Published online Jan 14, 2011. doi: 10.3748/wjg.v17.i2.231
Revised: May 24, 2010
Accepted: May 31, 2010
Published online: January 14, 2011
AIM: To investigate the role of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) in the diagnosis of small pancreatic cancer.
METHODS: This study involved 31 patients with proven invasive ductal cancer of the pancreas. The patients were divided into 3 groups according to the maximum diameter of the tumor: TS1 (maximum tumor size ≤ 2.0 cm), TS2 (> 2.0 cm and ≤ 4.0 cm) or TS3-4 (> 4.0 cm). The relationships between the TS and various diagnostic tools, including FDG-PET with dual time point evaluation, were analyzed.
RESULTS: The tumors ranged from 1.3 to 11.0 cm in diameter. Thirty of the 31 patients (97%) had a positive FDG-PET study. There were 5 patients classified as TS1, 15 as TS2 and 11 as TS3-4. The sensitivity of FDG-PET, computed tomography (CT) and magnetic resonance imaging (MRI) were 100%, 40%, 0% in TS1, 93%, 93%, 89% in TS2 and 100%, 100%, 100% in TS3-4. The sensitivity of FDG-PET was significantly higher in comparison to CT and MRI in patients with TS1 (P < 0.032). The mean standardized uptake values (SUVs) did not show a significant difference in relation to the TS (TS1: 5.8 ± 4.5, TS2: 5.7 ± 2.2, TS3-4: 8.2 ± 3.9), respectively. All the TS1 tumors (from 13 to 20 mm) showed higher SUVs in FDG-PET with dual time point evaluation in the delayed phase compared with the early phase, which suggested the lesions were malignant.
CONCLUSION: These results indicate that FDG-PET with dual time point evaluation is a useful modality for the detection of small pancreatic cancers with a diameter of less than 20 mm.
- Citation: Okano K, Kakinoki K, Akamoto S, Hagiike M, Usuki H, Yamamoto Y, Nishiyama Y, Suzuki Y. 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of small pancreatic cancer. World J Gastroenterol 2011; 17(2): 231-235
- URL: https://www.wjgnet.com/1007-9327/full/v17/i2/231.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i2.231
Pancreatic cancer is the 5th leading cause of cancer-related mortality in Japan, with an estimated 20 000 deaths attributable to the disease[1,2]. The annual mortality rate closely approximates the annual incidence, thereby reflecting a generally short survival time associated with pancreatic cancer, which is generally less than 1 year. Cancer of the pancreas has the shortest median survival time out of all cancer types in a stage for stage basis. Early diagnosis is the most important factor for improving the overall survival and quality of life in patients with pancreatic cancer.
Recently, positron emission tomography (PET) has demonstrated superiority to computed tomography (CT), ultrasonography (US), and endoscopic US (EUS) in its sensitivity and specificity in diagnosing pancreatic cancer[3-6]. Furthermore, the metabolic activity of the tumor may be of prognostic significance. We have been reported the efficacy of delayed additional 18F-fluorodeoxyglucose PET (FDG-PET) imaging in the differential diagnosis of malignant from benign lesions in patients who are suspected of having pancreatic cancer[7]. Furthermore, the detection rate of liver metastases smaller than 1 cm in diameter from pancreatic cancer was only 33% on early image and 58% on delayed image[7]. However, the role of dual time point FDG-PET in the diagnosis of small pancreatic cancers has yet to be established.
Therefore, the present study investigated whether small cancers of the pancreas could be accurately diagnosed by FDG-PET with dual time point evaluation.
Thirty-one patients with pancreatic carcinoma suspected on the basis of conventional radiological studies (22 males and 9 females; mean age, 65 years; age range, 44-82 years) and who underwent FDG-PET between 2003 and 2007 were retrospectively selected. Patients were excluded from this study if they had poorly controlled diabetes mellitus (presenting with blood glucose level > 200 mg/dL prior to PET imaging). Conventional radiological staging was performed by means of CT or magnetic resonance imaging (MRI). The location of the cancer was in the head of the pancreas in 17 patients and in the body and tail in 14 patients. Twelve of the 31 cancers were diagnosed to be unresectable, and 19 patients eventually underwent surgery with a curative intention, although the cancer turned out to be unresectable in 7 because of intraoperative findings.
The patients were divided into 3 groups according to the maximum diameter of the tumor: TS1 (maximum size ≤ 2.0 cm), TS2 (> 2.0 cm and ≤ 4.0 cm) or TS3-4 (> 4.0 cm) as indicated by the classification system of the Japan Pancreas Society. FDG-PET was analyzed semi-quantitatively using the standardized uptake values (SUVs). The sensitivity of diagnosing pancreatic cancer was examined for FDG-PET, CT, MRI and the serum levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) with regard to the size of the tumor. The details of SUVs, the histological findings and correlation of CT findings were evaluated in patients with TS1 pancreatic cancer. This study was performed retrospectively by collecting and analyzing data from the patient records.
The FDG-PET images were acquired with a PET machine (Siemens EXACT HR+, CTI, Knoxville, TN, USA). The patients were required to fast for at least 4 h before PET imaging. The emission images were acquired (early image) 1 h after the intravenous administration of 5 mCi of FDG. Delayed PET emission images of the upper abdomen were acquired at 2 h after administration of 18F-FDG, using 2 or 3 bed positions with a 3-min acquisition at each[7]. This acquisition was immediately followed by a transmission scan of the same transverse planes, using a 2-min acquisition at each bed position. The early and delayed PET images were reviewed independently and consecutively by 2 radiologists with extensive experience in FDG-PET imaging. PET images were compared with the corresponding CT and/or MRI images for accurate anatomical identification of the tumor. The findings were considered to be positive when both radiologists strongly suspected malignant disease. In addition, the images were analyzed semi-quantitatively using the SUV, as reported elsewhere. Briefly, for semi-quantitative analysis, a region of interest was placed over the entire FDG-avid lesion including the largest amount of radioactivity using the transverse PET image. The SUV was calculated as: SUV = (activity in region of interest in mCi)/(injected dose in mCi/weight in kg).
CT studies were performed with a multidetector row CT scanner (Aquilion, Toshiba, Tokyo, Japan). Helical images of the abdomen were routinely obtained and reconstructed with 5 mm thickness. After pre-contrast CT scans, arterial dominant phase images of dynamic CT were obtained starting 40 s after the beginning of the intravenous bolus injection (3 mL/s) of 100 mL of iodized contrast medium at 350 mg/mL. The pancreatic phase and the late phase (near equilibrium phase) were also obtained, starting at 60 and 180 s after injection, respectively. The CT images were interpreted independently and consecutively by 2 radiologists with extensive experience of more than 10 years in CT scanning. The findings of the CT scans were considered positive when both radiologists strongly suspected malignant disease due to a discrete low-attenuation mass within the pancreas.
Two 1.5 T superconducting units, Signa Advantage (General Electric, Milwaukee, WI, USA, USA) and Visart (Toshiba, Tokyo, Japan), were used for MRI. T1-weighted gradient-echo imaging; FS-T2-weighted turbo SE imaging and heavily T2-weighted turbo SE images were acquired in the order of scan after initial T1-weighted localizing images were obtained in the coronal and trans-axial directions.
The χ2 test was employed for a statistical comparison of the sensitivity of FDG-PET, CT, MRI, CEA and CA19-9. The Student t test was used to compare the values of the SUV between the groups. All statistical analyses were performed using SPSS software (SPSS, Chicago, USA). A P value < 0.05 was considered to be statistically significant.
Table 1 shows the clinicopathological profiles of the 31 patients. The sensitivity of FDG-PET, CT, MRI, the serum levels of CEA and CA19-9 were 100%, 40%, 0%, 0%, 40% in TS1, 93%, 93%, 89%, 20%, 73% in TS2 and 100%, 100%, 100%, 73%, 91% in TS3-4 (Table 2). The sensitivity of PET for detecting TS1, TS2, and TS3 tumors was 100%, 93%, and 100%, respectively. The sensitivity of FDG-PET was significantly higher in comparison to CT, MRI and the serum levels of CEA and CA19-9 in the patients with TS1 (P = 0.002 vs MRI or CEA, P = 0.038 vs CT or CA19-9).
| mean ± SD (range) or n (%) | |
| Age (yr) | 65 ± 9 (44-82) |
| Gender (M:F) | 22:9 |
| Tumor location | |
| Head | 17 (55) |
| Body | 11 (35) |
| Tail | 3 (10) |
| Maximum tumor diameter (cm) | 3.8 ± 2.0 (1.3-11.0) |
| SUV | 6.5 ± 3.3 (2.5-15.8) |
| TS (cm) | n | PET (%) | CT (%) | MRI (%) | CEA (%) | CA19-9 (%) |
| TS1 ( ≤ 2) | 5 | 100a | 40 | 0 | 0 | 40 |
| TS2 (> 2, ≤ 4) | 15 | 93 | 93 | 89 | 20 | 73 |
| TS3-4 (> 4) | 11 | 100 | 100 | 100 | 73 | 91 |
Although the sensitivity was higher for larger tumors, the SUV was not significantly associated with the TS factor. The mean SUV did not show a significant difference in relation to the TS (TS1: 5.8 ± 4.5, TS2: 5.7 ± 2.2, TS3-4: 8.2 ± 3.9), respectively. The diagnosis of pancreatic adenocarcinoma was histologically confirmed in all patients with TS1 cancer (Table 3). The tumor was well differentiated in 4 patients and poorly differentiated in one patient. The tumor diameter ranged from 13 to 20 mm. All the TS1 tumors showed higher SUVs in the delayed phase compared with that in the early phase. The SUV pattern suggested the small lesions were malignant tumors.
| Age (yr) | Gender | Size (mm) | Tumor differentiation | SUV early | SUV delayed | |
| 1 | 77 | F | 13 | Poor | 3.59 | 4.16 |
| 2 | 77 | M | 20 | Well | 5.53 | 7.10 |
| 3 | 82 | F | 20 | Well | 2.74 | 3.14 |
| 4 | 68 | M | 18 | Well | 2.87 | 3.06 |
| 5 | 81 | M | 20 | Well | 12.79 | 13.78 |
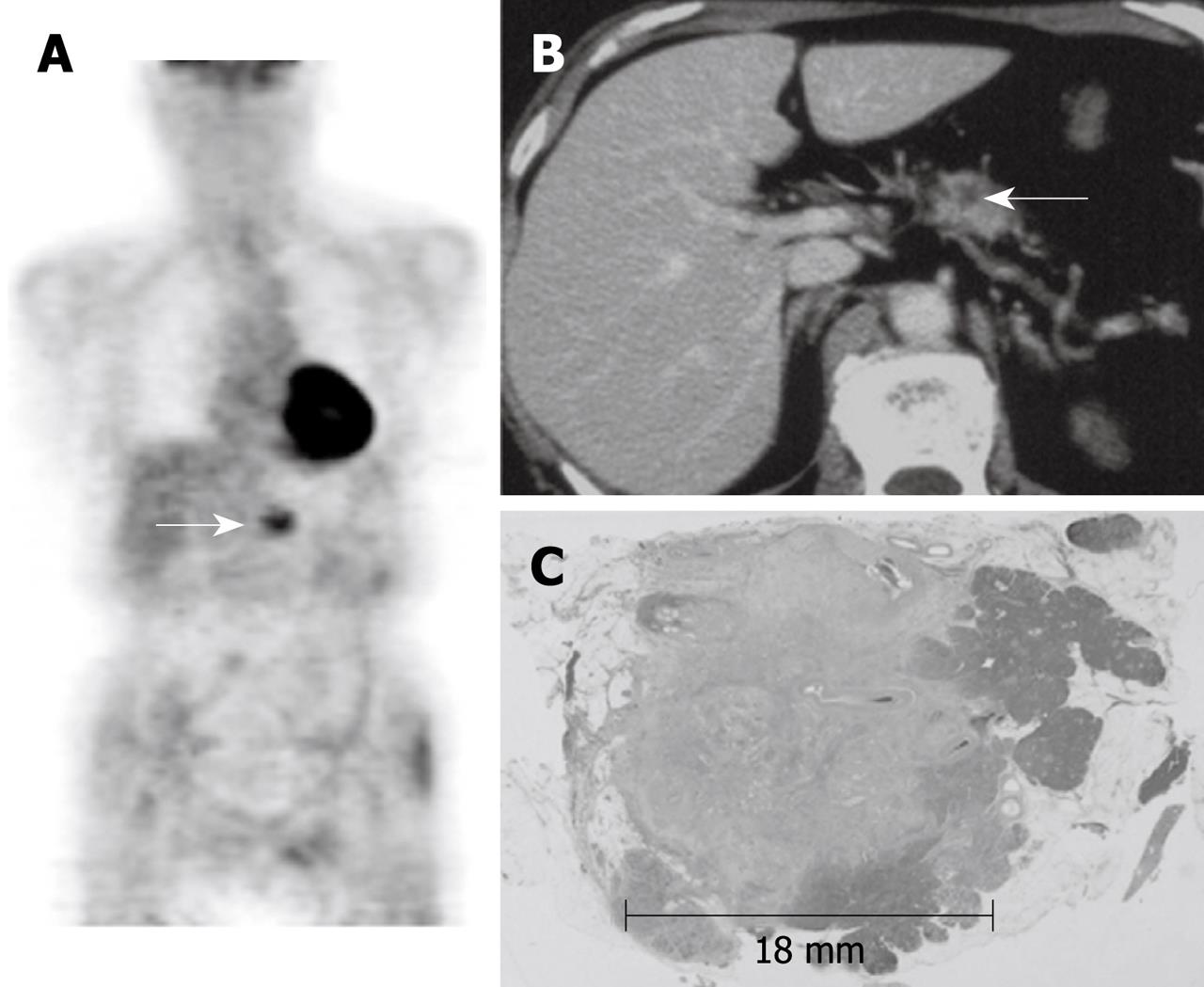
Representative images of one patient (case 4 in Table 3) with TS1 pancreas cancer are shown in Figure 1. A 68-year-old male was transferred to our hospital for evaluation and further management of diabetes mellitus. A whole body FDG-PET image shows apparent increased uptake in the tumor (delayed point SUV, 3.06) (Figure 1A). An axial CT image with contrast enhancement shows a small low-density mass in the pancreas body (Figure 1B). The histological findings (HE staining) of the pancreas revealed invasive ductal cancer in the body of pancreas with a diameter of 18 mm (Figure 1C).
The usefulness of FDG-PET in diagnosing distant disease from advanced pancreatic cancer has been previously reported, although the poor spatial resolution of FDG-PET is known to limit the local staging of pancreatic cancer[3]. CT is better suited to demonstrate the relationship of the tumor, adjacent organs, and vascular structure in advanced pancreatic cancer, but it is relatively insensitive for detecting pancreatic cancers < 2 cm in size[8-11]. Although the sensitivity of contrast-enhanced helical CT in the detection of pancreatic carcinoma is reported to vary from 76% to 92%, the sensitivity declines to 58% to 67% for tumors smaller than 2 cm[8-10,12]. The sensitivity of EUS or MRI has been reported to be the same or slightly better in comparison to that of CT[13,14].
Patients with small pancreatic carcinoma have no typical symptoms, which make it very difficult to detect. In contrast to the inherent limitations of this anatomic imaging modality, functional imaging using FDG PET appears to represent a significant advance in the detection of small pancreatic cancers < 2 cm in size. Seo et al[15] reported the effectiveness of FDG-PET for the detection of small pancreatic cancers with a sensitivity of 81% for tumors smaller than 2 cm. Although there have been a few reports indicating the value of FDG-PET in the diagnosis of small pancreatic cancer, the efficacy of dual phase FDG-PET in small pancreatic cancer has not been fully evaluated.
Dual time point FDG-PET is a more reliable method than single time point FDG-PET for differentiating pancreatic cancer from a mass identified to be chronic pancreatitis. In addition, delayed PET imaging is also helpful for identifying more lesions in patients with pancreatic cancer[7]. Dual time point evaluation is routinely performed in our institution for patients with pancreatic cancer. There were 5 tumors smaller than 2 cm in the current series, and the sensitivity of FDG-PET for the detection of these tumors was 100%, although there was no tumor smaller than 1 cm. A dual time point evaluation may help to increase the sensitivity in the diagnosis of small pancreatic cancer.
The increased uptake of FDG due to the enhanced glucose metabolism of cancer cells is a sensitive marker of tumor viability or biological behavior. The SUV is an independent prognostic factor in various malignant tumors. Sperti et al[16] demonstrated that a high SUV (> 4.0) was associated with shorter survival. Maemura et al[17] reported that pancreatic tumors with distant metastases showed significantly higher SUV levels than tumors without metastases. The present study showed the SUVs of pancreatic cancer did not differ significantly in relation to tumor size. The results indicate that FDG-PET may, therefore be useful even in patients with small pancreatic cancers that can not be visualized by either CT or other modalities. The present study did not provide data on the specificity because there were no benign lesions. In our previous study[7], the specificity of FDG-PET for detection of pancreatic cancer was 65%. Benign lesions such as chronic pancreatitis and autoimmune-related pancreatitis can also accumulate FDG and result in false-positive interpretations of PET studies. Further studies including benign lesions are required to clarify the diagnostic accuracy of FDG-PET.
The routine use of PET is not believed to be cost-effective and thus has not been accepted as a standard screening examination for small pancreatic cancer. Although the etiology of pancreatic cancer has not yet been completely elucidated, several factors are thought to be associated with cancer[18-21]. Smoking is a consistently identified environmental risk factor which doubles the risk of pancreatic cancer[19,20]. Dietary factors, such as high energy intake, cholesterol, and high meat consumption are known to increase the risk. Long-standing diabetes, chronic pancreatitis and certain hereditary conditions can affect the risk of developing pancreatic cancer. FDG-PET screening is therefore recommended if the patients are elderly and have been identified to be at risk for pancreatic cancer. FDG-PET screening for the detection of pancreatic cancers should therefore be considered for patients with chronic pancreatitis, because such patients are 16 times more likely to develop pancreatic cancer than healthy controls. Dual time point FDG-PET is a reliable method for differentiating pancreatic cancer from a mass identified to be chronic pancreatitis[22]. However, there is a limitation in our study. This study was performed by a PET scanner. The coregistration of CT and PET images or integrated PET/CT devices may help to improve some diagnostic problems. Further evolution of PET scanner technology, including the PET/CT hybrid scanner, should provide superior diagnostic performance.
These results indicate that FDG-PET is a useful modality for the detection of small pancreatic cancers with a diameter of less than 20 mm. However, this study was limitated due to the small population of patients. As a result, further prospective studies with PET/CT involving a larger population of patients should therefore be conducted to substantiate the results of this study.
Early diagnosis is the most important factor for improving the overall survival and quality of life in patients with pancreatic cancer. Positron emission tomography (PET) has demonstrated superiority to computed tomography (CT), ultrasonography (US), and endoscopic US (EUS) in its sensitivity and specificity in diagnosing pancreas cancer.
Delayed additional 18F-fluorodeoxyglucose PET (FDG-PET) imaging is a useful method in differential diagnosis of malignant from benign lesions. However, the role of dual time point FDG-PET in the diagnosis of small pancreatic cancers has yet to be established.
The usefulness of FDG-PET in diagnosing distant disease from advanced pancreatic cancer has previously been reported, although the poor spatial resolution of FDG-PET is known to limit the local staging of pancreatic cancer. This is the first study to describe the usefulness of dual time point FDG-PET in detection of small pancreatic cancers with a diameter of less than 20 mm.
The ability to diagnose the early stage of pancreas cancer can be improved by using the dual time point FDG-PET in combination with CT, US and EUS. Early diagnosis is the most important factor for improving the overall survival and quality of life in patients with pancreatic cancer.
Dual time point FDG-PET: FDG, a glucose analog, is taken up by high-glucose-using cells such as brain, kidney, and cancer cells, where phosphorylation prevents the glucose from being released intact. FDG-PET can be used for diagnosis, staging, and monitoring treatment of cancers. PET scans detect the areas with increased glucose uptake. The standardized uptake value of FDG is measured from two sequential time points.
This article is a retrospective analysis concerning a diagnostic value of PET for small pancreatic cancer. It is well-written but there are several issues to be resolved.
Peer reviewer: Itaru Endo, Professor, Gastroenterological Surgery, Yokohama City University, Graduate School of Medicine, Yokohama 236-0004, Japan
S- Editor Wang YR L- Editor Cant MR E- Editor Lin YP
| 1. | Matsuno S, Egawa S, Arai K. Trends in treatment for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2001;8:544-548. |
| 2. | Yamamoto M, Ohashi O, Saitoh Y. Japan Pancreatic Cancer Registry: current status. Pancreas. 1998;16:238-242. |
| 3. | Wakabayashi H, Nishiyama Y, Otani T, Sano T, Yachida S, Okano K, Izuishi K, Suzuki Y. Role of 18F-fluorodeoxyglucose positron emission tomography imaging in surgery for pancreatic cancer. World J Gastroenterol. 2008;14:64-69. |
| 4. | Pakzad F, Groves AM, Ell PJ. The role of positron emission tomography in the management of pancreatic cancer. Semin Nucl Med. 2006;36:248-256. |
| 5. | Hanbidge AE. Cancer of the pancreas: the best image for early detection--CT, MRI, PET or US? Can J Gastroenterol. 2002;16:101-105. |
| 6. | Sato M, Okumura T, Kaito K, Kiyoshima M, Asato Y, Uchiumi K, Iijima H, Hashimoto I, Kaburagi T, Amemiya R. Usefulness of FDG-PET/CT in the detection of pancreatic metastases from lung cancer. Ann Nucl Med. 2009;23:49-57. |
| 7. | Nishiyama Y, Yamamoto Y, Monden T, Sasakawa Y, Tsutsui K, Wakabayashi H, Ohkawa M. Evaluation of delayed additional FDG PET imaging in patients with pancreatic tumour. Nucl Med Commun. 2005;26:895-901. |
| 8. | Ichikawa T, Haradome H, Hachiya J, Nitatori T, Ohtomo K, Kinoshita T, Araki T. Pancreatic ductal adenocarcinoma: preoperative assessment with helical CT versus dynamic MR imaging. Radiology. 1997;202:655-662. |
| 9. | Sheridan MB, Ward J, Guthrie JA, Spencer JA, Craven CM, Wilson D, Guillou PJ, Robinson PJ. Dynamic contrast-enhanced MR imaging and dual-phase helical CT in the preoperative assessment of suspected pancreatic cancer: a comparative study with receiver operating characteristic analysis. AJR Am J Roentgenol. 1999;173:583-590. |
| 10. | Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, Coste J, Louvel A, Roseau G, Couturier D. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315-1322. |
| 11. | Tabuchi T, Itoh K, Ohshio G, Kojima N, Maetani Y, Shibata T, Konishi J. Tumor staging of pancreatic adenocarcinoma using early- and late-phase helical CT. AJR Am J Roentgenol. 1999;173:375-380. |
| 12. | Kaneko K, Honda H, Hayashi T, Fukuya T, Ro T, Irie H, Masuda K. Helical CT evaluation of arterial invasion in pancreatic tumors: comparison with angiography. Abdom Imaging. 1997;22:204-207. |
| 13. | DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D, Kopecky K. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753-763. |
| 14. | Erturk SM, Ichikawa T, Motosugi U, Sou H, Araki T. Diffusion-weighted MR imaging in the evaluation of pancreatic exocrine function before and after secretin stimulation. Am J Gastroenterol. 2006;101:133-136. |
| 15. | Seo S, Doi R, Machimoto T, Kami K, Masui T, Hatano E, Ogawa K, Higashi T, Uemoto S. Contribution of 18F-fluorodeoxyglucose positron emission tomography to the diagnosis of early pancreatic carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:634-639. |
| 16. | Sperti C, Pasquali C, Chierichetti F, Ferronato A, Decet G, Pedrazzoli S. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg. 2003;7:953-959; discussion 959-960. |
| 17. | Maemura K, Takao S, Shinchi H, Noma H, Mataki Y, Kurahara H, Jinnouchi S, Aikou T. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006;13:435-441. |
| 18. | Chowdhury P, Rayford PL. Smoking and pancreatic disorders. Eur J Gastroenterol Hepatol. 2000;12:869-877. |
| 19. | Lowenfels AB, Maisonneuve P. Epidemiologic and etiologic factors of pancreatic cancer. Hematol Oncol Clin North Am. 2002;16:1-16. |
| 21. | Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev. 2003;27:87-93. |