targeted lifestyle intervention or medical therapy. In concordance with our longitudinal findings, fast-MRI has also been found to identify longitudinal liver fat changes in adults during pioglitazone treatment for biopsy-proven NAFLD and in obese children and adolescents after a 1-year nutrition-behavior intervention[25,30].
Fibrosis and inflammation may be present in patients with hepatic steatosis. In adult patients with heterogeneity of underlying pathologies including NAFLD, Fishbein et al[8] showed that hepatic MRI, based upon chemical shift imaging, is not influenced by the presence of fibrosis and was able to accurately quantify the hepatic fat content in the patients who also had significant hepatic fibrosis. Our present results confirm and expand on the findings of the above report. In fact, we found that in our obese children with NAFLD, neither inflammation nor fibrosis had an influence on the estimates of steatosis.
Our study has some limitations. Firstly, our results were obtained in a selected population of children with and without NAFLD. Therefore, one could argue that by doing so we would maximize the differences between cases and controls. If we had included patients from the general pediatric population, the results would have been more conclusive. However, obtaining hepatic biopsy for research purposes in such patients would not be feasible or ethical. Secondly, another restriction is that we did not perform MRI measurements at exactly the same locations in the liver that were used for histopathologic assessment. This could affect the results we reported in diagnostic performance. However, we obtained large-wedge liver biopsy specimens which provided accurate data. Lastly, we used a T1-weighted dual-echo chemical shift MRI method to study hepatic steatosis. No corrections for T1, T2*, or fat spectral complexity were made, and consequently only MR signal intensities were evaluated. Recent studies have indicated that T1-weighting (flip angle) and T2-weighting (iron deposition) may interfere with accurate fat quantification[16,31]. However, in the study by van Werven et al[21], a strong correlation between T1-weighted dual gradient-echo MR imaging and histopathologic results was demonstrated in the absence of any correction for T1, T2*, or fat spectral complexity. In that study as well as in ours, the mean signal intensity decay of 12 and 9 ROIs throughout the liver was measured, respectively. The T2 correction is important in cases where iron overload problems might lead to T2 changes. As liver iron deposition is a common secondary feature of many chronic liver diseases, signal intensity loss on in-phase gradient-echo MR images caused by the presence of liver iron is a potential pitfall in the determination of liver fat percentage at opposed-phase MR imaging in chronic liver diseases[16]. Thus the T2* correction is very important in cases where iron overload might lead to T2 changes, but none of our young patients with NAFLD had histological evidence of iron accumulation, consistent with a previous report of adult patients with NAFLD who were seen at a referral center without a special interest in disorders of iron storage[32]. In that report, significant iron histological accumulation was not observed in the majority of patients with NAFLD or its various subtypes.
In conclusion, this study with histopathologic validation shows that the modified Dixon method provides high diagnostic and fat-grading accuracy in obese children with NAFLD. Even if the small number of patients included in our study must be taken into account, the results obtained are highly encouraging and may provide a basis for stimulating further studies which would include a larger number of children.
Nonalcoholic fatty liver disease (NAFLD) has been increasing over the past three decades, both in children and adolescents, presenting a worldwide problem. NAFLD is characterized by lipid accumulation in the liver, and it represents a disease spectrum that ranges from simple hepatic steatosis to steatohepatitis, and eventually to cirrhosis and liver failure.
Currently, liver biopsy is considered the gold standard to accurately determine, in a semiquantitative manner, the amount of fatty liver infiltration. However, the authors cannot perform liver biopsy as a screening method to detect NAFLD in the general pediatric population. This is an invasive procedure with the potential of complications, being also prone to sampling error and interobserver variability. Consequently, there is a need in children for non-invasive, safe diagnostic tools to detect and quantify hepatic steatosis as well as to identify hepatic fat regression or accumulation over time.
To date, several imaging techniques are used to detect hepatic steatosis. Ultrasonography is a low-cost, widely used technique for the qualitative assessment of steatosis in children. However, it cannot provide reliable quantitative data, and its sensitivity is reduced in subjects with small amounts of fatty liver infiltration. Standard magnetic resonance imaging (MRI), proton magnetic resonance spectroscopy, and computed tomography may not be feasible for children because of their long scan time, reliance on compliance of the patient, and ionizing radiation. In the pediatric population, among the MRI methods, the 2-point Dixon method as modified by Fishbein is an accepted technique for measuring hepatic fat content. It can also be helpful in identifying fat regression or progression in children, and it has been found useful in differentiating increased liver echogenicity due to simple steatosis from that related to glycogen storage disease. However, no previous studies in children have used the degree of hepatic steatosis at histologic analysis as the reference standard.
The authors’ present results obtained in a homogeneous population with NAFLD indicate that the dual-echo MRI may be a good alternative to biopsy for quantifying fat liver content in obese youngsters and for assessing the relation between hepatic fat fraction and metabolic outcomes in these patients. Furthermore, the authors’ results highlight the potential application of this method for tracking longitudinal changes in liver fat content in patients under targeted lifestyle intervention or medical therapy.
This study with histopathologic validation shows that the dual-echo MRI provides high diagnostic and fat-grading accuracy in obese children with NAFLD. MRI is easy to perform and interpret, and, therefore, may be suitable for widespread use.
Dr. Lucia Pacifico and colleagues quantified the amount of liver fat in pediatric NAFLD by using T1-weighted dual-echo MRI, and assessed the validation of MRI quantification by comparing liver biopsy specimens. The noninvasive quantification method with high sensitivity and specificity is very important to assess the degree of fat accumulation in the liver, specifically in uncooperative or high-risk patients for invasive procedures. This study arouses interest for readers and provides an important clue to evaluate the degree of NAFLD or the improvement of the disease in the treatment or follow-up observation.
Peer reviewer: Akihito Tsubota, Assistant Professor, Institute of Clinical Medicine and Research, Jikei University School of Medicine, 163-1 Kashiwa-shita, Kashiwa, Chiba 277-8567, Japan
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Dunn W, Schwimmer JB. The obesity epidemic and nonalcoholic fatty liver disease in children. Curr Gastroenterol Rep. 2008;10:67-72. |
| 2. | Pacifico L, Poggiogalle E, Cantisani V, Menichini G, Ricci P, Ferraro F, Chiesa C. Pediatric nonalcoholic fatty liver disease: A clinical and laboratory challenge. World J Hepatol. 2010;2:275-288. |
| 3. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. |
| 4. | Cassidy FH, Yokoo T, Aganovic L, Hanna RF, Bydder M, Middleton MS, Hamilton G, Chavez AD, Schwimmer JB, Sirlin CB. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics. 2009;29:231-260. |
| 5. | Mehta SR, Thomas EL, Bell JD, Johnston DG, Taylor-Robinson SD. Non-invasive means of measuring hepatic fat content. World J Gastroenterol. 2008;14:3476-3483. |
| 6. | Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433-445. |
| 7. | Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87-97. |
| 8. | Fishbein M, Castro F, Cheruku S, Jain S, Webb B, Gleason T, Stevens WR. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005;39:619-625. |
| 9. | Mennesson N, Dumortier J, Hervieu V, Milot L, Guillaud O, Scoazec JY, Pilleul F. Liver steatosis quantification using magnetic resonance imaging: a prospective comparative study with liver biopsy. J Comput Assist Tomogr. 2009;33:672-677. |
| 10. | McPherson S, Jonsson JR, Cowin GJ, O'Rourke P, Clouston AD, Volp A, Horsfall L, Jothimani D, Fawcett J, Galloway GJ. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J Hepatol. 2009;51:389-397. |
| 11. | Lee SS, Park SH, Kim HJ, Kim SY, Kim MY, Kim DY, Suh DJ, Kim KM, Bae MH, Lee JY. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579-585. |
| 12. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. |
| 13. | Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, Anania C, Ferrara E, Dvisic G, Chiesa C. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res. 2008;63:423-427. |
| 14. | Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240-1243. |
| 15. | Fishbein MH, Gardner KG, Potter CJ, Schmalbrock P, Smith MA. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging. 1997;15:287-293. |
| 16. | Westphalen AC, Qayyum A, Yeh BM, Merriman RB, Lee JA, Lamba A, Lu Y, Coakley FV. Liver fat: effect of hepatic iron deposition on evaluation with opposed-phase MR imaging. Radiology. 2007;242:450-455. |
| 17. | Qayyum A, Goh JS, Kakar S, Yeh BM, Merriman RB, Coakley FV. Accuracy of liver fat quantification at MR imaging: comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques--initial experience. Radiology. 2005;237:507-511. |
| 18. | Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89:419-422. |
| 19. | Reinehr T, Kiess W, Kapellen T, Andler W. Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics. 2004;114:1569-1573. |
| 20. | Kreft BP, Tanimoto A, Baba Y, Zhao L, Chen J, Middleton MS, Compton CC, Finn JP, Stark DD. Diagnosis of fatty liver with MR imaging. J Magn Reson Imaging. 1992;2:463-471. |
| 21. | van Werven JR, Marsman HA, Nederveen AJ, Smits NJ, ten Kate FJ, van Gulik TM, Stoker J. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010;256:159-168. |
| 22. | Fishbein MH, Miner M, Mogren C, Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr. 2003;36:54-61. |
| 23. | Kim H, Taksali SE, Dufour S, Befroy D, Goodman TR, Petersen KF, Shulman GI, Caprio S, Constable RT. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point dixon and three-point IDEAL. Magn Reson Med. 2008;59:521-527. |
| 24. | Cali AM, De Oliveira AM, Kim H, Chen S, Reyes-Mugica M, Escalera S, Dziura J, Taksali SE, Kursawe R, Shaw M. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49:1896-1903. |
| 25. | Fishbein MH, Stevens WR. Rapid MRI using a modified Dixon technique: a non-invasive and effective method for detection and monitoring of fatty metamorphosis of the liver. Pediatr Radiol. 2001;31:806-809. |
| 26. | Pozzato C, Dall'asta C, Radaelli G, Torcoletti M, Formenti A, Riva E, Cornalba G, Pontiroli AE. Usefulness of chemical-shift MRI in discriminating increased liver echogenicity in glycogenosis. Dig Liver Dis. 2007;39:1018-1023. |
| 27. | Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X, Constable RT, Weiss R, Tamborlane WV, Savoye M. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287-4294. |
| 28. | D'Adamo E, Cali AM, Weiss R, Santoro N, Pierpont B, Northrup V, Caprio S. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33:1817-1822. |
| 29. | Pacifico L, Celestre M, Anania C, Paolantonio P, Chiesa C, Laghi A. MRI and ultrasound for hepatic fat quantification:relationships to clinical and metabolic characteristics of pediatric nonalcoholic fatty liver disease. Acta Paediatr. 2007;96:542-547. |
| 30. | Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188-196. |
| 31. | Schwenzer NF, Machann J, Martirosian P, Stefan N, Schraml C, Fritsche A, Claussen CD, Schick F. Quantification of pancreatic lipomatosis and liver steatosis by MRI: comparison of in/opposed-phase and spectral-spatial excitation techniques. Invest Radiol. 2008;43:330-337. |
| 32. | Younossi ZM, Gramlich T, Bacon BR, Matteoni CA, Boparai N, O'Neill R, McCullough AJ. Hepatic iron and nonalcoholic fatty liver disease. Hepatology. 1999;30:847-850. |