Published online Jun 7, 2011. doi: 10.3748/wjg.v17.i21.2602
Revised: September 9, 2010
Accepted: September 16, 2010
Published online: June 7, 2011
The histopathological diagnosis of gastric mucosal biopsy and endoscopic mucosal resection/endoscopic submucosal dissection specimens is important, but the diagnostic criteria, terminology, and grading system are not the same in the East and West. A structurally invasive focus is necessary to diagnose carcinoma for most Western pathologists, but Japanese pathologists make a diagnosis of cancer based on severe dysplastic cytologic atypia irrespective of the presence of invasion. Although the Vienna classification was introduced to reduce diagnostic discrepancies, it has been difficult to adopt due to different concepts for gastric epithelial neoplastic lesions. Korean pathologists experience much difficulty making a diagnosis because we are influenced by Japanese pathologists as well as Western medicine. Japan is geographically close to Korea, and academic exchanges are active. Additionally, Korean doctors are familiar with Western style medical terminology. As a result, the terminology, definitions, and diagnostic criteria for gastric intraepithelial neoplasia are very heterogeneous in Korea. To solve this problem, the Gastrointestinal Pathology Study Group of the Korean Society of Pathologists has made an effort and has suggested guidelines for differential diagnosis: (1) a diagnosis of carcinoma is based on invasion; (2) the most important characteristic of low grade dysplasia is the architectural pattern such as regular distribution of crypts without severe branching, budding, or marked glandular crowding; (3) if nuclear pseudostratification occupies more than the basal half of the cryptal cells in three or more adjacent crypts, the lesion is considered high grade dysplasia; (4) if severe cytologic atypia is present, careful inspection for invasive foci is necessary, because the risk for invasion is very high; and (5) other structural or nuclear atypia should be evaluated to make a final decision such as cribriform pattern, papillae, ridges, vesicular nuclei, high nuclear/cytoplasmic ratio, loss of nuclear polarity, thick and irregular nuclear membrane, and nucleoli.
- Citation: Kim JM, Cho MY, Sohn JH, Kang DY, Park CK, Kim WH, Jin SY, Kim KM, Chang HK, Yu E, Jung ES, Chang MS, Joo JE, Joo M, Kim YW, Park DY, Kang YK, Park SH, Han HS, Kim YB, Park HS, Chae YS, Kwon KW, Chang HJ, Pathologists TGPSGOKSO. Diagnosis of gastric epithelial neoplasia: Dilemma for Korean pathologists. World J Gastroenterol 2011; 17(21): 2602-2610
- URL: https://www.wjgnet.com/1007-9327/full/v17/i21/2602.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i21.2602
Techniques for endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have been developing rapidly, and pathologists more commonly encounter specimens derived from endoscopic resection. These procedures are sometimes performed for diagnostic purposes but mostly for therapeutic convenience compared with radical surgery. However, for both purposes, pathological diagnosis of gastric biopsies and EMR/ESD specimens is very important, because further treatment plans and a surveillance schedule must be established. The importance of a diagnosis is not only stressed from a clinical viewpoint, but also from an academic perspective. Many studies regarding early gastric neoplastic lesions based on a histopathological diagnosis have been performed, and they focus on various clinical and pathological aspects such as survival, recurrence, surveillance programs, and molecular pathology. However, if the pathological diagnosis is different among pathologists, the results cannot be compared. Additionally, socioeconomic problems are also important, because medical insurance is intimately associated with disease severity.
Although these issues are important, it is unfortunate that the definition, diagnostic criteria, and grading system for early stage gastric neoplastic lesions are not completely developed. In particular, it is well known that Eastern and Western pathologists use different criteria to make a gastric carcinoma diagnosis. A structurally invasive focus is necessary to diagnose carcinoma for most Western pathologists. The “Eastern” opinion is actually the “Japanese” concept, and diagnosis of gastric carcinoma is based on the cytological findings. We have realized that Korean pathologists have different diagnostic criteria for gastric epithelial neoplasia than Japanese or Western pathologists. In this article, we discuss the current problems for the pathological diagnosis of gastric neoplastic lesions, the Korean perspectives, and the path we should follow.
Initially “dysplasia” was used for inflammatory bowel disease, but, now it is used throughout the gastrointestinal tract as well as for other organs. Dysplasia means an unequivocally neoplastic but non-invasive lesion distinguished from regenerative changes[1]. The term “gastric dysplasia” was used for the first time by Grundmann in 1975 to describe an exclusively precancerous gastric lesion[2]. Shortly thereafter, a WHO committee published a definition that characterizes dysplasia as cellular atypia, abnormal differentiation, and disorganized architecture[3,4].
Most Western pathologists use the term “dysplasia” to describe a neoplastic premalignant abnormality[1,5-11]. However, in Japan, the terminology for non-invasive neoplastic lesions is different. Since Nakamura et al[12] established specific histological atypical gastric epithelium criteria, which were classified as definitely benign, borderline, or carcinoma, the Japanese Society for Research on Gastric Cancer has recommended that gastric neoplastic lesions be subdivided into one of five categories: normal or benign without cellular atypia, benign with slight atypia, borderline, probable carcinoma, and obvious carcinoma[13,14]. “Atypia” has been used more frequently than “dysplasia” in Japan. Subsequently, Japanese authors suggested the definition of “group 3 or 4 lesions”[14-16] (Table 1).
| References | Dysplasia and related lesions (from Rugge et al[40], modified) |
| Takagi et al[15] | Benign |
| Borderline | |
| Carcinoma | |
| Nagayo[14] | Atypical |
| Borderline | |
| Probable cancer | |
| Definitive cancer | |
| Grundmann[2] | Low-grade GED |
| High-grade GED | |
| Invasive cancer | |
| Oehlert et al[5] | Slight GED |
| Moderate GED | |
| Severer GED | |
| Invasive cancer | |
| Morson et al[4] | Regenerative |
| Mild GED | |
| Moderate GED | |
| Severe GED | |
| Invasive cancer | |
| Ming et al[7] | Grade 1 GED |
| Grade 2 GED | |
| Grade 3 GED | |
| Grade 4 GED | |
| Invasive cancer | |
| Japanese classification of gastric carcinoma[16] | Group I lesions |
| Group II lesions | |
| Group III-IV lesions | |
| Group V lesions | |
| Goldstein et al[9] | Reactive |
| Indefinite for GED | |
| Low-grade GED | |
| High-grade GED | |
| Invasive cancer | |
| Padova classification | Negative |
| Indefinite for dysplasia | |
| Noninvasive neoplasia | |
| Suspect for invasive carcinoma | |
| Invasive carcinoma | |
| Vienna classification | Negative |
| Indefinite for dysplasia | |
| Low grade neoplasia | |
| High grade neoplasia | |
| Invasive neoplasia |
In addition to the differences in terminology describing premalignant gastric lesions, some confusion exists for the terms adenoma and dysplasia. Originally, adenoma was considered a raised circumscribed lesion, either sessile or pedunculated, in contrast to dysplasia, which arose at flat or depressed mucosa. However, much confusing terminology has been introduced such as “flat adenoma”, “depressed adenoma”, “elevated dysplasia”, and “polypoid dysplasia”[17-31]. Thus, WHO defined adenoma as “a circumscribed benign neoplasm composed of tubular and/or villous structures lined by dysplastic epithelium”. In 1998, Lewin et al[10] suggested nomenclature using both adenoma and dysplasia; the former meant neoplastic circumscribed benign lesions unassociated with underlying inflammation whether pedunculated, sessile, flat or depressed, and the latter meant benign neoplastic lesions associated with underlying inflammation. Both were subdivided as low and high grade. Although there have been efforts to clarify the definition, a confusing situation still persists[9].
Another confusing concept is carcinoma in situ, which means carcinoma without invasion. However, a differential diagnosis of high grade dysplasia/atypia and carcinoma in situ is problematic. When cytological atypia and architectural complexity is marked, the term “carcinoma in situ” is used by some pathologists, but others do not make a distinction between “high grade dysplasia” and “carcinoma in situ” because the behavior and management are the same[10]. In the Japanese classification, there is no disease group describing adenocarcinoma in situ. The WHO International References Center for Histological/Classification of Precancerous Lesions of the Stomach met in 1978 and developed a consensus statement that stated that presumed precancerous lesions of the stomach should be termed “dysplasia” and that the term “intramucosal carcinoma” should replace “in situ carcinoma” for lesions that have invasive malignant cells confined to the lamina propria[3,4]. However, “adenocarcinoma in situ” is categorized in the AJCC and Vienna classifications as a non-invasive intraepithelial carcinoma, resulting in confusion.
The most important and surprising inconsistency between Western and Japanese criteria is in the diagnosis of adenocarcinoma. Japanese pathologists make a diagnosis of cancer based on severe dysplastic cytologic atypia with enlarged vesicular oval nuclei and prominent nucleoli irrespective of the presence of invasion. But, Western pathologists believe there must be evidence of invasion into the lamina propria to make a cancer diagnosis. This inconsistency causes serious problems understanding “early” cancer. Many investigators have pointed out this discrepancy and made some efforts to reduce the confusion. The Vienna classification was developed for common world terminology of gastrointestinal epithelial neoplasia[32]. Some Western pathologists agreed with the Japanese criteria and changed their view points[33].
Dysplasia is regarded as a precancerous lesion with increased risk of carcinoma, and the risk increases in parallel with the histological grade of the atypia. Various grading systems have been introduced to predict a prognosis of dysplasia/adenoma with more accuracy (Tables 1 and 2). The most popular grading system is the three-tiered (mild, moderate, and severe) or two-tiered (low and high) system; the latter shows better inter-observer agreement[1,33-37], and most management protocols are based on the two-tiered system. There is no distinctive management protocol according to the three-tiered system that is practically significant[1,34-37].
| Histology | Feature | Low-grade dysplasia | High-grade dysplsia | Carcinoma |
| Structural atypia | Gland size | Uniform | Variable | Variable |
| Gland arrangement | Regular | Slightly irregular | Irregular | |
| Glandular crowding | Slight | Moderate | Marked | |
| Glandular transition to surrounding mucosa | No | No | No | |
| Glandular branching/budding | Focal | Prominent | Prominent | |
| Glandular cribriform | No | Yes | Yes | |
| Surface maturation | No | No | No | |
| Nuclear atypia | Shape | Elongated | Elongated and/or irregular | Oval/round |
| Pseudostratification | Basal 1/2 | Over basal 1/2 | Irregular | |
| Membrane | Thin | Thick | Uneven | |
| Hyperchromasia | Hyperchromatic even | Hyperchromatic irregular | Vesicular | |
| Pleomorphism | No | Mild | Moderate to marked | |
| Prominent nucleoli | Absent | Present | Present | |
| Loss of polarity | No | No/yes | Yes | |
| Stroma | Invasion | No | No | Yes |
The morphological features of low grade dysplasia/adenoma are characterized by simple tubules with little branching, nuclear stratification below half of the cytoplasm, tall columnar cells with dense spindle-shaped hyperchromatic nuclei, ample amphophilic cytoplasm, and sparse mitotic figures. High-grade dysplasia/adenoma is composed of tubules with elongation and complex budding, cribriform in the most extreme cases, greatly enlarged round to oval nuclei, markedly increased nuclear/cytoplasmic ratio, and loss of nuclear polarity (Table 2).
Although well-established low and high-grade dysplasia criteria are present, there are large scale interobserver or intraobserver discrepancies. Sometimes, regeneration causes serious confusion with carcinoma. The category of “indefinite for dysplasia” is maintained in the Vienna classification[32], and the histopathological finding of regeneration has been well described in many studies[9,10,38].
Korea is geographically close to Japan, and academic exchanges are active. Korean endoscopists introduced the EMR/ESD technique from Japan, and many discussions and cooperation continues. In the pathology field, there are many conferences and collaborations between Japanese and Korean pathologists. Furthermore, Korea’s medical science is influenced by that in Western countries. Korean doctors are familiar with Western style medical terminology. As a result, the terminology, definition, and diagnostic criteria for gastric intraepithelial neoplasia are very heterogeneous in Korea.
To promote diagnostic consensus, The Gastrointestinal Pathology Study Group of the Korean Society of Pathologist (GIPS-KSP) established a grading system for gastric epithelial proliferative disease and produced a standardized pathological report for gastric cancer[38,39]. The standard guidelines for grading gastric epithelial proliferative disease are as follows: (1) proliferating gastric epithelium can be divided into hyperplastic and neoplastic; (2) the term “dysplasia” is reserved for the microscopic epithelial changes that are unequivocally neoplastic; (3) biopsy specimens are categorized as regenerative (negative for dysplasia), indefinite (questionable dysplasia), positive (positive for dysplasia) and overt carcinoma; and (4) the positive category is divided into two groups; high-grade dysplasia and low-grade dysplasia[40]. Another important criterion for the differential diagnosis of low and high-grade dysplasia is the extent of nuclear stratification; nuclear stratification below half of the cytoplasm is characteristic of low-grade dysplasia. If nuclear stratification above half of the cytoplasm is present at more than three contiguous glands, it is considered high-grade dysplasia[39]. This criterion is based on the definition of high-grade dysplasia associated with inflammatory bowel disease.
In Korea, most pathologists use the term “tubular/villous/villotubular adenoma with low/high grade dysplasia” to describe intraepithelial precancerous disease. “Dysplasia” is used to describe atypia due to neoplastic etiology, excluding regenerative changes. This concept of dysplasia unassociated with adenoma (foveolar type dysplasia) is not well established and needs further study. The term “carcinoma in situ” is used by some pathologists to describe a highly anaplastic lesion without lamina propria invasion, but this is not widely accepted.
At the 8th Japan-Korea Pathologist Symposium in 2008 in Yokohama, Japan, there was a consensus conference to discuss diagnostic differences in gastrointestinal neoplasia, and Japanese and Korean pathologists confirmed their different viewpoints.
In 2009, GIPS-KSP began to establish new Korean diagnostic criteria for gastric epithelial proliferative disease, and the effort is ongoing. We gathered 117 cases of gastric biopsy specimens and ESD specimens from 14 institutes. Six pathologists screened the slides and selected 42 cases, which showed the difficulty of diagnosis. The selected cases were circulated and answers were gathered from 45 pathologists. The answer sheet was composed of five categories of diagnosis; regenerative atypia, low grade dysplasia, high grade dysplasia, carcinoma in situ, and carcinoma. In most cases, there was a wide range of interobserver discrepancy. We tried to simplify the diagnostic criteria to enhance diagnostic consistency but realized that it was impossible because determining low-grade dysplasia, high-grade dysplasia, and carcinoma was a complex process based on many kinds of diagnostic criteria. A consensus conference was held eight times and the pathological findings of each case were discussed and voted on anonymously. After the consensus conferences, the agreement rate increased (Table 3). Before the consensus conferences, only 10 cases among 42 showed a high agreement rate (more than 70%). After the conferences, the cases showing high agreement rate increased to 25 cases (Table 3). Although these data were not enough for a conclusion, it was suggested that there could be agreement for a pathologic diagnosis among Korean pathologists.
| Agreement rate (%) | Before (%) | After (%) |
| 0-50 | 16 (38.1) | 1 (2.4) |
| 51-60 | 13 (31.0) | 4 (9.5) |
| 61-70 | 3 (7.1) | 8 (19.0) |
| 71-80 | 5 (11.9) | 6 (14.3) |
| 81-90 | 3 (7.1) | 6 (14.3) |
| 91-100 | 2 (4.8) | 17 (40.5) |
| Total | 42 (100) | 42 (100) |
Many histological factors are helpful for the differential diagnosis of low and high-grade dysplasia, but these factors sometimes conflicted with each other. We attempted to identify a more simple and reproducible way to determine the dysplasia grade. We propose guidelines for differential diagnosis: (1) a diagnosis of carcinoma is based on invasion; (2) the most important characteristic of low-grade dysplasia is a regular distribution of crypts without severe branching, budding, or marked glandular crowding; (3) if nuclear pseudostratification occupies more than the basal half of the cryptal cells in three or more adjacent crypts, the lesion is considered high-grade dysplasia (this rule was based according to the previously mentioned Korean Standard of Pathology Report of Gastric Cancer[39]); (4) if severe cytologic atypia is present, careful inspection for invasive foci is necessary, because the risk of invasion is very high; and (5) other structural or nuclear atypia should be evaluated to make a final decision such as cribriform pattern, papillae, ridges, vesicular nuclei, high nuclear/cytoplasmic ratio, loss of nuclear polarity, thick and irregular nuclear membrane, and nucleoli. Based on these principles, the consensus rate was markedly increased, although not in every case.
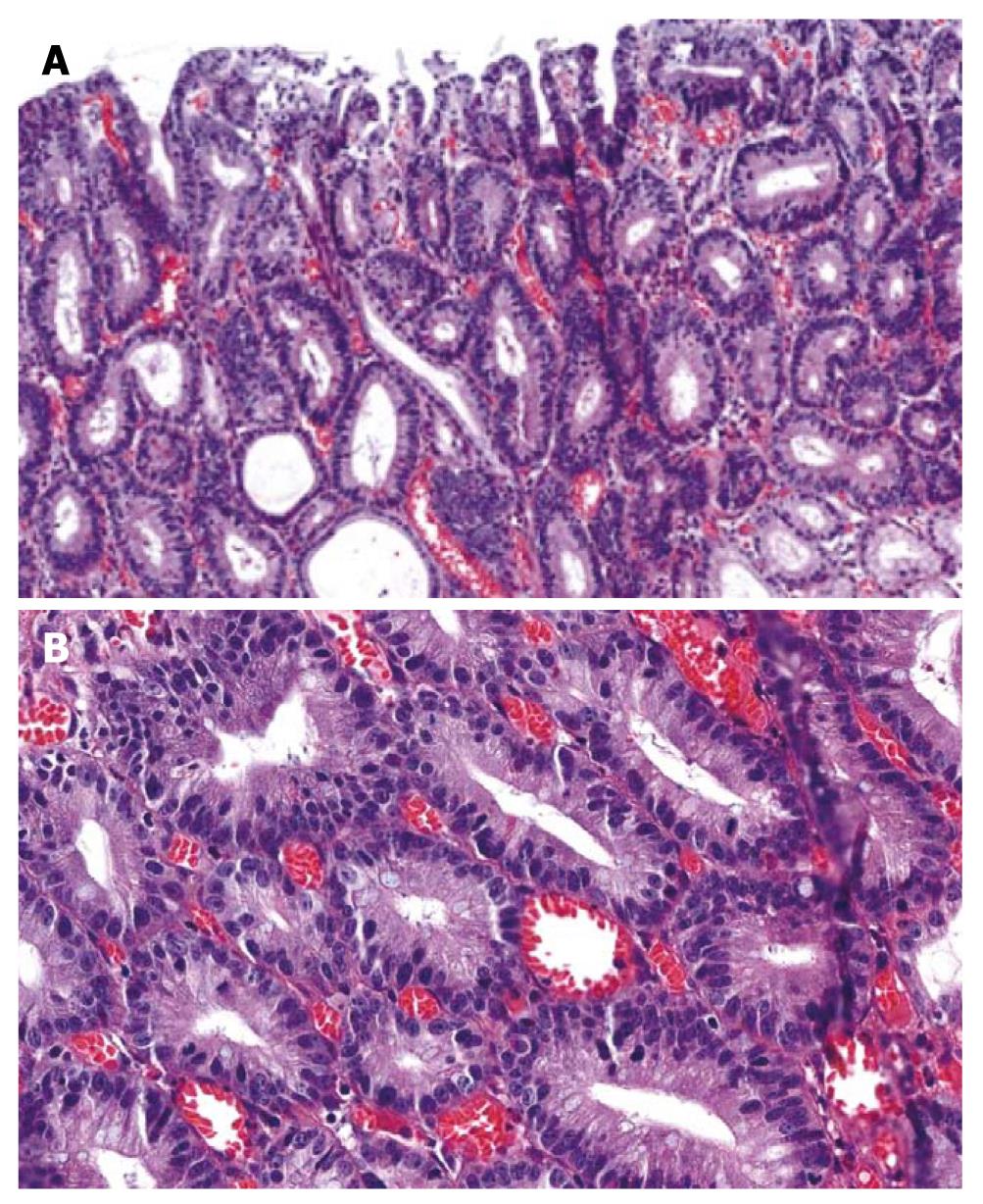
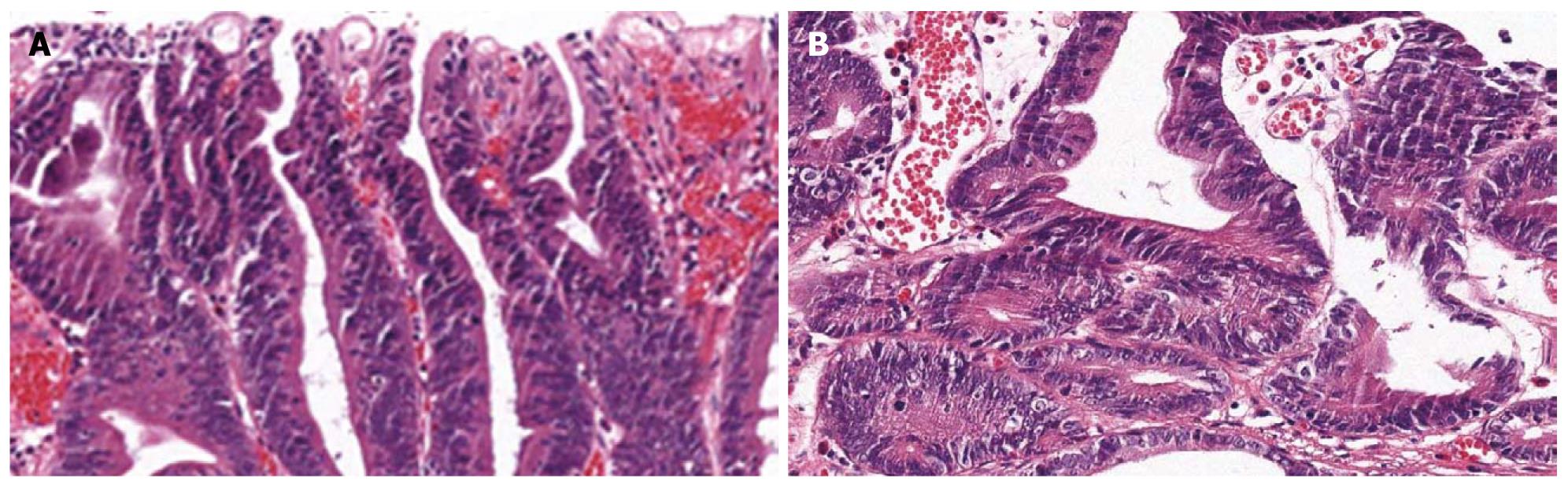
An ESD specimen revealed a regular distribution of small proliferative glands without budding or branching (Figure 1A). The nuclei were elongated and stratified below half of the cytoplasm (Figure 1B). Hyperchromasia and mitoses were present; 57.8% and 100% of the pathologists agreed with a diagnosis of tubular adenoma with low grade dysplasia before and after the consensus conference, respectively.
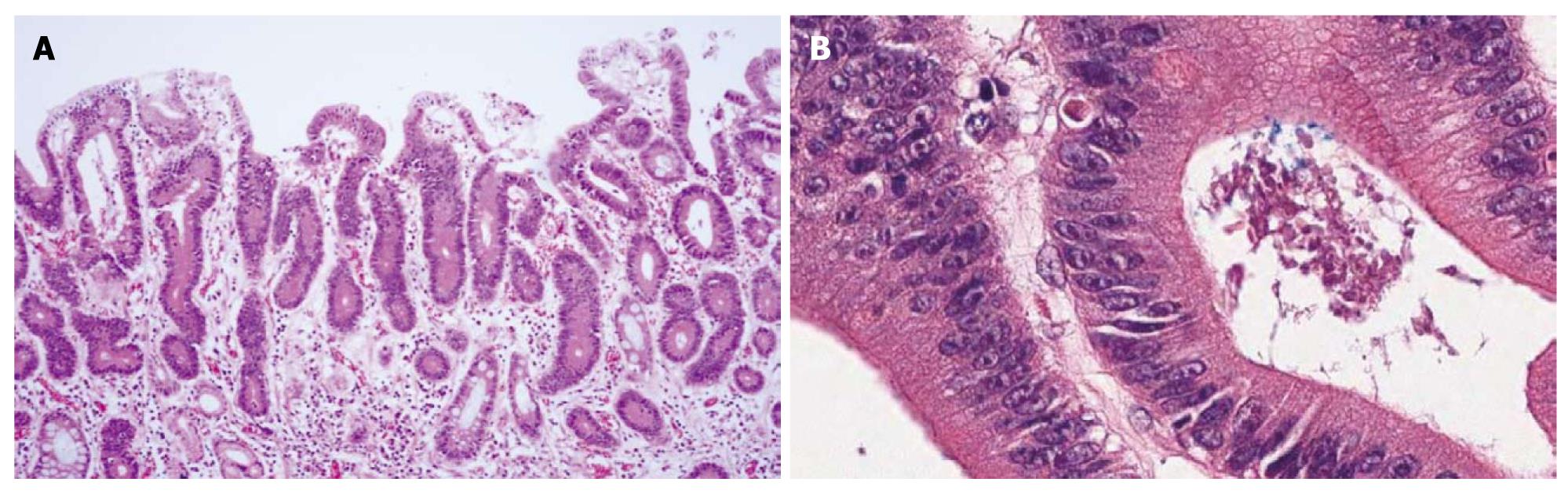
An ESD specimen revealed regular distribution of small proliferative glands without budding or branching (Figure 2A). Glandular crowding was mild. The nuclei were ovoid and vesicular with conspicuous nucleoli but nuclear stratification did not exceed the basal half of the cell (Figure 2B); 73.3% and 60.9% of the pathologists agreed with a diagnosis of tubular adenoma with low-grade dysplasia before and after the consensus conference, respectively.
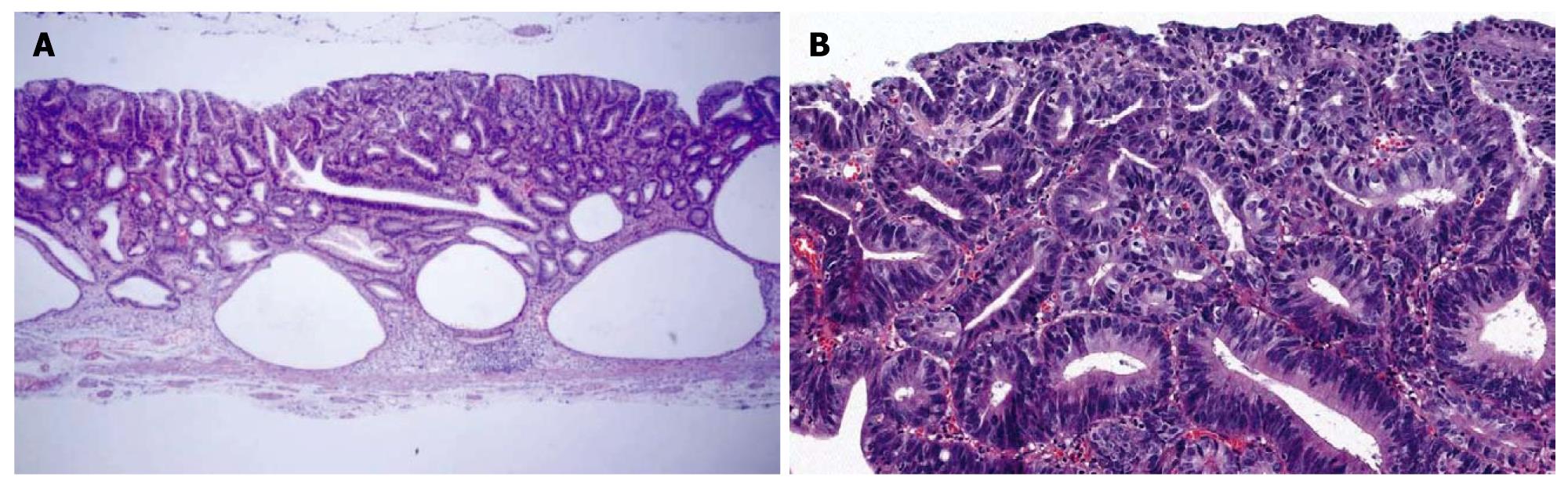
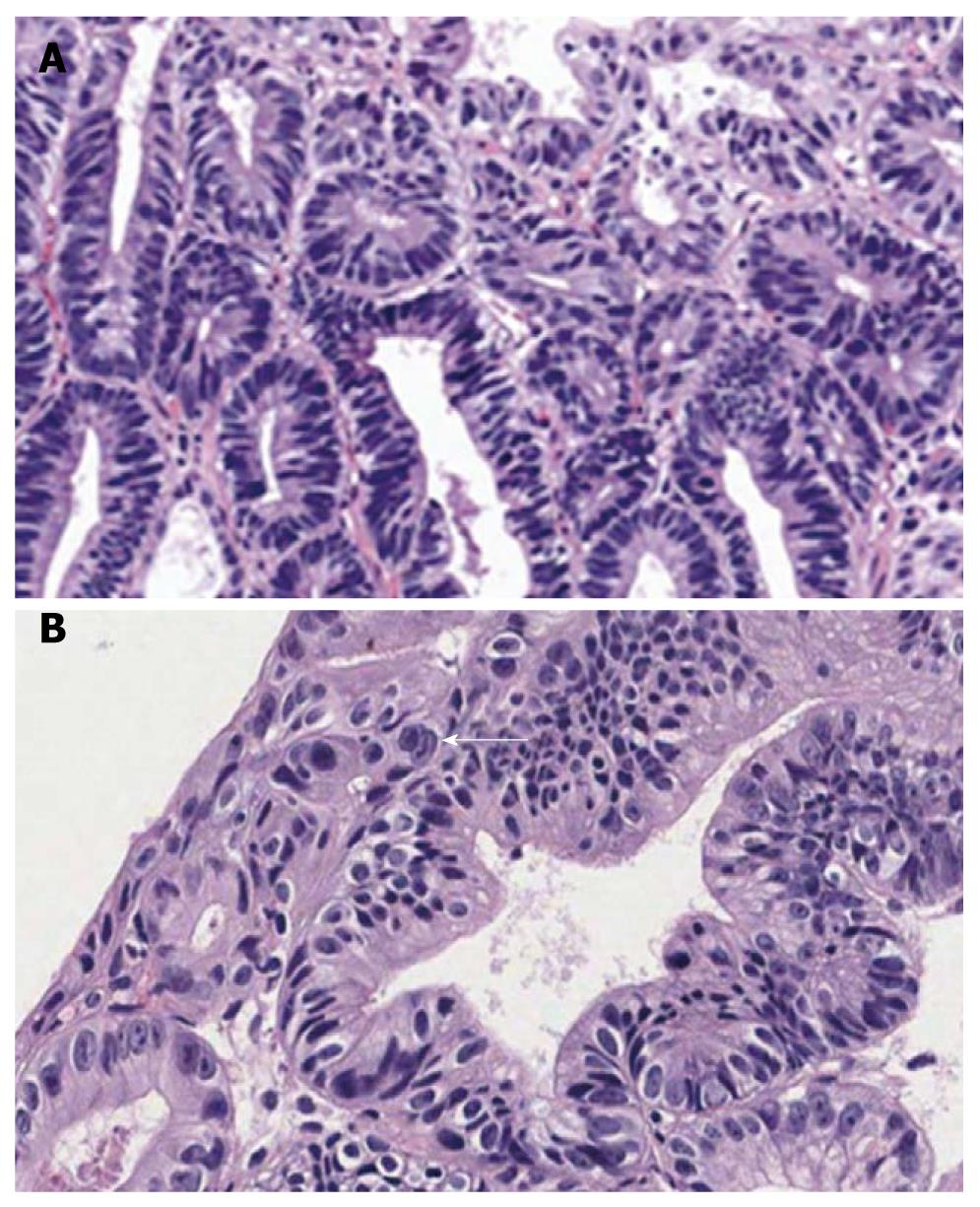
An ESD specimen revealed compact small glandular proliferation with some variation in gland size (Figure 3A). Budding or branching was present. The nuclei were elongated and stratified with some ovoid nuclei. More than three contiguous glands showed nuclear stratification above half of the cytoplasm (Figure 3B). Hyperchromasia and mitoses were present; 44.4% and 100% of the pathologists agreed with a diagnosis of tubular adenoma with high-grade dysplasia before and after the consensus conference, respectively.
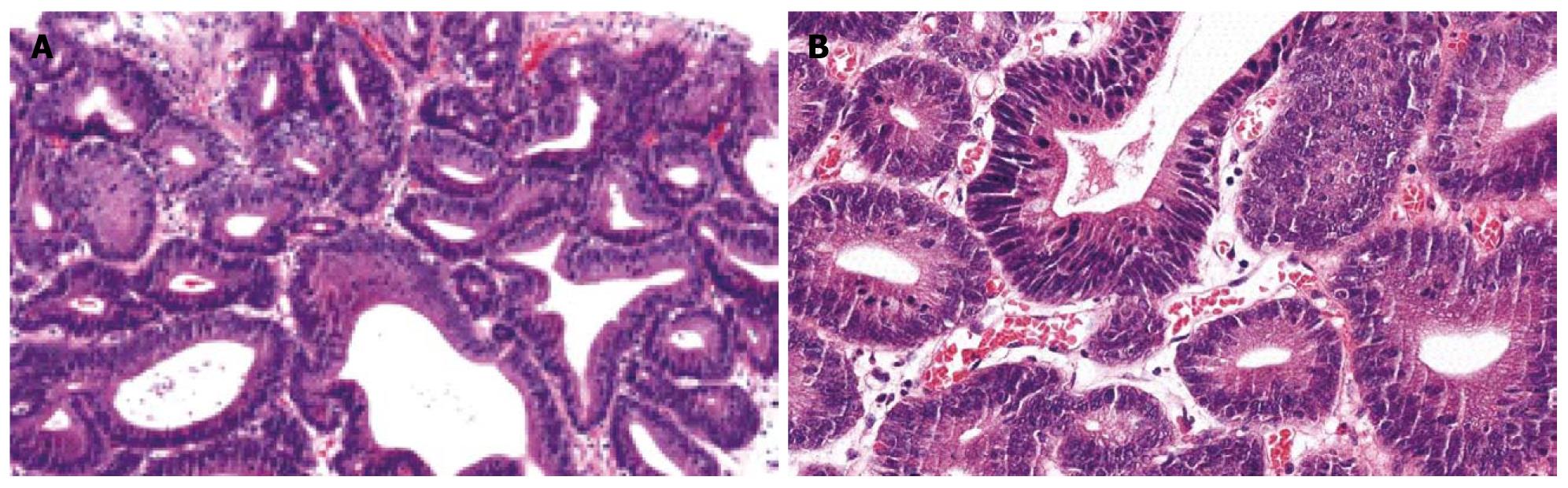
An ESD specimen revealed glandular crowding with some variation in gland size and budding (Figure 4A). The nuclei were elongated and stratified with some ovoid nuclei. Nuclear stratification above the basal half of the cytoplasm was present (Figure 4B). Hyperchromasia and mitoses were noted. Before the consensus conference, 44.4% of pathologists agreed with a diagnosis of tubular adenoma with high-grade dysplasia, which increased to 75% after the conference.
An ESD specimen revealed compact small glandular proliferation with variation in gland size, budding and branching (Figure 5A). The nuclei were elongated and stratified with some ovoid nuclei (Figure 5B). More than three contiguous glands showed nuclear stratification above the basal half of the cytoplasm. Hyperchromasia and mitoses were present. Glandular complexity was present but definite invasion was not identified; 42.5% and 62.5% of the pathologists agreed with a diagnosis of tubular adenoma with high-grade dysplasia before and after the consensus conference, respectively.
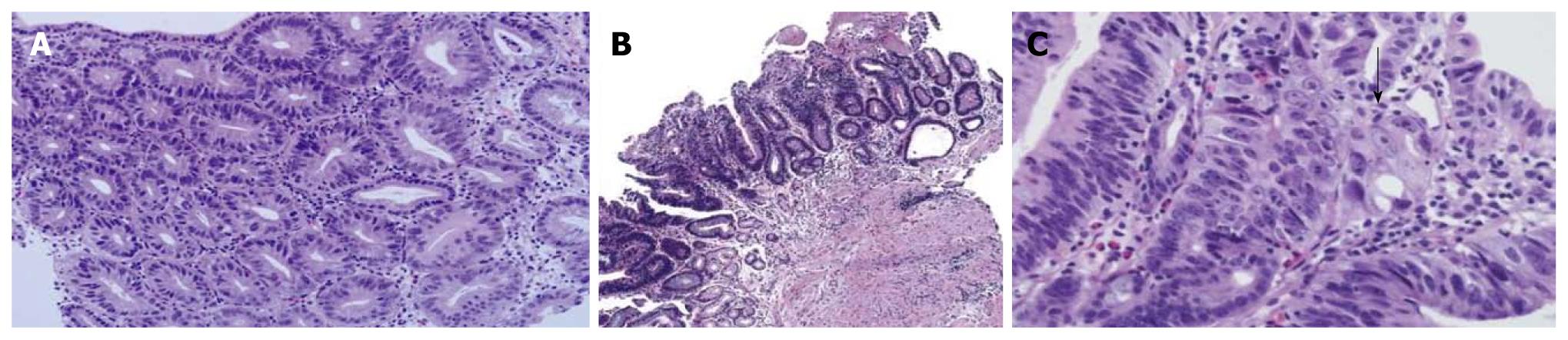
A mucosal biopsy specimen revealed compact small glandular proliferation without budding or branching (Figure 6A). Another section showed a villous configuration (Figure 6B). The glandular distribution was relatively regular but gland size was mildly variable. The nuclei were enlarged, oval to round, and pleomorphic. Nuclear stratification was not severe, but enlarged nuclei occupied more than the basal half of the cytoplasm. Hyperchromasia and mitoses were present. Invasion into the lamina propria was present (Figure 6C, arrow); 22.2% and 100% of the pathologists agreed with a diagnosis of adenocarcinoma before and after the consensus conference, respectively.
A mucosal biopsy specimen revealed compact small glandular proliferation with budding or branching (Figure 7A). Glandular size and distribution were irregular. The nuclei were enlarged, oval to round, with vesicular chromatin. Severe nuclear stratification approaching the top of the cytoplasm in more than three contiguous glands was present. Marked hyperchromasia and mitoses were noted with invasion into the lamina propria (Figure 7B, arrow); 26.7% and 56.3% of the pathologists agreed with a diagnosis of adenocarcinoma before and after the consensus conference, respectively.
The rate of agreement markedly increased after many consensus conferences (Table 3). However, this guideline has some limitations; (1) focal invasion into the lamina propria may not be detected on a biopsy specimen, which causes diagnostic discrepancy between a biopsy and resection specimen; and (2) a gray zone due to overlapping or mismatching of diagnostic criteria lowers the agreement rate. We must conduct a further study to verify the hypothesis in expanded cases and to determine that the guideline lowers inter and intraobserver discrepancies and correlates with clinical outcome. If more reliable pathological findings suggesting possible invasion into an adjacent area could be found, it would be very useful for small biopsy specimens. We will attempt to define the pathological criteria in a more simple and subjective way, and we would like to develop a diagnostic algorithm. Education is also important. Symposiums, workshops, and publishing of articles will be helpful.
We have additional problems to be solved, such as how to measure the invasion depth if submucosal invasion is present, the diagnostic policy for differentiation, judgment on lymphovascular invasion, and a fixation method for ESD specimens, which are all important decisions to develop a further treatment plan after EMR/ESD.
Eastern and Western pathologists have different terminology and diagnostic criteria for gastric intraepithelial neoplasia. In Korea, pathologists experience much difficulty when making a diagnosis, and have made efforts to increase the interobserver agreement rate. As a result, we have achieved improved diagnostic consensus, although it is not yet perfect. We tentatively suggest the guidelines for differential diagnosis: (1) a diagnosis of carcinoma is based on invasion; (2) the most important characteristic of low grade dysplasia is the architectural pattern such as regular distribution of crypts without severe branching, budding, or marked glandular crowding; (3) if nuclear pseudostratification occupies more than the basal half of the cryptal cells in three or more adjacent crypts, the lesion is considered high grade dysplasia; (4) if severe cytologic atypia is present, careful inspection for invasive foci is necessary, because the risk for invasion is very high; and (5) other structural or nuclear atypia should be evaluated to make a final decision such as cribriform pattern, papillae, ridges, vesicular nuclei, high nuclear/cytoplasmic ratio, loss of nuclear polarity, thick and irregular nuclear membrane, and nucleoli. Further study on the pathological findings and clinicopathological correlations as well as a follow-up study are necessary to increase diagnostic accuracy.
Peer reviewers: Fabio Grizzi, PhD, Laboratories of Quantitative Medicine, Istituto Clinico Humanitas IRCCS, Via Manzoni 56, 20089 Rozzano, Milan, Italy; Vittorio Ricci, MD, PhD, Department of Physiology, Human Physiology Section, University of Pavia Medical School, Via Forlanini 6, Pavia, 27100, Italy
S- Editor Shi ZF L- Editor O’Neill M E- Editor Zheng XM
| 1. | Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931-968. |
| 2. | Grundmann E. Histologic types and possible initial stages in early gastric carcinoma. Beitr Pathol. 1975;154:256-280. |
| 3. | Serck-Hanssen A. Precancerous lesions of the stomach. Scand J Gastroenterol Suppl. 1979;54:104-105. |
| 4. | Morson BC, Sobin LH, Grundmann E, Johansen A, Nagayo T, Serck-Hanssen A. Precancerous conditions and epithelial dysplasia in the stomach. J Clin Pathol. 1980;33:711-721. |
| 5. | Oehlert W, Keller P, Henke M, Strauch M. [Gastric mucosal dysplasias: what is their clinical significance (author’s transl)]. Dtsch Med Wochenschr. 1975;100:1950-1956. |
| 6. | Jass JR. A classification of gastric dysplasia. Histopathology. 1983;7:181-193. |
| 7. | Ming SC, Bajtai A, Correa P, Elster K, Jarvi OH, Munoz N, Nagayo T, Stemmerman GN. Gastric dysplasia. Significance and pathologic criteria. Cancer. 1984;54:1794-1801. |
| 8. | Riddell RH. Premalignant and early malignant lesions in the gastrointestinal tract: definitions, terminology, and problems. Am J Gastroenterol. 1996;91:864-872. |
| 9. | Goldstein NS, Lewin KJ. Gastric epithelial dysplasia and adenoma: historical review and histological criteria for grading. Hum Pathol. 1997;28:127-133. |
| 10. | Lewin KJ. Nomenclature problems of gastrointestinal epithelial neoplasia. Am J Surg Pathol. 1998;22:1043-1047. |
| 12. | Nakamura K, Sugano H, Takagi K, Fuchigami A. Histopathological study on early carcinoma of the stomach: criteria for diagnosis of atypical epithelium. Gann. 1966;57:613-620. |
| 13. | Sugano H, Nakamura K, Takagi K. An atypical epithelium of the stomach: A clinico-pathological entity. Gann Monogr Cancer Res. 1971;2:257-269. |
| 14. | Nagayo T. Histological diagnosis of biopsied gastric mucosae with special reference to that of borderline lesions. Gann Monogr Cancer Res. 1971;11:245-256. |
| 15. | Takagi K, Kumakura K, Sugano H, Nakamura K. [Polypoid lesions of the stomach--with special reference to atypical epithelial lesions]. Gan No Rinsho. 1967;13:809-817. |
| 16. | Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma. Tokyo: Kanehara & Co., Ltd 1995; . |
| 17. | Schade ROK. The borderline between benign and malignant lesions of the stomach. Early Gastric Cancer. New York: Springer Verlag 1974; 45–53. |
| 18. | Davaris P, Petraki K, Archimandritis A, Haritopoulos N, Papacharalampous N. Mucosal hyperplastic polyps of the stomach. Do they have any potential to malignancy? Pathol Res Pract. 1986;181:385-389. |
| 19. | Ming SC, Goldman H. Gastric polyps; A histogenetic classification and its relation to carcinoma. Cancer. 1965;18:721-726. |
| 20. | Nagayo T. Dysplasia of the gastric mucosa and its relation to the precancerous state. Gann. 1981;72:813-823. |
| 21. | Hattori T. Morphological range of hyperplastic polyps and carcinomas arising in hyperplastic polyps of the stomach. J Clin Pathol. 1985;38:622-630. |
| 22. | Nakamura T, Nakano G. Histopathological classification and malignant change in gastric polyps. J Clin Pathol. 1985;38:754-764. |
| 23. | Usha SD, Shukla HS, Singh RG, Khanna S, Gupta RM. Pre-cancerous lesions of stomach. Indian J Pathol Microbiol. 1989;32:75-80. |
| 24. | Ming SC. Adenocarcinoma and other malignant epithelial tumors of the stomach. Pathology of the Gastrointestinal Tract. Philadelphia, PA: Saunders 1992; 584-617. |
| 25. | Ito H, Yasui W, Yoshida K, Nakayama H, Tahara E. Depressed tubular adenoma of the stomach: pathological and immunohistochemical features. Histopathology. 1990;17:419-426. |
| 26. | Correa P. Clinical implications of recent developments in gastric cancer pathology and epidemiology. Semin Oncol. 1985;12:2-10. |
| 27. | Freeny PC, Vimont TR. Villous tumors of the stomach and small bowel. Arch Surg. 1978;113:255-259. |
| 28. | Xuan ZX, Ambe K, Enjoji M. Depressed adenoma of the stomach, revisited. Histologic, histochemical, and immunohistochemical profiles. Cancer. 1991;67:2382-2389. |
| 30. | Ito H, Yokozaki H, Ito M, Tahara E. Papillary adenoma of the stomach. Pathologic and immunohistochemical study. Arch Pathol Lab Med. 1989;113:1030-1034. |
| 31. | Tsujitani S, Furusawa M, Hayashi I. Morphological factors aid in therapeutic decisions concerning gastric adenomas. Hepatogastroenterology. 1992;39:56-58. |
| 32. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. |
| 33. | Stolte M. Diagnosis of gastric carcinoma: Japanese fairy tales or Western deficiency? Virchows Arch. 1999;434:279-280. |
| 34. | de Dombal FT, Price AB, Thompson H, Williams GT, Morgan AG, Softley A, Clamp SE, Unwin BJ. The British Society of Gastroenterology early gastric cancer/dysplasia survey: an interim report. Gut. 1990;31:115-120. |
| 35. | Lewin KJ, Appleman HD. Carcinoma of the stomach. Tumors of the esophagus and stomach. Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology 1996; 245-321. |
| 36. | Tosi P, Baak JP, Luzi P, Miracco C, Lio R, Barbini P. Morphometric distinction of low- and high-grade dysplasias in gastric biopsies. Hum Pathol. 1989;20:839-844. |
| 37. | Burke AP, Sobin LH, Shekitka KM, Helwig EB. Dysplasia of the stomach and Barrett esophagus: a follow-up study. Mod Pathol. 1991;4:336-341. |
| 38. | Kim H, Jin SY, Jang JJ, Kim WH, Song SY, Kim KR, Yu ES, Shin HS, Kim HK, Sohn JH. Grading system for gastric epithelial proliferative diseases standardized guidelines proposed by Korean Study Group for Pathology of Digestive Diseases. Korean J Pathol. 1997;31:389-400. |
| 39. | Kim WH, Park CK, Kim YB, Kim YW, Kim HG, Bae HI, Song KS, Chang HK, Chang HJ, Chae YS. A standardized pathology report for gastric cancer. Korean J Pathol. 2005;39:106-113. |
| 40. | Rugge M, Nitti D, Farinati F, di Mario F, Genta RM. Non-invasive neoplasia of the stomach. Eur J Gastroenterol Hepatol. 2005;17:1191-1196. |