Published online May 21, 2011. doi: 10.3748/wjg.v17.i19.2417
Revised: February 6, 2011
Accepted: February 13, 2011
Published online: May 21, 2011
AIM: To investigate the role of p53 antibodies (p53Abs), metallothioneins (MTs) and oxidative stress markers in the early detection of dysplasia in chronic ulcerative colitis (UC).
METHODS: The study included 30 UC patients, 15 without dysplasia (group II) and 15 with dysplasia (group III), in addition to 15 healthy volunteers (group I, control subjects). The enzyme-linked immunosorbent assay technique was used to measure serum p53Abs and MTs, while advanced oxidation protein products (AOPPs), and reduced glutathione (GSH) levels were measured by spectrophotometric method in all subjects.
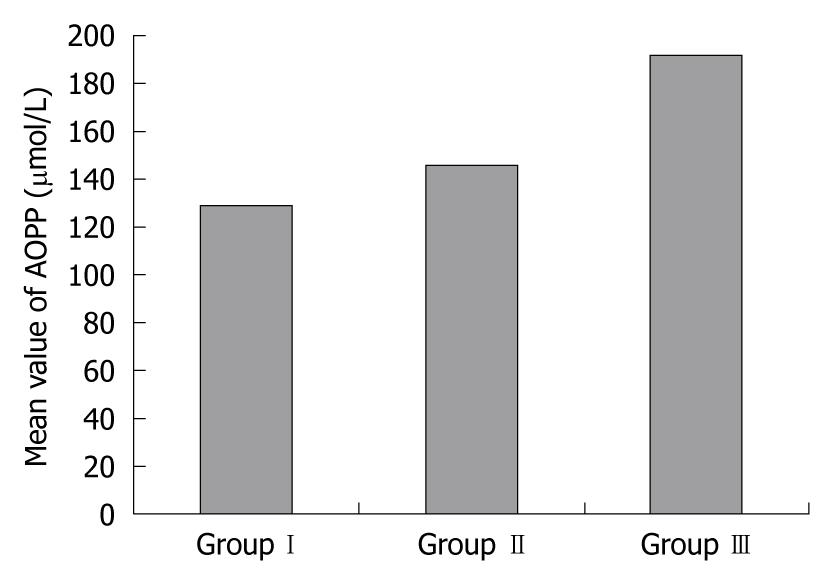
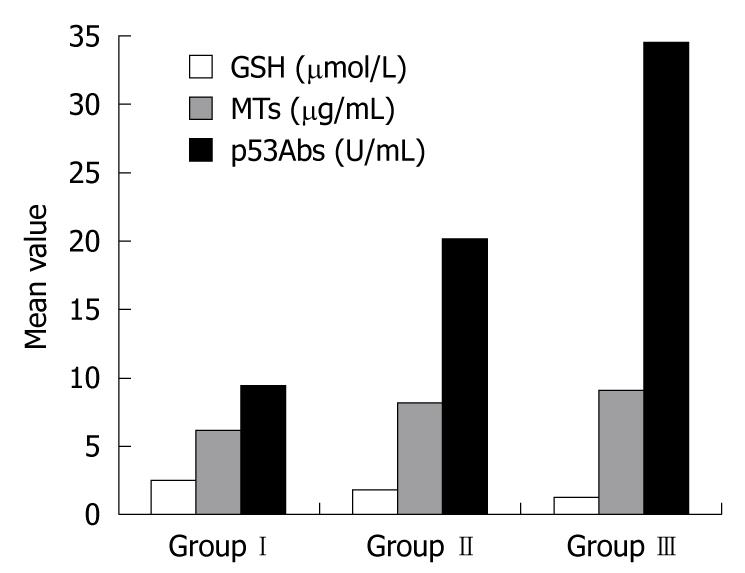
RESULTS: In group II and group III compared to group I, there were significant increases in serum levels of AOPPs (145.94 ± 29.86 μmol/L and 192.21 ± 46.71 μmol/L vs 128.95 ± 3.06 μmol/L, P < 0.002 and P < 0.001, respectively), MTs (8.18 ± 0.35 μg/mL and 9.20 ± 0.58 μg/mL vs 6.12 ± 0.25 μg/mL, P < 0.05 and P < 0.05, respectively), and p53Abs (20.19 ± 3.20 U/mL and 34.66 ± 1.34 U/mL vs 9.42 ± 1.64 U/mL, P < 0.001 and P < 0.001, respectively). There were significantly higher levels of AOPPs (P < 0.05) and p53Abs (P < 0.001) in UC patients with dysplasia compared to those without dysplasia, while MTs showed no significant difference between the 2 groups (P > 0.096). In contrast, GSH levels showed a significant decrease in both patients’ groups (1.87 ± 0.02 μmol/mL and 1.37 ± 0.09 μmol/mL vs 2.49 ± 0.10 μmol/mL, P < 0.05 and P < 0.05 in groups II and III, respectively) compared with group I, and the levels were significantly lower in group III than group II (P < 0.05). There was a positive correlation between AOPPs and both MTs (r = 0.678, P < 0.001) and p53Abs (r = 0.547, P < 0.001), and also between p53Abs and MTs (r = 0.739, P < 0.001). There was a negative correlation between AOPPs and GSH (r = -0.385, P < 0.001), and also between GSH and both MTs (r = -0.662, P < 0.001) and p53Abs (r = -0.923, P < 0.001).
CONCLUSION: Oxidative stress and oxidative cellular damage play an important role in the pathogenesis of chronic UC and the associated carcinogenetic process. p53Abs levels could help in early detection of dysplasia in these conditions.
- Citation: Hamouda HE, Zakaria SS, Ismail SA, Khedr MA, Mayah WW. p53 antibodies, metallothioneins, and oxidative stress markers in chronic ulcerative colitis with dysplasia. World J Gastroenterol 2011; 17(19): 2417-2423
- URL: https://www.wjgnet.com/1007-9327/full/v17/i19/2417.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i19.2417
Ulcerative colitis (UC) is a chronic inflammatory bowel disease limited to the colon and affecting only mucosa and submucosa except in the most severe cases. Although the exact pathogenesis of UC remains unknown, various hypotheses have been put forward, and a number of factors are associated with its occurrence[1,2]. The development of dysplasia and cancer is one the most concerning complications of longstanding UC[3].
Both genetic and environmental factors contribute to the pathogenesis of colorectal cancer in UC. Oxidative stress also seems to be involved in the pathogenesis of UC because the inflammatory cells, neutrophils, and macrophages produce large amounts of reactive oxygen species (ROS)[2]. Oxidative stress in inflamed tissue can pave the way for malignant tumors, and it is a major pathogenetic factor in the well-established correlation between inflammatory diseases and cancer[4]. As it is difficult to detect UC-associated dysplasia endoscopically, the development of new diagnostic modalities at an early or precancerous stage is crucial to improve the prognosis of UC-associated neoplasia[5,6].
Advanced oxidation protein products (AOPPs) are new protein markers of oxidative stress with pro-inflammatory properties, which accumulate in many pathological conditions[7,8]. AOPPs are formed mainly as a consequence of the action of chlorinated compounds, leading to the formation of dityrosine residues and protein crosslinking[9,10]. Being the products of oxidative imbalance themselves, AOPPs further participate in the potentiation and perpetuation of both oxidative stress and inflammation[11].
Glutathione (GSH) and its related enzymes are essential enzymatic defense systems in the colonic mucosa that have many important functions, such as maintaining the reduced state of proteins and protecting the cells against ROS, drugs or heavy metal ions[12,13].
Metallothioneins (MTs) are a superfamily of small proteins that are present in virtually every living organism and have highly a conserved number and position of cysteine residues, enabling them to incorporate monovalent and divalent metal atoms and to reduce reactive oxygen and nitrogen species[14]. MTs are known to participate in fundamental cellular processes such as cell proliferation and apoptosis[15].
Altered genes may not only lead to a functional change that contributes to the appearance of a malignant phenotype, but may also generate molecules that will induce humoral or cell-mediated specific immune responses[16]. p53 is the most striking tumor suppressor gene. Mutations of the p53 gene are the most frequently reported somatic gene alterations in human cancer, leading to accumulation of p53 gene products in tumor cells that can initiate an immune response with generation of circulating anti-p53 antibodies (p53Abs)[17,18]. The earlier observation of p53Abs in sera of patients with lung, liver, colon, and breast cancer[17,19] not only raised the question of the relationship between p53 gene mutation, p53 accumulation, and the anti-p53 humoral response, but also opened the way to the development of new markers for cancer diagnosis[16].
The aim of this study was to investigate the role of p53Abs, MTs and some oxidative stress markers in the early detection of dysplasia in chronic UC patients.
The study included 45 subjects in 3 groups. Group I (control group) comprised 15 healthy volunteers (8 male, 7 female), aged 40.3 ± 14.6 years. Group II comprised 15 UC patients (9 male, 6 female), aged 52.9 ± 17.8 years without colonic dysplasia. Group III comprised 15 UC patients (7 male, 8 female), aged 65.5 ± 11.2 years with mild to moderate degrees of dysplasia.
Patients were selected from the inpatients and outpatients of the Tropical Medicine Department of Tanta University Hospital, Egypt. Written consent was obtained from every investigated subject. Disease duration was calculated from the date of diagnosis of UC, taken from the medical records.
Patients with autoimmune disease, any malignant tumor, and/or chronic liver diseases and those with concurrent infections, previous surgery, chemotherapy or who had undergone colorectal surgery and those with nutritional support were excluded from the study. The diagnosis of UC was based upon a clinical history of diarrhea and/or rectal bleeding for 6 wk or more, typical radiological and endoscopic findings, and characteristic microscopic changes on biopsy specimens.
All patients underwent colonic biopsy sampling during surveillance colonoscopy, which was performed according to established American Gastroenterological Association guidelines for UC surveillance colonoscopy[20]. An aliquot of the colon sample was immediately fixed in formalin for the histopathological evaluation which was performed by the pathologist in a blinded fashion throughout the study. Dysplasia was diagnosed in line with classification proposed by the Inflammatory Bowel Disease Dysplasia Morphology Study Group[21]. At the time of the endoscopic procedure, blood samples were taken from each subject under complete aseptic conditions, and the sera obtained by centrifugation were stored at -80°C until the time of use.
All groups were subjected to the following: (1) A thorough medical history-taking, full clinical examination and routine laboratory investigations including complete blood picture and serum albumin, and lipid profile including serum total cholesterol, triglycerides, LDL-cholesterol and HDL-cholesterol; (2) Estimation of serum AOPP level: determination of AOPPs based on spectrophotometric detection according to Witko-Sarsat et al[9] (1998). Briefly, 200 μL of serum (diluted 1:5 with phosphate-buffered saline (PBS), 200 μL of chloramin T (0-100 μmol/L) for calibration and 200 μL of PBS as blank were applied on a microtiter plate; 10 μL of 1.16 mol/L potassium iodide and 20 μL of acetic acid were added to each well and absorbance at 340 nm was measured immediately. The concentration of AOPPs was expressed as μmol/L chloramine T equivalents units; (3) Spectrophotometric determination of serum GSH level: the method is based on the reduction of 5.5’-dithiobis-(2-nitrobenzoic acid) by GSH to produce a yellow compound. The reduced chromogen is directly proportional to GSH concentration and its absorbance was measured at 405 nm using a commercial kit (Biodiagnostic, Egypt)[22]; (4) Estimation of serum metallothionein level: MTs in the serum were measured by an enzyme-linked immunosorbent assay (ELISA) kit (Ray Biotech, Inc) after acid (1 mol/L HCl) and heat (100°C, 10 min) treatment to eliminate the coexistent antibody-reactive protein according to Cousins[23] (1991); and (5) Assay of serum p53Abs: p53Abs were detected by a commercially available ELISA kit (Quantkine) kit supplied by Clinilab according to Kirsch et al[24] (1998). Cut-off absorbance value was calculated according to the manufacturer’s instructions (cut-off = 26.3). Therefore, we judged samples to be positive for serum when the p53Abs level was higher than 26.3 U/mL.
Statistical analysis was performed using SPSS for Windows 10.0. One way analysis of variance was used to compare groups and Tukey’s test was used to determine the significance between 2 groups. The Pearson correlation coefficient (r) was used to identify a correlation between different parameters.
The demographics and biochemical characteristics in all studied groups are shown in Table 1.
| Group I (n = 15) | Group II(n = 15) | Group III(n = 15) | |
| Age (yr) | 40.3 ± 14.6 | 52.9 ± 17.8 | 65.5 ± 11.2 |
| Sex (male/female) | 8/7 | 9/6 | 7/8 |
| Disease duration (yr) | ------ | 6 ± 1.1 | 7 ± 2.1 |
| TG (mg/dL) | 90.3 ± 14.5 | 92.0 ± 10.8 | 96.5 ± 10.8 |
| TC (mg/dL) | 160.5 ± 13.76 | 167.6 ± 15.3 | 170.5 ± 13.8 |
| LDL-C (mg/dL) | 124 ± 16.46 | 142.33 ± 24.41 | 142.66 ± 24.33 |
| HDL-C (mg/dL) | 54.50 ± 11.81 | 44.06 ± 9.12 | 41.60 ± 10.85 |
| Hb (g/dL) | 13.1 ± 1 | 11.78 ± 1.2 | 11.3 ± 0.9 |
| WBC (/mm3) | 5180 ± 967 | 5630 ± 1252 | 5033.3 ± 976 |
| PLT (104/mm3) | 34.8 ± 5 | 36 ± 4.9 | 38 ±4.2 |
| Albumin (g/dL) | 4.1 ± 0.23 | 4.08 ± 0.35 | 2.9 ± 0.29 |
Serum AOPP levels were significantly increased in UC groups II and III compared with the control group (P < 0.002 and P < 0.001, respectively), with significantly higher levels in patients with dysplasia than in those without dysplasia (P < 0.001). GSH serum levels were significantly reduced in groups II and III compared with controls (P < 0.05 and P < 0.05, respectively), with significantly lower levels in patients with dysplasia than in those without dysplasia (P < 0.05). MT serum levels were significantly increased in groups II and III compared with controls (P < 0.05 and P < 0.05, respectively), with no significant difference between the UC groups (P > 0.096). p53Ab serum levels were significantly increased in groups II and III compared with controls (P < 0.001 and P < 0.001, respectively) with significantly higher levels in patients with dysplasia than in those without dysplasia (P < 0.001, Table 2, Figures 1 and 2).
| Group I (n = 15) | Group II(n = 15) | Group III(n = 15) | P | |
| AOPP (μmol/L) | 128.95 ± 3.06 | 145.94 ± 29.86 | 192.21 ± 46.71 | < 0.0021a |
| < 0.0011b | ||||
| < 0.0011c | ||||
| GSH (μmol/L) | 2.49 ± 0.10 | 1.87 ± 0.02 | 1.37 ± 0.09 | < 0.051a |
| < 0.051b | ||||
| < 0.051c | ||||
| MT (μg/mL) | 6.12 ± 0.25 | 8.18 ± 0.35 | 9.20 ± 0.58 | < 0.051a |
| < 0.051b | ||||
| > 0.096c | ||||
| p53Abs (U/mL) | 9.42 ± 1.64 | 20.19 ± 3.20 | 34.66 ± 1.34 | < 0.0011a |
| < 0.0011b | ||||
| < 0.0011c |
Correlation studies in UC patients (Table 3) showed a positive correlation between AOPPs and both MTs (r = 0.678, P < 0.001) and p53Abs (r = 0.547, P < 0.001), and also between p53Abs and MTs (r = 0.739, P < 0.001). On the other hand, there was a negative correlation between AOPPs and GSH (r = -0.385, P < 0.001), and also between GSH and both MTs (r = -0.662, P < 0.001) and p53Abs (r = -0.923, P < 0.001).
Using a cut-off value (≥ 26 U/mL), we demonstrated that p53Abs were present in 40.0% of UC patients with dysplasia and in 13.3% without dysplasia.
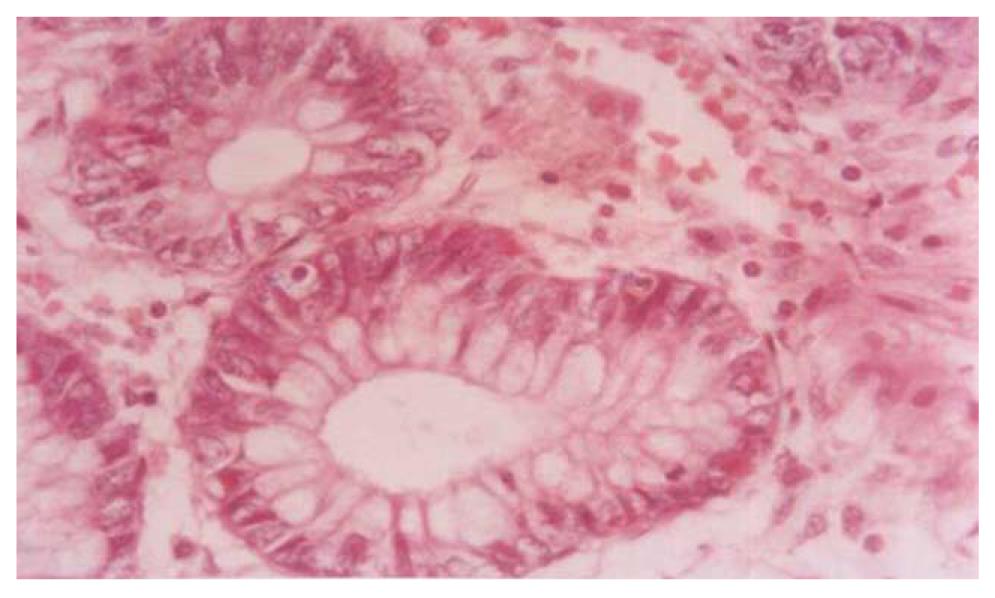
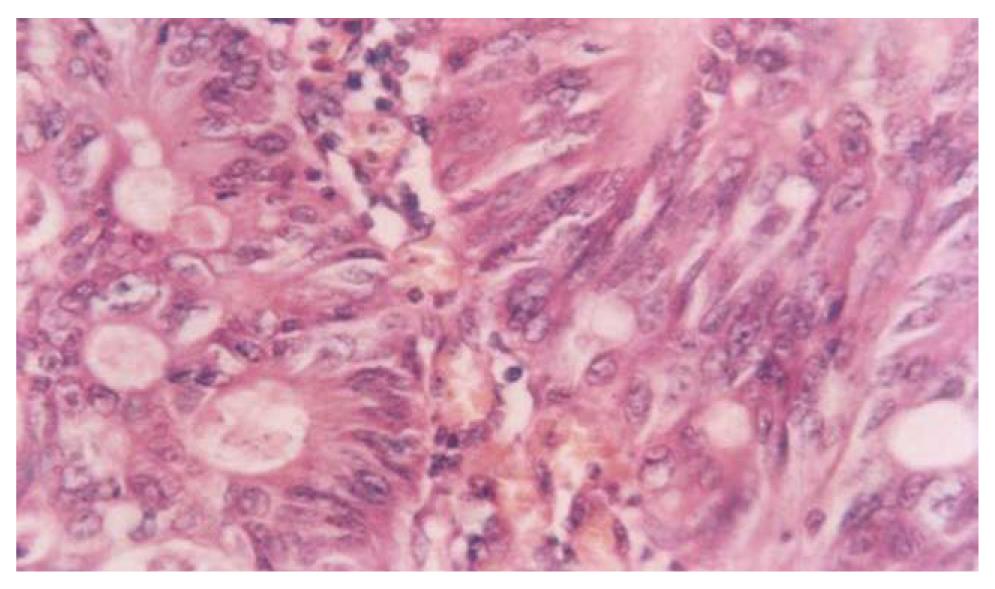
Figures 3 and 4 show photomicrographs of the mucosa in UC without and with dysplasia.
Chronic UC is associated with an increased risk of developing colorectal cancer. The risk of developing cancer, or its precursor lesion, dysplasia, increases exponentially with the duration of the disease[6]. For early detection of UC-associated colorectal cancer, surveillance colonoscopy is recommended in UC patients at high risk. However, poor acceptability by patients reduces its effectiveness. In addition, it is difficult to detect UC-associated dysplasia endoscopically; therefore, a suitable marker for selecting patients at high risk is needed[4]. Accordingly, this study aimed to investigate the value of AOPPs, GSH, MTs and p53Abs levels for the early detection of dysplasia in chronic UC patients.
The activities of phagocytic leukocytes are greatly increased in the colons of UC patients resulting in enhanced generation of pro-oxidant molecules that have been known to play an important role in the initiation and promotion of multi-step carcinogenesis through specific gene alterations, genetic instability and aberrant methylation[25-27]. Indices of oxidative damage are widely used as markers of oxidative stress. AOPPs are new protein markers of oxidative stress with pro-inflammatory properties, accumulated in many pathological conditions[11,13]. In the present study, serum AOPP levels were significantly increased in UC patients, with significantly higher levels in UC patients with dysplasia than in those without dysplasia. Our results are in agreement with other previous studies which showed an increased formation of AOPPs in inflammatory bowel disease[28,29], and those which showed that colorectal cancer is associated with oxidative stress with increased AOPP levels[30-32].
The epithelium of the colon contains multiple antioxidant systems, including antioxidant enzymes and low-molecular-weight antioxidant molecules such as GSH[33]. GSH and enzymes associated with it play crucial role in cell defense against ROS, which are implicated in various types of cancer[34,35]. In the present study, the significant decrease in GSH level in UC patients with significantly lower levels in patients with dysplasia are in accordance with previous studies, which showed depletion of the GSH system in UC patients, leading to increased susceptibility towards toxic or carcinogenic compounds[13]. The significant decrease in GSH level in UC patients may result from an increased turnover of GSH to prevent oxidative damage in these patients. The lower levels of GSH in patients with dysplasia may still favor an overproduction of free radicals which in turn may induce damage to the cell membrane and cellular molecules (DNA, RNA) leading to neoplasia[34,35].
The thiol-rich protein MT plays a major role in detoxification of toxic metals and in protection against oxidative damage. In normal tissues, MT expression is usually undetectable, except in certain types of cells such as myoepithelial, renal and thyroid epithelial cells. It has been observed that the large bowel epithelial cells express MT in up to 40% of patients with UC, and a correlation has been found between the degree of MT expression and severity of inflammation[36].
Our study showed a significant increase in MT levels in both groups of UC patients, but a non significant difference between them. This finding is in agreement with the studies of Brüwer et al[36] and Waeytens et al[37], who found an increased expression of MT in epithelial cells of the intestinal mucosa in inflammatory bowel disease patients. The increased MT concentration in UC patients suggests induction of MT synthesis in response to the potential harmful effects of ROS and nitrogen intermediates produced during the inflammatory response. The generated ROS may activate MT expression through multiple pathways, including directly by stimulating an antioxidant response element and specific metal response elements in the promoter region as well as indirectly by events associated with second-messenger protein kinase pathways[17,18].
Several lines of evidence indicated that many types of tumors have high concentrations of MT and this may play a role in various carcinogenic processes. Also high levels of MT expression have been reported in initiation, progression, and metastasis of cancer[14].
MT overexpression may represent an important early step in the development of colorectal carcinoma in UC patients and those with low grade dysplasia[38]. It has been hypothesized that mutation-induced metallothionein overexpression may interfere with the function of zinc finger DNA binding transcription factors involved in the control of the expression of a wide range of genes regulating cell proliferation and apoptosis, such as p53, and conferring a growth advantage on the mutated cells[39].
The p53 gene mutations seem to be an early event in cancerous change in UC patients[25]. These mutations lead to inactivation of the p53 protein, and cellular accumulation in the nucleus which can initiate an immune response with generation of circulating p53Abs. These p53Abs reflect a humoral response that occurs early in tumor development. Previous studies reported high diagnostic sensitivity and specificity of p53Abs in patients with various types of cancers[40].
In the present study serum p53Abs showed significantly increased levels in both groups of UC patients, with significantly higher levels in patients with dysplasia. The widely accepted hypothesis for increased serum p53Abs is as follows; a point mutation of the p53 gene is associated with overexpression of p53 protein. Overexpressed mutant p53 is considered a nonself protein, triggering the immunocompetent cells and secretion of p53Abs[40]. The type and location of p53 gene mutations may impact on the generation of p53Abs. Most of these mutations lead to the synthesis of a stable protein which accumulates in tumor cells and may be important for the development of the immune response[40]. It has also been suggested that mutations in exons 5 and 6 (coding for mutant protein that binds to heat shock protein 70) are immunogenic, while mutations in exons 7 and 8 are not related to the generation of p53Abs[41]. Our results are in line with the study of Cioffi et al[15], 2004 who suggested that assessment of serum p53Abs is an indirect marker for p53 gene mutations, and the abnormally high p53 protein levels could be considered to have a potential for use as a complementary test to improve surveillance program performance in UC patients. Normally p53 comes into action when DNA is damaged and arrests the cell cycle so as to allow repair. In the case of failure of repair, p53 directs the cell to apoptosis; therefore, p53 mutation leads to uncontrolled proliferation of cells[42].
Using a cut-off value (≥ 26 U/mL), we demonstrated that p53Abs were present in 40.0% of UC patients with dysplasia and in 13.3% without dysplasia. Further investigations on a large scale are needed to better define the sensitivity, specificity, positive and negative predictive values of p53 alterations in patients with UC with and without dysplasia.
In conclusion, oxidative stress and oxidative cellular damage play an important role in the pathogenesis of chronic UC and the associated carcinogenetic process. p53Ab levels could help in early detection of dysplasia in UC patients.
Patients with longstanding ulcerative colitis (UC) have an increased risk of developing dysplasia and colorectal cancer. This risk appears to be related to the cumulative effect of chronic inflammation and correlates directly with the extent and duration of disease. Earlier colonoscopy screening and surveillance for detecting mucosal dysplasia is important to select colorectal cancer-prone individuals for prophylactic colectomy but, to date, this has not been translated into a positive survival benefit. p53 antibodies (p53Abs), metallothioneins (MTs) and some oxidative stress markers may aid in the early detection of dysplasia in chronic UC patients and could be used as complementary tests to improve surveillance program.
Despite several acknowledged limitations, periodic surveillance colonoscopy continues to be used to diagnose dysplasia and colorectal cancer complications of longstanding UC. Investigating the role of p53Abs, MTs and some oxidative stress markers may open the way to the development of new cancer diagnosis.
The present study revealed that the decreased serum protective antioxidant glutathione (GSH) level associated with an increased production of oxygen free radicals results in protein oxidation and hence advanced oxidation protein products (AOPPs) with subsequent increase of MTs leading to p53 instability and the development of high levels of p53Abs in longstanding UC, that may precede and accompany dysplasia. These data are in line with previous studies and call for wider research on the associations in UC.
AOPPs, GSH and MTs are associated with dysplastic changes in chronic UC. p53Ab levels could be used as a complementary test in a surveillance program. Further investigations on large scale populations are needed to compare their sensitivity, specificity, positive and negative predictive values, in addition to, alterations of the p53 tumor suppressor gene, which occurs early in patients with UC and may precede dysplasia.
p53: the p53 gene is the tumor suppressor gene whose abnormalities are the most frequently reported gene alterations in human cancer. MTs: a superfamily of small proteins with a highly conserved number of cysteine residues and they reduce reactive oxygen and nitrogen species. GSH: essential enzymatic defense system in the colonic mucosa. AOPPs are protein markers of oxidative stress with pro-inflammatory properties.
This is an excellent study which is based on the early detection of serological biomarkers in order to predict the risk of developing colorectal cancer in individuals with UC.
Peer reviewer: Jay Pravda, MD, Inflammatory Disease Research Center, Gainesville, Florida, 32614-2181, United States
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
| 1. | Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255-281, vii. |
| 2. | Risques RA, Rabinovitch PS, Brentnall TA. Cancer surveillance in inflammatory bowel disease: new molecular approaches. Curr Opin Gastroenterol. 2006;22:382-390. |
| 3. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. |
| 4. | Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52:2015-2021. |
| 5. | Fujii S, Katsumata D, Fujimori T. Limits of diagnosis and molecular markers for early detection of ulcerative colitis-associated colorectal neoplasia. Digestion. 2008;77 Suppl 1:2-12. |
| 6. | Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511-524. |
| 7. | Baskol G, Demir H, Baskol M, Kilic E, Ates F, Karakukcu C, Ustdal M. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem Funct. 2006;24:307-311. |
| 8. | Wykretowicz A, Adamska K, Krauze T, Guzik P, Szczepanik A, Rutkowska A, Wysoki H. The plasma concentration of advanced oxidation protein products and arterial stiffness in apparently healthy adults. Free Radic Res. 2007;41:645-649. |
| 9. | Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drüeke T, Descamps-Latscha B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524-2532. |
| 10. | Kalousová M, Zima T, Tesar V, Dusilová-Sulková S, Skrha J. Advanced glycoxidation end products in chronic diseases-clinical chemistry and genetic background. Mutat Res. 2005;579:37-46. |
| 11. | Peng KF, Wu XF, Zhao HW, Sun Y. Advanced oxidation protein products induce monocyte chemoattractant protein-1 expression via p38 mitogen-activated protein kinase activation in rat vascular smooth muscle cells. Chin Med J (Engl). 2006;119:1088-1093. |
| 12. | Morgenstern I, Raijmakers MT, Peters WH, Hoensch H, Kirch W. Homocysteine, cysteine, and glutathione in human colonic mucosa: elevated levels of homocysteine in patients with inflammatory bowel disease. Dig Dis Sci. 2003;48:2083-2090. |
| 13. | Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627-647. |
| 14. | Roesijadi G. Metal transfer as a mechanism for metallothionein-mediated metal detoxification. Cell Mol Biol (Noisy-le-grand). 2000;46:393-405. |
| 15. | Cioffi M, Riegler G, Vietri MT, Pilla P, Caserta L, Carratù R, Sica V, Molinari AM. Serum p53 antibodies in patients affected with ulcerative colitis. Inflamm Bowel Dis. 2004;10:606-611. |
| 16. | Soussi T. The humoral response to the tumor-suppressor gene-product p53 in human cancer: implications for diagnosis and therapy. Immunol Today. 1996;17:354-356. |
| 17. | Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777-1788. |
| 18. | El-Sayed ZA, Farag DH, Eissa S. Tumor suppressor protein p53 and anti-p53 autoantibodies in pediatric rheumatological diseases. Pediatr Allergy Immunol. 2003;14:229-233. |
| 19. | Coomber D, Hawkins NJ, Clark M, Meagher A, Ward RL. Characterisation and clinicopathological correlates of serum anti-p53 antibodies in breast and colon cancer. J Cancer Res Clin Oncol. 1996;122:757-762. |
| 20. | Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544-560. |
| 21. | Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931-968. |
| 22. | Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192-205. |
| 23. | Cousins RJ. Measurement of human metallothionein by enzyme-linked immunosorbent assay. Methods Enzymol. 1991;205:131-140. |
| 24. | Kirsch DG, Kastan MB. Tumor-suppressor p53: implications for tumor development and prognosis. J Clin Oncol. 1998;16:3158-3168. |
| 25. | Fricke H, Urban S, Noehl N, Folwaczny C. Serum p53 antibodies in patients with chronic inflammatory bowel disease. Gut. 1998;42:899. |
| 26. | Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353-362. |
| 27. | Schreiber S, MacDermott RP, Raedler A, Pinnau R, Bertovich MJ, Nash GS. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991;101:1020-1030. |
| 29. | Krzystek-Korpacka M, Neubauer K, Berdowska I, Boehm D, Zielinski B, Petryszyn P, Terlecki G, Paradowski L, Gamian A. Enhanced formation of advanced oxidation protein products in IBD. Inflamm Bowel Dis. 2008;14:794-802. |
| 30. | Chang D, Wang F, Zhao YS, Pan HZ. Evaluation of oxidative stress in colorectal cancer patients. Biomed Environ Sci. 2008;21:286-289. |
| 31. | Avinash SS, Anitha M, Vinodchandran , Rao GM, Sudha K, Shetty BV. Advanced oxidation protein products and total antioxidant activity in colorectal carcinoma. Indian J Physiol Pharmacol. 2009;53:370-374. |
| 32. | Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease--radicals or ridiculous? Aliment Pharmacol Ther. 2002;16:1997-2015. |
| 33. | Holmes EW, Yong SL, Eiznhamer D, Keshavarzian A. Glutathione content of colonic mucosa: evidence for oxidative damage in active ulcerative colitis. Dig Dis Sci. 1998;43:1088-1095. |
| 34. | Saygili EI, Akcay T, Konukoglu D, Papilla C. Glutathione and glutathione-related enzymes in colorectal cancer patients. J Toxicol Environ Health A. 2003;66:411-415. |
| 35. | Scibior D, Skrzycki M, Podsiad M, Czeczot H. Glutathione level and glutathione-dependent enzyme activities in blood serum of patients with gastrointestinal tract tumors. Clin Biochem. 2008;41:852-858. |
| 36. | Brüwer M, Schmid KW, Metz KA, Krieglstein CF, Senninger N, Schürmann G. Increased expression of metallothionein in inflammatory bowel disease. Inflamm Res. 2001;50:289-293. |
| 37. | Waeytens A, De Vos M, Laukens D. Evidence for a potential role of metallothioneins in inflammatory bowel diseases. Mediators Inflamm. 2009;2009:729172. |
| 38. | Bruewer M, Schmid KW, Krieglstein CF, Senninger N, Schuermann G. Metallothionein: early marker in the carcinogenesis of ulcerative colitis-associated colorectal carcinoma. World J Surg. 2002;26:726-731. |
| 39. | Kim HJ, Chang SK. p53 mutation in patients with ulcerative colitis in rectal biopsy. Korean J Intern Med. 1998;13:110-116. |
| 40. | Yoshizawa S, Matsuoka K, Inoue N, Takaishi H, Ogata H, Iwao Y, Mukai M, Fujita T, Kawakami Y, Hibi T. Clinical significance of serum p53 antibodies in patients with ulcerative colitis and its carcinogenesis. Inflamm Bowel Dis. 2007;13:865-873. |
| 41. | Kulić A, Sirotković-Skerlev M, Jelisavac-Cosić S, Herceg D, Kovac Z, Vrbanec D. Anti-p53 antibodies in serum: relationship to tumor biology and prognosis of breast cancer patients. Med Oncol. 2010;27:887-893. |
| 42. | Radović S, Vukobrat-Bijedić Z, Selak I, Babić M. Expression of p53, bcl-2, and Ki-67 proteins in the inflammatory regenerative and dysplastic epithelial lesions of flat colonic mucosa. Bosn J Basic Med Sci. 2006;6:39-45. |