Published online May 21, 2011. doi: 10.3748/wjg.v17.i19.2424
Revised: December 23, 2010
Accepted: December 30, 2010
Published online: May 21, 2011
AIM: To assess the clinical role of monoclonal immunoscintigraphy for the detection of metastasis and recurrence of colorectal cancer.
METHODS: Monoclonal immunoscintigraphy was performed in patients operated on for colorectal adenocarcinoma suspected of local recurrence and metastatic disease. The results were compared with conventional diagnostics.
RESULTS: Immunoscintigraphic investigation was done in 53 patients. Tumor recurrence occurred in 38 patients, and was confirmed by other diagnostic modalities in 35. In 15 patients, immunoscintigraphic findings were negative, and confirmed in 14 with other diagnostic methods. Comparative analysis confirmed good correlation of immunoscintigraphic findings and the results of conventional diagnostics and the level of tumor marker carcinoembryonic antigen. Statistical analysis of parameters of radiopharmaceutical groups imacis, indimacis and oncoscint presented homogenous characteristics all of three radiopharmaceuticals. The analysis of immunoscintigraphic target focus was clearly improved using tomography.
CONCLUSION: Immunoscintigraphy is highly specific and has a good predictive value in local recurrence of colorectal cancer.
- Citation: Artiko V, Marković AK, Šobić-Šaranović D, Petrović M, Antić A, Stojković M, Žuvela M, Šaranović D, Stojković M, Radovanović N, Galun D, Milovanović A, Milovanović J, Bobić-Radovanović A, Krivokapic Z, Obradović V. Monoclonal immunoscintigraphy for detection of metastasis and recurrence of colorectal cancer. World J Gastroenterol 2011; 17(19): 2424-2430
- URL: https://www.wjgnet.com/1007-9327/full/v17/i19/2424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i19.2424
Radiolabeled monoclonal antibodies (mAbs) against tumor-associated antigens enable imaging of primary tumors of the gastrointestinal system, and their metastases and/or recurrence, with high sensitivity and specificity. Whole body immunoscintigraphy and/or single photon emission computed tomography (SPECT) are accessible imaging methods focusing on specific type of tumors. Nuclear medicine imaging enables determination of the pathophysiological and biochemical parameters of the viable tumor tissue, including metabolic changes as well as the presence of specific proteins/receptors on the surface of the tumor cells. Positron emission tomography (PET) is the best method for imaging metabolic changes based on increased rate of tumor glycolysis and/or protein metabolism. Dual modality hybrid imaging techniques PET/CT enable precise morphological and anatomical localization.
Radio-immunoguided surgery has been introduced as a method of more accurate detection of tumor extension and enables radical resection. Radioimmunotherapy with mAbs as postoperative adjuvant treatment is currently been investigated[1].
The aim of the present study was to evaluate the clinical reliability of immunoscintigraphy for detection of metastasis and recurrence of colorectal carcinoma, using three different radiopharmaceuticals.
Imacis 1 contains a cocktail of (111 MBq 131I) mAb 19-9 F (ab’) 2 and mAb anti CEA F (ab’) 2. It is labeled with131I. Its half-life of 8 d and β-minus emission leads to significant radiation exposure of the patient. In addition, its high energy (364 KeV) makes it less than optimal for imaging, necessitating special collimation for contemporary gamma cameras. The two other radiopharmaceuticals used in this study were labeled with111In. Indimacis 19-9 contains 19-9 F(ab’)2/DTPA fragments of mAbs. It is a pure γ emitting isotope with a physical half life of 67 h, an abundance of photon emissions at 173 and 247 keV, while Oncoscint CR 103 is an immunoconjugate produced by site-specific modification of the mAb B72.3, which is a murine immunoglobulin that is able to recognize high molecular weight glycoprotein (TAG-72) expressed by a majority of adenocarcinomas[2-6].
Imacis 1 was administered by slow injection for approximately 30 min. Potassium iodide (600 mg/d) was administered orally for 10 d (starting 24 h before injection) to block the uptake of free iodine into the thyroid gland. Imaging was carried out after 96-120 h. Planar images (6 min/image or at least 200 000 counts over the whole field of view) including anterior and posterior projections of the thorax, abdomen and pelvis, were taken using large field-of-view cameras, fitted with parallel hole high energy collimators. Indimacis 19-9 at a dose of 185 MBq was administered by slow infusion of 100 mL 0.9% sodium chloride over 30 min. Anterior and posterior spot views of the abdomen, pelvis and/or chest (500 000 counts/view) were obtained 24 h and 48 h following the infusion. SPECT of abdominal and pelvic regions, including 360° rotating orbit, sampling every 6° with an 40-s acquisition per stop, using a 128 × 128 or 64 × 640 word matrix was carried out. Reconstruction was performed using Butterworth filter, order 6-10. Oncoscint CR 103 at a dose of 185-200 MBq was administered by slow injection for approximately 5 min, following the same acquisition protocol as above. To achieve more precise localization of the pathological lesions, as well as to increase target-to-background ratio, the dual isotope acquisition and subsequent subtraction of the obtained images were carried out. Thus, images of the vascular system (99mTc-red blood cells), liver and spleen (99mTc-sulfur colloid) or kidney (99mTc-DTPA) are obtained and used for subtraction. Scintigraphy was performed with a ROTA/Orbiter scintillation camera and Micro Delta computer.
The selection of patients was based upon complete diagnostic records [anamnestic data, physical examination, blood analysis, ultrasonography, contrast radiography, rectoscopy/colonoscopy, CT, magnetic resonance imaging (MRI), tumor marker assay] and clinical follow-up of at least 6 mo. The investigation was performed whenever there was a rise in serum levels of tumor markers [carcinoembryonic antigen (CEA) and carbohydrate antigen (CA 19-9)], and metastasis or recurrence could not be located according to clinical, radiological (chest X-rays), sonographic or endoscopic findings.
In all the patients, tumor marker (CEA and CA 19-9) blood levels were estimated every month in the same laboratory. Blood samples for tumor marker estimation were taken from the cubital vein of the patients and stored at -20°C until analysis. Physiological values of CEA were considered up to 7 U/L, while for CA 19-9, they were up to 33 U/mL. Fifteen patients were treated with Imacis 1, 18 with Indimacis 19-9, and 20 with Oncoscint CR 103.
The study data were analyzed in program R version 2.8.1. Tables and graphs were created in Microsoft Office Excel 2007. For statistical analysis of inter-rater agreement of samples with normal distribution, the following graphs were used: Normal Q-Q plot, histogram, and Kolmogorov-Smirnov’s and Shapiro-Wilk’s tests. In order to test the differences between parameters based on their nature, we used Kruskal-Wallis’s test, exact Wilcoxon’s rank sum test and Fisher’s exact test. The value of α = 0.05 was accepted as statistically significant. In case of multiple testing of the same set of data, Bonferoni’s correction was used (α1 = 0.05/β = 0.0167). For the inter-rater agreement of significant parameters, Cohen’s κ coefficient test was used.
Data from various patients were statistically analyzed using Fisher’s exact test in a study group consisting of 53 patients investigated with three radiopharmaceuticals. Analyzed parameters were: age, sex, surgical treatment, pathological verification and diagnostic examination findings. These analyses showed the homogeneity between the three different radiopharmaceuticals (Table 1).
| Parameter | Imacis 1 | Indimacis 19-9 | Oncoscint CR 20 | Test |
| No. of patients | 15 | 18 | 20 | - |
| CEA (μg/L) | Kruskal-Wallis | |||
| Mean (SD) | 11.6 (12.2) | 8.6 (6.5) | 41.2 (60.4) | χ2 = 5.71 |
| Median (rang) | 8.9 (1.2-40) | 5.9 (3.2-21) | 14 (1.3-234) | P = 0.0577 |
| NA | 2/15 (13.3%) | 8/18 (44.4%) | 1/20 (5.0%) | |
| CA 19-9 (U/mL) | Kruskal Wallis | |||
| Mean (SD) | 27.6 (10.4) | 16.2 (4.5) | 49.3 (56.5) | χ2 = 10.71 |
| Median (rang) | 22.0 (15-42) | 14.6 (11.2-27) | 24.5 (14-183) | P = 0.0047 |
| NA | 6/15 (40.0%) | 8/18 (44.4%) | 6/20 (30.0%) | |
| US | Fisher’s eact | |||
| 0 | 9 (60.0%) | 12 (66.7%) | 16 (80.0%) | P = 0.5838 |
| 1 | 5 (33.3%) | 6 (33.3%) | 4 (20.0%) | |
| NA | 1 (6.7%) | - | - | |
| CT | ||||
| 0 | 8 (53.3%) | 6 (33.3%) | 8 (40.0%) | |
| 1 | 5 (33.3%) | 5 (27.8%) | 3 (15.0%) | Fisher’s exact |
| 2 | - | 5 (27.8%) | 6 (30.0%) | P = 0.1787 |
| 3 | - | 1 (5.6%) | 2 (10.0%) | |
| 4 | - | - | 1 (5.0%) | |
| NA | 2 (13.3%) | 1 (5.6%) | - | |
| MR | ||||
| 0 | 2 (13.3%) | - | 4 (20.0%) | |
| 1 | - | - | 3 (15.0%) | Fisher’s exact |
| 2 | - | - | 1 (5%) | P = 0.6 |
| NA | 13 (86.67%) | 12 (60.0%) | ||
| Colonoscopy | ||||
| 0 | 2 (13.3%) | 9 (50.0%) | 5 (25.0%) | |
| 1 | 1 (6.67%) | 3 (15.0%) | 3 (15.0%) | Fisher’s exact |
| 2 | 3 (20.0%) | 2 (11.1%) | 2 (10.0%) | P = 0.55942 |
| NA | 9 (60.0%) | 4 (22.2%) | 10 (50.0%) | |
| Rectoscopy | ||||
| 0 | 5 (33.3%) | 8 (44.4%) | 5 (25.0%) | |
| 1 | 4 (26.7%) | 1 (5.6%) | 2 (10.0%) | Fisher’s exact |
| 2 | 4 (26.7%) | 1 (5.6%) | 1 (5.0%) | P = 0.42891 |
| NA | 2 (13.3%) | 8 (44.4%) | 12 (60.0%) | |
| Immunoscintgraphy | ||||
| 0 | 7 (46.7%) | 5 (27.8%) | 4 (20.0%) | |
| 1 | 2 (13.3%) | 5 (27.8%) | 3 (15.0%) | Fisher’s exact |
| 2 | 5 (33.3%) | 6 (33.3%) | 5 (25.0%) | P = 0.31134 |
| 3 | 1 (6.7%) | 2 (11.1%) | 7 (35.0%) | |
| NA | ||||
| SPECT | ||||
| 0 | - | 2 (11.1%) | 3 (15.0%) | |
| 1 | - | 6 (33.0%) | 1 (5.0%) | |
| 2 | - | 6 (33.0%) | 3 (15.0%) | Fisher’s exact |
| 3 | - | 2 (11.1%) | 7 (35.0%) | P = 0.06137 |
| NA | 2 (11.1%) | 6 (30.0%) |
With Kruskal-Wallis’s test, levels of tumor markers CEA and CA 19-9 were analyzed in 53 patients. CA 19-9 level in the Indimacis 19-9 group was lower than in the other two groups, but it was still elevated. CEA level was elevated in all patients, but was significantly lower in those without pathological findings, and elevated in those with metastatic disease and/or recurrence (Table 2).
| Type of disease | n | CEA median |
| 0 | 14 | 5 |
| 1 | 8 | 16.25 |
| 2 | 10 | 9.45 |
| 3 | 9 | 10 |
| 4 | 1 | 40 |
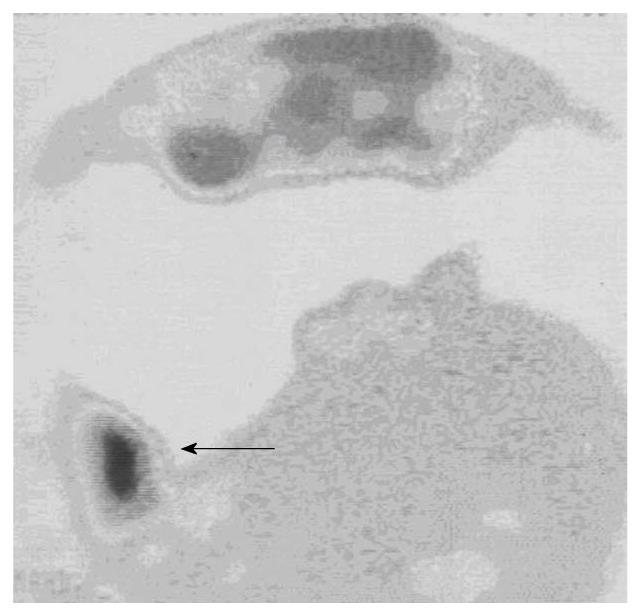
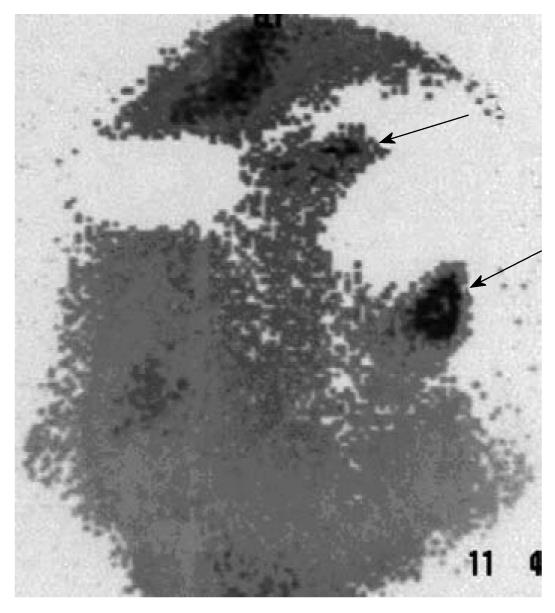
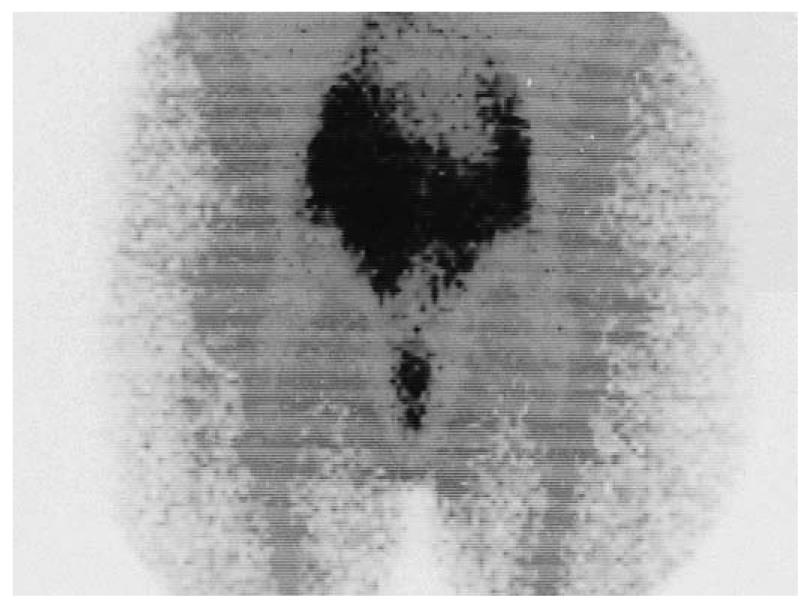
The findings of the complementary diagnostic methods and immunoscintigraphy were analyzed with Cohen’s κ coefficient for the statistical analysis of inter-rater agreement: ultrasonography, slight; rectoscopy, substantial; colonoscopy, moderate; CT, fair; and MRI, substantial (Table 3). Whole body immunoscintigraphy was superior in correlation with complementary diagnostic methods for the detection of pelvic and extrahepatic metastases. Tumor recurrence occurred in 38 patients, and was confirmed by other diagnostic modalities in 35. In three patients, immunoscintigraphic findings were false positive, because hepatic metastases were not confirmed by other imaging modalities. This can be explained by the local inflammation after liver surgery. Thus, sensitivity of the method was 97%, specificity 82%, positive predictive value 92%, negative predictive value 93%, and accuracy 92% (Figure 1, Figure 2, Figure 3).
| Method | n | κ |
| US | 52 | 0.157 |
| CT | 50 | 0.384 |
| MRI | 10 | 0.667 |
| Colonoscopy | 31 | 0.469 |
| Rectoscopy | 30 | 0.655 |
In 28 patients with positive immunoscintigraphic findings (both Indimacis 19-9 and Oncoscint), target/background (tg/bg) ratio was analyzed. Higher tg/bg ratio was found in metastatic foci with Indimacis 19-9 (Table 4). Further analysis of SPECT tg/bg ratio and planar tg/bg ratio points out the advantages of SPECT acquisition in immunoscintigraphic detection of metastases (exact Wilcoxon’s rank sum test: planar images W = 169, P = 0.0005 and SPECT images W = 174.5, P = 0.0001).
| Planar | SPECT | n | |
| Indimacis 19-9 mean (SD) | |||
| Recurrence | 1.2 | 3.5 | 1/13 |
| Metastasis | 2.03 (0.44) | 3.04 (0.8) | 13/13 |
| Oncoscint mean (SD) | |||
| Recurrence | 1.6 (0.24) | 2.15 (0.29) | 4/5 |
| Metastasis | 1.45 (0.26) | 1.84 (0.28) | 15/15 |
The analyses showed homogeneity between the groups for the three different radiopharmaceuticals. CEA and CA 19-9 were analyzed in 53 patients. The CA 19-9 level was lower in the Indimacis 19-9 group compared with the other two groups, but it was still elevated. Tumor marker CEA was elevated in all patients, but significantly lower in those without pathological findings, and elevated in those with metastatic disease and/or recurrence. Thus, we can conclude that both parameters are valuable for evaluation and follow-up of disease.
The findings of the complementary diagnostic methods and immunoscintigraphy were analyzed and whole body immunoscintigraphy was superior in correlation with complementary diagnostic methods for the detection of pelvic and extrahepatic metastases.
Tumor recurrence occurred in 38 patients, and was confirmed by other diagnostic modalities in 35 (Figures 1-3). In three patients, immunoscintigraphic findings were false positive due to local inflammation after liver surgery. In 15 patients, findings were negative, which were confirmed in 14 patients using other diagnostic methods, and one patient had a false-negative result, which was a small lesion in the rectal lumen (1 cm) that was confirmed by rectoscopy. Thus, sensitivity of the method was 97%, specificity 82%, positive predictive value 92%, negative predictive value 93%, and accuracy 92%.
A higher tg/bg ratio was found for metastatic foci with Indimacis 19-9. Further analysis pointed out the advantages of SPECT acquisition for immunoscintigraphic detection of metastases.
In most of the investigated cases, immunoscintigraphy was complementary to other imaging methods and significantly influenced the patient management. The most appropriate applications of this method should be the detection of recurrence, assessment of viability, as well as follow-up of disease progression and regression after therapy. Its diagnostic role is complementary to the radiological methods, which show limitations such as viability assessment after surgery, radio- and chemotherapy (CT, MRI), as well as when contrast radiography and colonoscopy cannot be performed (patients with colostomas and strictures), or when recurrence has an extraluminal position. However, other morphological methods (CT, ultrasongraphy, MRI) are superior for detection of liver metastases, while immunoscintigraphy is more sensitive and specific for the discovery of recurrences of colorectal carcinoma.
With regard to the false-negative findings for small intraluminal tumors, we conclude that for intraluminal tumors, endoscopic methods such as rectoscopy are the methods of choice. Apart from localization, the disadvantage of immunoscintigraphy is low spatial resolution of gamma cameras, which can lead to overlooking of small lesions (1 cm). These disadvantages can be overcome by using new generation gamma cameras with increased resolution as well as fusion images with CT and MRI, and especially hybrid systems. Thus, the recent introduction of a hybrid imaging device that contains a low-dose CT system and a gamma camera (SPECT/CT) on a single gantry has enabled the sequential acquisition of the two imaging modalities, with subsequent merging of data into a composite image display. These hybrid studies have led to a revolution in the field of imaging, with highly accurate localization of tumor sites, assessment of invasion into surrounding tissues, and characterization of their functional status[7-9]. In the absence of SPECT/CT systems, tomography (SPECT) for a better distinction of the tumor is highly recommended. Further improvement in the detection of small tumor recurrences with immunoscintigraphy, using radioimmunoguided surgery (RIGS) and intraoperative detection of tumor deposits using special gamma probe systems, after i.v. application of radiopharmaceuticals, is discussed later.
For accurate diagnosis, it is necessary to estimate tg/bg ratio, especially for the detection of liver metastases (subtraction method is highly recommended), and non-specific uptake of the radiopharmaceuticals in organs due to metabolism and excretion (liver and kidneys), and tissues mainly due to local inflammation. In our patients, false-positive findings can be attributed to the accumulation of radiopharmaceuticals in inflammation. We must emphasize that in all three false-positive patients, tg/bg ratio was on the lower edge of values for positive findings, which means lower than in tumor tissue, but the difference was not obvious and it was not easy to make a clear cut-off. All three patients underwent liver surgery during 6 mo before our investigation, which caused local inflammation with increased accumulation of radiopharmaceuticals. This means that, to prevent false-positive findings, the time of investigation should be longer after surgery, or repeated after 1 or 2 mo, with the expectation of obtaining lower values in non-specific accumulation and higher values in the case of tumor tissue, with obligatory quantification, i.e. estimation of tg/bg ratio[10,11]. Although the antibodies are tumor specific, there is a certain non-specific accumulation in tissues, due to increased vascularization and local inflammation because specific radioimmunotherapy has never been employed widely[12]. Also, as in previously described methods recommended for detection of false-negative cases, apart from physiological methods (immunoscintigraphy with SPECT), additional morphological investigation is recommended, such as hybrid SPECT/CT imaging, in order to distinguish accumulation in the unchanged, inflamed tumor tissue from newly developed tumor formation. Furthermore, even tumor marker/antigen (CEA, CA19-9) levels can be moderately increased due to local inflammation, which results in binding with specific radiolabeled antibodies[13,14]. This also confirms the importance of follow-up in unclear cases. However, even with the above-mentioned limitations, accuracy of the method is very high.
The results from the literature mainly correspond to ours. Thus, some authors have confirmed the significance of the method for detection of recurrence, but have not confirmed its validity for detection of liver metastases[15,16], whereas many[17-19] have emphasized the significance of tomography. However, on the contrary, some investigations[20-22] have found immunoscintigraphy inferior to other imaging methods, especially for the detection of lymph node metastases and for planning adequate surgical approaches for recurrent colorectal carcinoma.
Our previous results, as well as those of other authors[23-29] have pointed out the particular application of these antibodies for disease staging, and detection of local recurrence and extra-hepatic metastases in colorectal carcinoma, and that they have an important role in the therapeutic decision making process. The clinical value of PET and immunoscintigraphy with ¹³¹I or ¹¹¹In anti-CEA mAb for diagnosis of recurrent colorectal cancer has been confirmed by Ito et al[30], who have concluded that PET/CT reflects more accurately the biological character of tumors, but cannot provide the specificity of immunoscintigraphy that enables us to distinguish patients for antibody-based therapy. The superior value of PET with fluorodeoxyglucose for detection of distant metastases (liver, bone, and lung) and lymph node involvement has been estimated in comparison to 99mTc-labeled anti-CEA Fab for detection of recurrence of colorectal carcinoma[31]. Immunoscintigraphy is superior for detection of local recurrent colorectal cancer, whereas PET is better for detection of distal metastases[32].
RIGS[33] enables localization of small tumor deposits. Roveda et al[34] have performed immunoscintigraphy with ¹³¹I or ¹¹¹In anti-CEA and 19.9 mAb using a gamma probe, and have found it particularly useful for endoscopic study of the pelvis after anterior resection, which is difficult to achieve by other diagnostic procedures. Both immunoscintigraphy and RIGS enable a more accurate diagnosis according to Hladic et al[35]. Florio et al[36] have found positive intraoperative gamma probe detection, although negative for immunoscintigraphy. RIGS applied in primary colorectal cancer enables the detection of occult lymph node metastases[37].
In summary, imaging methods (CT, US, MRI) have an advantage for detection of liver metastases, whereas immunoscinitgraphy is more specific for the assessment of recurrence of abdominal tumors. Thus, immunoscintigraphy should be applied in patients with suspected local recurrence and inconclusive results of routine diagnostic workup.
Considering that some tumors produce characteristic antigens, scintigraphy with monoclonal antibodies to these antigens seems to be a very promising method for detection. The aim of this study was to evaluate the clinical reliability of immunoscintigraphy for the detection of metastasis and recurrence of colorectal carcinoma, using three different radiopharmaceuticals.
The results demonstrate that immunoscintigraphy is an accurate method for the detection of cancer recurrence. Together with single photon emission computed tomography (SPECT)/CT and radioimmunoguided surgery, it could have potential for selection of patients for immunotherapy or, in the future, radioimmunotherapy.
Imaging methods (CT, ultrasonography, magnetic resonance imaging) have advantages for detection of liver metastases, whereas immunoscintigraphy is more specific for the assessment of recurrence of abdominal tumors.
Immunoscintigraphy should be used in patients with suspected local recurrence and inconclusive results from routine diagnostic workup.
Monoclonal immunoscintigraphy is scintigraphy with radiolabeled monoclonal antibodies on tumor markers/antigens.
The research article by Artiko and his team deals with the usefulness of immnunoscintigraphy for the detection of metastases and the recurrence of colorectal cancer. The results indicate that immunoscintigraphy is reliable and has a specific advantage for the detection of tumor recurrence, and can also be useful for suspected local recurrence. There was a good correlation between immunoscintigraphic evaluation and the results of conventional diagnostic methods.
Peer reviewer: Dr. Devinder Kumar Dhawan, Professor, Department of Biophysics and Coordinator, Nuclear Medicine, Panjab University, Chandigarh 160014, India
S- Editor Sun H L- Editor Kerr C E- Editor Ma WH
| 1. | Stocchi L, Nelson H. Diagnostic and therapeutic applications of monoclonal antibodies in colorectal cancer. Dis Colon Rectum. 1998;41:232-250. |
| 2. | Storto G, Buchegger F, Waibel R, Kuenzi G, Offord RE, Schubiger PA, Gillet M, Delaloye AB. Biokinetics of a F(ab')3 iodine-131 labeled antigen binding construct (Mab 35) directed against CEA in patients with colorectal carcinoma. Cancer Biother Radiopharm. 2001;16:371-379. |
| 3. | Colcher D, Hand PH, Nuti M, Schlom J. A spectrum of monoclonal antibodies reactive with human mammary tumor cells. Proc Natl Acad Sci USA. 1981;78:3199-3203. |
| 4. | Johnson VG, Schlom J, Paterson AJ, Bennett J, Magnani JL, Colcher D. Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3. Cancer Res. 1986;46:850-857. |
| 5. | Kholin VV, Stoliarova IV. [Current state and approaches to increasing the effectiveness of combined radiotherapy of uterine cancer]. Med Radiol (Mosk). 1986;31:62-69. |
| 6. | Thor A, Ohuchi N, Szpak CA, Johnston WW, Schlom J. Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B72.3. Cancer Res. 1986;46:3118-3124. |
| 7. | Keidar Z, Israel O, Krausz Y. SPECT/CT in tumor imaging: technical aspects and clinical applications. Semin Nucl Med. 2003;33:205-218. |
| 8. | Chen MH, Chang CH, Chang YJ, Chen LC, Yu CY, Wu YH, Lee WC, Yeh CH, Lin FH, Lee TW. MicroSPECT/CT imaging and pharmacokinetics of 188Re-(DXR)-liposome in human colorectal adenocarcinoma-bearing mice. Anticancer Res. 2010;1:65-72. |
| 9. | Chang YJ, Chang CH, Yu CY, Chang TJ, Chen LC, Chen MH, Lee TW, Ting G. Therapeutic efficacy and microSPECT/CT imaging of 188Re-DXR-liposome in a C26 murine colon carcinoma solid tumor model. Nucl Med Biol. 2010;37:95-104. |
| 10. | Mansberg R, Sorensen N, Mansberg V, Van der Wall H. Yttrium 90 Bremsstrahlung SPECT/CT scan demonstrating areas of tracer/tumour uptake. Eur J Nucl Med Mol Imaging. 2007;34:1887. |
| 11. | Aqueveque AC, González E P, Gutiérrez B D, Jaimovich F R, Díaz P JC, Csendes G P, Orellana P P, Lavados M H, Alliende G I, Araya L S. [Fusion of SPECT with computed tomography or magnetic resonance for the interpretation of abnormal tracer uptake]. Rev Med Chil. 2007;135:725-734. |
| 12. | Schoffelen R, van der Graaf WT, Franssen G, Sharkey RM, Goldenberg DM, McBride WJ, Rossi EA, Eek A, Oyen WJ, Boerman OC. Pretargeted 177Lu radioimmunotherapy of carcinoembryonic antigen-expressing human colonic tumors in mice. J Nucl Med. 2010;51:1780-1787. |
| 13. | Koide R, Taniguchi M, Ueki Y, Isozaki E, Hayashi H. [A case of lumbar intradural and epidural abscesses presenting with elevated serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9)]. No To Shinkei. 2003;55:443-447. |
| 14. | Chen CY, Shiesh SC, Tsao HC, Lin XZ. The assessment of biliary CA 125, CA 19-9 and CEA in diagnosing cholangiocarcinoma--the influence of sampling time and hepatolithiasis. Hepatogastroenterology. 2002;49:616-620. |
| 15. | Riva P, Moscatelli G, Agostini M, Spinelli A, Franceschi G. Immunoscintigraphy of primary and metastatic colorectal cancers with radiolabelled monoclonal antibodies anti-CEA. Acta Gastroenterol Belg. 1989;52:497-505. |
| 16. | Buraggi G, Callegaro L, Turrin A, Gennari L, Bombardieri E, Mariani G, Deleide G, Dovis M, Gasparini M, Doci R. Immunoscintigraphy of colorectal carcinoma with F (ab')2 fragments of anti-CEA monoclonal antibody. Cancer Detect Prev. 1987;10:335-345. |
| 17. | Winzelberg GG, Grossman SJ, Rizk S, Joyce JM, Hill JB, Atkinson DP, Sudina K, Anderson K, McElwain D, Jones AM. Indium-111 monoclonal antibody B72.3 scintigraphy in colorectal cancer. Correlation with computed tomography, surgery, histopathology, immunohistology, and human immune response. Cancer. 1992;69:1656-1663. |
| 19. | Nabi HA, Erb DA, Cronin VR. Superiority of SPET to planar imaging in the detection of colorectal carcinomas with 111In monoclonal antibodies. Nucl Med Commun. 1995;16:631-639. |
| 20. | Hölting T, Schlag P, Steinbächer M, Kretzschmar U, Georgi P, Herfarth C. The value of immunoscintigraphy for the operative retreatment of colorectal cancer. Limitations of a new diagnostic method. Cancer. 1989;64:830-833. |
| 21. | Hölting T, Schlag P, Georgi P. Current status of immunoscintigraphy in colorectal cancer--results of 5 years' clinical experiences. Eur J Surg Oncol. 1990;16:312-318. |
| 22. | Schlag P, Hölting T, Steinbächer M, Kretzschmar U, Georgi P. [Current role of immunoscintigraphy for surgical therapy of the recurrence of colorectal cancers]. Chirurg. 1987;58:594-596. |
| 23. | Edlin JP, Kahn D. Detection of recurrent colorectal carcinoma with In-111 CYT-103 scintigraphy in a patient with nondiagnostic MRI and CT. Clin Nucl Med. 1994;19:1004-1007. |
| 24. | Goldenberg DM. Perspectives on oncologic imaging with radiolabeled antibodies. Cancer. 1997;80:2431-2435. |
| 25. | Artiko V, Obradović V, Davidović B, Petrović N, Petrović M, Krivokapić Z, Pavlov M, Adanja G, Sobić D, Vlajković M. [Indium 111-labeled antibodies in the detection of colorectal carcinoma]. Acta Chir Iugosl. 2003;50:43-46. |
| 26. | Artiko V, Obradovic V, Davidovic B, Petrovic N, Petrovic M, Krivokapic Z, Kecmanovic D, Pesko P, Djukic V, Milosavljevic T. Radioimmunodetection of colorectal carcinoma. Hepatogastroenterology. 2003;50:1029-1031. |
| 27. | Obradovic V, Artiko V, Petrovic M, Lausevic Z, Stojkovic M, Sobic-Saranovic D, Petrovic N, Vlajkovic M, Krivokapic Z. Radioimmunoscintigraphy of colorectal carcinomas with three different radiopharmaceuticals. Neoplasma. 2006;53:444-449. |
| 28. | Artiko VM, Sobić-Saranović DP, Krivokapić ZV, Petrović MN, Obradović VB. Is there a future role for immunoscintigraphy in the diagnosis of colorectal carcinoma? Neoplasma. 2009;56:1-8. |
| 29. | Obradovic V, Aritko V. Metastases and recurrence of colorectal cancer: diagnostic role of immunoscintigraphy. Colorectal Cancer. Springer Netherlands. 2009;4:43-63. |
| 30. | Ito K, Nakata K, Watanabe T, Hibi K, Kasai Y, Akiyama S, Takagi H. [Diagnosis of local recurrence of colorectal cancer, using PET and immunoscintigraphy by means of 131I or 111In anti-CEA monoclonal antibody]. Nippon Geka Gakkai Zasshi. 1997;98:373-379. |
| 31. | Willkomm P, Bender H, Bangard M, Decker P, Grünwald F, Biersack HJ. FDG PET and immunoscintigraphy with 99mTc-labeled antibody fragments for detection of the recurrence of colorectal carcinoma. J Nucl Med. 2000;41:1657-1663. |
| 32. | Sarikaya I, Povoski SP, Al-Saif OH, Kocak E, Bloomston M, Marsh S, Cao Z, Murrey DA, Zhang J, HallNC . Combined use of preoperative 18F FDG-PET imaging and intraoperative gamma probe detection for accurate assessment of tumor recurrence in patients with colorectal cancer. World J Surg Oncol. 2007;5:80. |
| 33. | Muxi A, Pons F, Vidal-Sicart S, Setoain FJ, Herranz R, Novell F, Fernandez RM, Trias M, Setoain J. Radioimmunoguided surgery of colorectal carcinoma with an 111In-labelled anti-TAG72 monoclonal antibody. Nucl Med Commun. 1999;20:123-130. |
| 34. | Roveda L. [Radioimmunoscintigraphy with monoclonal antibodies in recurrences and metastases of colorectal tumors]. Medicina (Firenze). 1990;10:160-161. |
| 35. | Hladik P, Vizda J, Bedrna J, Simkovic D, Strnad L, Smejkal K, Voboril Z. Immunoscintigraphy and intra-operative radioimmunodetection in the treatment of colorectal carcinoma. Colorectal Dis. 2001;3:380-386. |
| 36. | Gioffrè Florio MA, Baldari S, Famà F, Giacobbe G, Pollicino A. [Radioimmunoguided surgery (R.I.G.S.) in colorectal cancer. Preliminary results]. Chir Ital. 2002;54:323-329. |
| 37. | Aarts F, Boerman OC, Sharkey RM, Hendriks T, Chang CH, McBride WJ, Bleichrodt RP, Oyen WJ, Goldenberg DM. Pretargeted radioimmunoscintigraphy in patients with primary colorectal cancer using a bispecific anticarcinoembryonic antigen CEA X anti-di-diethylenetriaminepentaacetic acid F(ab')2 antibody. Cancer. 2010;116:1111-1117. |