Published online May 21, 2011. doi: 10.3748/wjg.v17.i19.2372
Revised: February 26, 2011
Accepted: March 5, 2011
Published online: May 21, 2011
Accompanying rapid developments in hepatic surgery, the number of surgeries and identifications of histological types of primary hepatic space-occupying lesions (PHSOLs) have increased dramatically. This has led to many changes in the surgicopathological spectrum of PHSOLs, and has contributed to a theoretical basis for modern hepatic surgery and oncological pathology. Between 1982 and 2009 at the Eastern Hepatobiliary Surgery Hospital (EHBH) in Shanghai, 31 901 patients underwent surgery and were diagnosed as having a PHSOL. In this paper, we present an analysis of the PHSOL cases at the EHBH for this time period, along with results from a systematic literature review. We describe a surgicopathological spectrum comprising more than 100 types of PHSOLs that can be stratified into three types: tumor-like, benign, and malignant. We also stratified the PHSOLs into six subtypes derived from hepatocytes; cholangiocytes; vascular, lymphoid and hemopoietic tissues; muscular, fibrous and adipose tissues; neural and neuroendocrine tissues; and miscellaneous tissues. The present study provides a new classification system that can be used as a current reference for clinicians and pathologists to make correct diagnoses and differential diagnoses among various PHSOLs.
- Citation: Cong WM, Dong H, Tan L, Sun XX, Wu MC. Surgicopathological classification of hepatic space-occupying lesions: A single-center experience with literature review. World J Gastroenterol 2011; 17(19): 2372-2378
- URL: https://www.wjgnet.com/1007-9327/full/v17/i19/2372.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i19.2372
Liver neoplasms are one of the most common tumors worldwide, especially in China and other developing countries. Rapid developments in liver surgery and liver pathology have led to many new types of primary hepatic space-occupying lesions (PHSOLs) being surgically resected and pathologically diagnosed, which has greatly increased the surgicopathological spectrum of PHSOLs. Indeed, insights into tumor pathological characteristics have illuminated the need for an improved practical guide for oncological clinicians and pathologists to make correct diagnoses and differential diagnoses among PHSOLs[1]. However, to the best of our knowledge, there is no report in the English literature that thoroughly assesses the whole spectrum of PHSOLs.
During the period from January 1982 to December 2009, 31 901 surgically resected PHSOLs were deposited in the archives of the Department of Pathology, Eastern Hepatobiliary Surgery Hospital (EHBH) in Shanghai. In this paper, we present an analysis of the above 31 901 PHSOL cases, along with results from a systematic literature review. To the best of our knowledge, this is the largest series of PHSOLs presented from a single center. Based on the EHBH archival data and literature reviews using MEDLINE and PUBMED, more than 100 types of PHSOLs have been described. In this article, we suggest a surgicopathological classification of PHSOLs comprising three types: tumor-like PHSOLs, benign PHSOLs, and malignant PHSOLs. We also stratified the PHSOLs into six subtypes: lesions derived from hepatocytes; cholangiocytes; vascular, lymphoid and hemopoietic tissues; muscular, fibrous and adipose tissues; neural and neuroendocrine tissues; and miscellaneous tissues.
Tumor-like PHSOLs are usually a type of space-occupying lesion within the hepatic parenchyma or intrahepatic bile ducts, but without a truly neoplastic nature. At least 31 kinds of tumor-like PHSOLs have been reported, as summarized in Table 1[2-25]. In the EHBH series, tumor-like PHSOLs accounted for 4.3% (n = 1370) of the 31 901 cases. Of the tumor-like PHSOLs, focal nodular hyperplasia (FNH) accounted for 51.5% (n = 705), solitary necrotic nodules accounted for 19.6% (n = 269), and hepatic inflammatory pseudotumors (HIP) accounted for 12.0% (n = 165). These are the three most common tumor-like PHSOLs.
| Hepatocellular lesions |
| Focal nodular hyperplasia[2] |
| Nodular regenerative hyperplasia[2] |
| Partial nodular transformation[3] |
| Adenomatoid hyperplasia (dysplastic nodules)[2] |
| Compensatory lobar or segmental hyperplasia[4] |
| Focal fatty change[2] |
| Accessory lobe[5] |
| Bile duct lesions |
| Biliary microhamartoma (Von Meyenburg complex)[2] |
| Cyst and polycystic liver[6] |
| Ciliated foregut cyst[7] |
| Epidermoid cyst[8] |
| Endometrial cyst[9] |
| Intrahepatic peribiliary gland cyst[2] |
| Mesothelial cyst[10] |
| Cystic echinococcosis[11] |
| Biloma[12] |
| Miscellaneous lesions |
| Mesenchymal hamartoma[2] |
| Inflammatory pseudotumor[2] |
| Pseudolymphoma[13] |
| Solitary necrotic nodule[14] |
| Peliosis hepatis[15] |
| Hereditary hemmorrhagic telangiectasia[16] |
| Sarcoidosis[17] |
| Nodular extramedullary hematopoiesis[18] |
| Abscess[19] |
| Tuberculoma[20] |
| Botryomycosis[21] |
| Malacoplakia[22] |
| Ectopic tissue[23] and adrenal rest tumor[24] |
| Pseudolipoma[2] |
| Granulomas[25] |
In the latest edition of the World Health Organization (WHO) classification report (2010 Edition), FNH and HIP were grouped as benign liver tumors[26]. However, most scholars, and the present authors, prefer to regard FNH and HIP as a kind of non-neoplastic lesion or a tumor-like lesion[6,27]. FNH is a regenerative hepatocellular nodule that is frequently related to factors that stimulate the hyperperfusion of either the artery or the portal vein. Clonal analysis using the human androgen receptor locus test demonstrated the reactive polyclonal nature in 50%-100% of the FNH cases. Genetic analysis of FNH failed to identify somatic gene mutations that occurred in hepatocellular adenoma (HCA)[28]. Currently, most FNH are considered as polyclonal, and there was neither recurrence nor substantiated malignant transformation in all 705 FNH cases included in the EHBH series after surgery, even though FNH may occasionally coexist with hepatocellular carcinoma (HCC)[29].
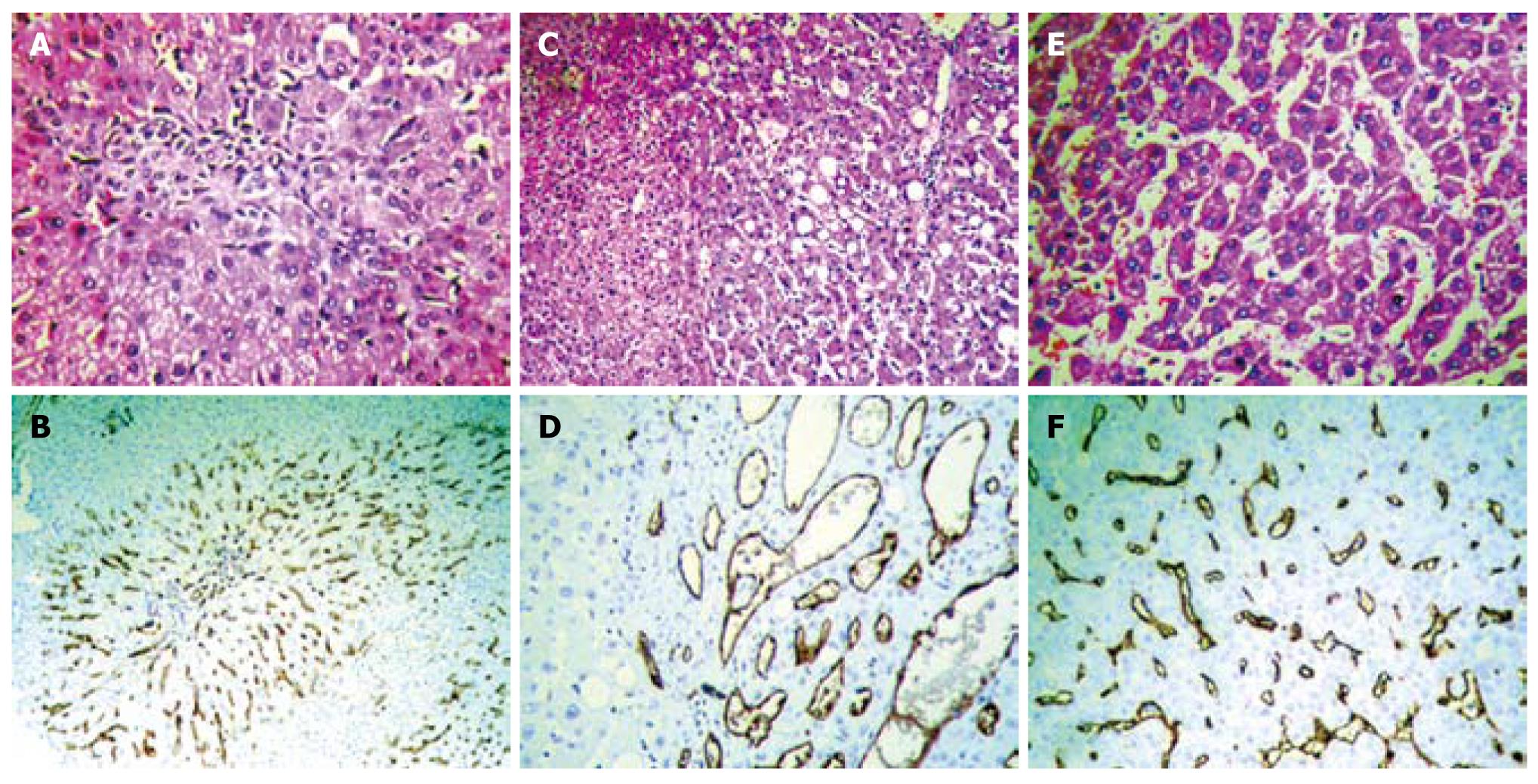
Either clinically or pathologically, FNH should be distinguished from other hepatocellular nodules, such as HCA and highly differentiated HCC. Hepatocyte paraffin 1 (Hep Par 1) and polyclonal carcinoembryonic antigen (CEA) are special hepatocellular markers, which cannot, however, differentiate between benign and malignant natures; therefore, we prefer to use CD34 immunostaining to sensitively and specifically outline microvasculatures to differentiate hepatocellular nodules[6,30,31]. FNH usually presents in a focal distribution pattern of microvasculatures around fibrous scars (Figure 1A and B), whereas HCA shows a chaotic distribution pattern, usually with thin-walled vascular staining (Figure 1C and D). HCC presents in a diffuse distribution pattern occupying a greater proportion of the lesion area (Figure 1E and F). Although glypican-3 (GPC-3) has recently been reported to be overexpressed in HCC, the lack of GPC-3 immunostaining could not exclude the diagnosis in at least 25%-30% of HCC[32].
At least 30 types of benign PHSOLs have been reported, as summarized in Table 2[2,33-56]. In the EHBH series, benign tumors accounted for 12.1% of the cases (n = 3847), among which hepatic cavernous hemangioma (n = 3191, 82.9%), hepatic angiomyolipoma (HAML, n = 153, 4.0%), and HCA (n = 148, 3.8%) were the most frequent types in this group.
| Hepatocellular tumors |
| Hepatocellular adenoma[2] and hepatic adenomatosis[33] |
| Intrahepatic bile duct tumors |
| Bile duct cystadenoma[2] |
| Intraductal papillary neoplasm[34] and intraductal papillomatosis[2] |
| Bile duct adenoma[2] |
| Biliary adenofibroma[35] |
| Vascular and lymphoid tumors |
| Cavernous hemangioma[2] |
| Perivascular epithelioid cell tumor[36] |
| Hemangioblastoma[37] |
| Infantile hemangioendothelioma[2] |
| Lymphangioma and lymphangiomatosis[2] |
| Muscle, fibrous and adipose tumors |
| Angiomyolipoma[2] |
| Leiomyoma[38] |
| Solitary fibrous tumor[2] |
| Lipoma[39] |
| Myelolipoma[40] |
| Neuronal and neuroendocrine tumors |
| Neurilemmoma[41] |
| Plexiform neurofibroma[42] and plexiform neurofibromatosis[43] |
| Paraganglioma[44] |
| Pheochromocytoma[45] |
| Gastrinoma[46] |
| Vascoactive intestinal peptide tumor[47] |
| Somatostatinoma[48] |
| Miscellaneous tumors |
| Teratoma[49] |
| Mesothelioma[50] |
| Endometrioma[51] |
| Chondroma[52] |
| Myxoma[53] |
| Langerhan’s cell histiocytosis[54] |
| Desmoplastic nested spindle cell tumor[55] |
| Spongiotic pericytoma[56] |
In Western countries, patients with HCA or hepatic adenomatosis are mostly estrogen/androgen dependent types, with a female gender bias (> 90%). Among them, 78% have a history of taking contraceptive drugs, and 4% to 4.7% may develop HCC[57]. However, the 148 cases of Chinese HCA in the EHBH series were the spontaneous type with a female:male ratio of 1:2.2. Recently, HCA has been categorized into three molecular subgroups including those with: (1) hepatocyte nuclear factor 1α mutations; (2) β-catenin mutations; and (3) no mutation, with or without inflammatory infiltrates. HCA with a β-catenin mutation has a risk odds of malignant transformation of 46%[58]. It has also been reported that 4% to 17.6% of HCA may have had histologically confirmed malignant transformation[58]. However, after being followed up for more than 5 years after surgery, there was neither a recurrence nor malignant transformation in all 148 cases of HCA in the EHBH series. No case, so far, has had recurrence or tumor canceration, suggesting that Chinese patients with HCA may have differences in etiology, genetics, and HCA-related HCC risk, compared to Western countries.
The above research suggests that the detection of molecular biological or immunohistochemical markers before or after surgery is essential for providing an active radical radiotherapy cure. In addition, more attention should be paid to the careful follow-up of patients with a high potential for transformation of β-catenin activated HCA to prevent HCA transformation or recurrence[26]. Thus, the treatment roadmap based on HCA molecular characteristics has also been described[59].
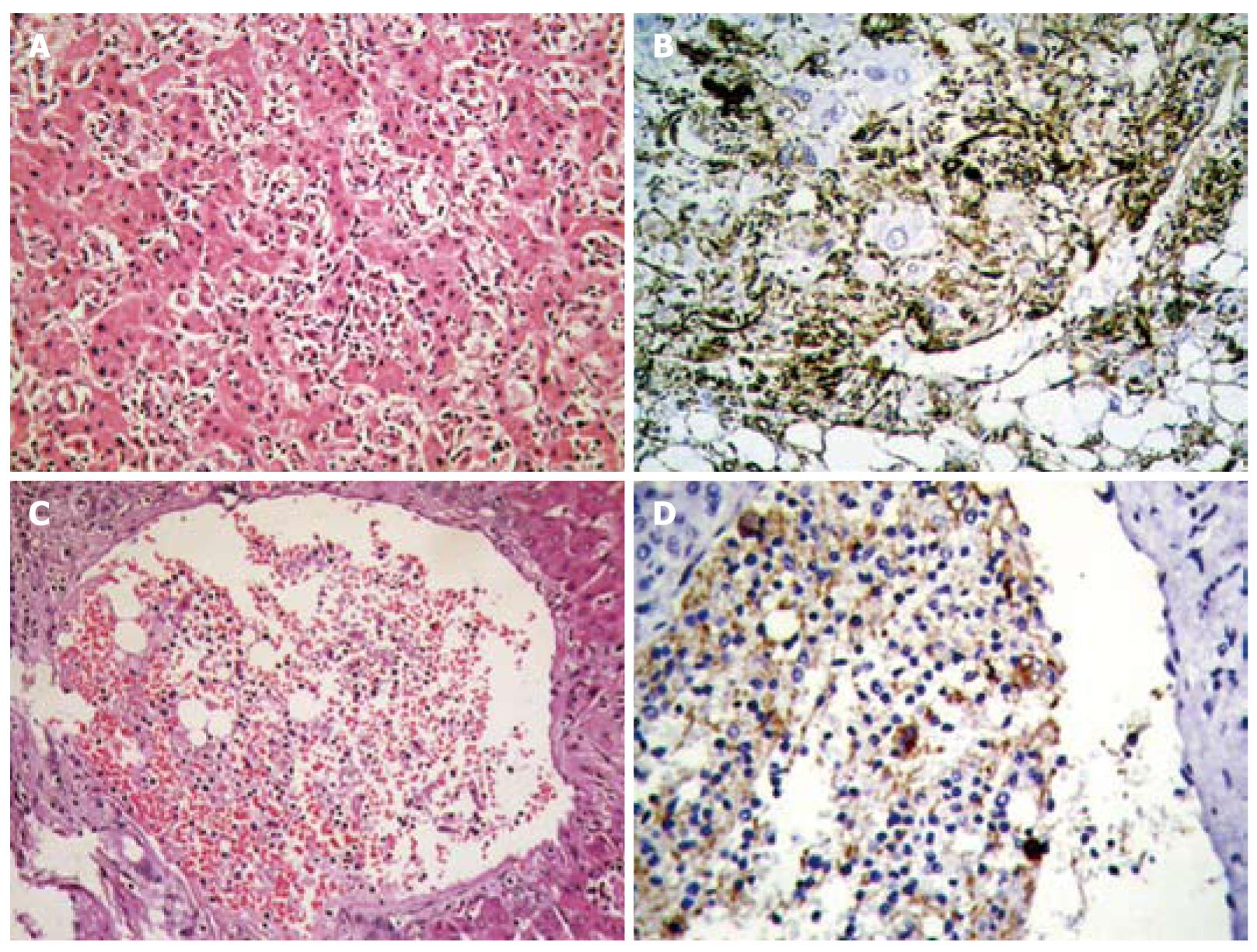
In 1993, we reported the first case of primary HAML in China. During the last 3 years of the study period, 85 cases of primary HAML and 66 cases of HCA were surgically resected at the EHBH. HAML is generally considered as a miscellaneous benign tumor; however, we find that some cases of HAML can show doubtful growth patterns, such as multi-focus, boundary infiltration along the sinusoids (Figure 2A and B), or even intravascular aggregation of conspicuous HMB45 positive cells (Figure 2C and D), which are similar to malignant behaviors. However, none of the 153 cases of HAML in the EHBH series showed evidence of malignant transformation or postoperative recurrence up to the time of the termination of this study. The presence of malignant HAML or malignant transformation of HAML[60,61] indicates that surgical excision should be considered as a preferred therapeutic, and a long-term follow-up after liver surgery is needed.
At least 41 malignant PHSOLs were reported, as summarized in Table 3[2,60-92]. In the EHBH series, malignant PHSOLs accounted for 83.6% (n = 26 684) of the cases, among which, HCC (n = 24 075, 90.2%) and intrahepatic cholangiocarcinoma (ICC, n = 2188, 8.2%) were the two most common malignant tumors. In contrast, undifferentiated embryonal sarcoma (UES, n = 34, 0.1%) and hepatoblastoma (HB, n = 33, 0.1%) ranked third, with a similar incidence.
| Hepatocellular tumors |
| Hepatocellular carcinoma[2] |
| Hepatoblastoma[2] |
| Combined hepatocellular and cholangiocarcinoma[2] |
| Intrahepatic bile duct tumors |
| Intrahepatic cholangiocarcinoma[2] |
| Cholangiolocellular carcinoma[62] |
| Bile duct cystadenocarcinoma[2] |
| Biliary rhabdomyosarcoma[63] |
| Solid-pseudopapilary tumor[64] |
| Vascular, lymphoid and haemopoietic tumors |
| Angiosarcoma[2] |
| Malignant angiomyolipoma[60]/malignant perivascular epithelioid |
| cell tumor[61] |
| Malignant hemangiopericytoma[65] |
| Epithelioid hemangioendothelioma[2] |
| Kaposi’s sarcoma[2] |
| Lymphoma[2] |
| Follicular dendritic cell sarcoma/tumor[66] |
| Extramedullary plasmacytoma[67] |
| Muscle, fibrous and adipose tumors |
| Leiomyosarcoma[68] |
| Rhabdomyosarcoma[69] |
| Fibrosarcoma[70] |
| Malignant fibrous histocytoma[71] |
| Liposarcoma[72] |
| Neuronal and neuroendocrine tumors |
| Carcinoid tumor[73] |
| Malignant neurilemmoma[74] |
| Miscellaneous tumors |
| Undifferentiated embryonal sarcoma[75] |
| Undifferentiated carcinoma[76] |
| Carcinosarcoma[2] |
| Lymphoepithelioma-like carcinoma[77] |
| Squamous cell carcinoma[78] |
| Germ cell tumor[79] |
| Chorioepithelioma[80] |
| Yolk sac tumor[81] |
| Immature teratoma[82] |
| Malignant rhabdoid tumor[83] |
| Malignant mesothelioma[84] |
| Synovial sarcoma[85] |
| Epithelial-myoepithelial carcinoma[86] |
| Gastrointestinal stromal tumor[87] |
| Osteosarcoma[88] |
| Osteoclast-like giant cell tumor[89] |
| Desmoplastic small round cell tumor[90] |
| Nested stromal-epithelial tumor[91]/ossifying stromal epithelial tumor[92] |
Histopathologically, HCC, which comprises more than 10 histological varieties[6], is always the central point of differentiated diagnoses among PHSOLs and metastatic tumors. We propose that CD34 immunostaining is one of the most effective methods to distinguish well-differentiated HCC from benign hepatocellular tumors (Figure 1)[6,26]. When HCC appears as a tubular-like arrangement, with solid nest structures and a pseudoglandular pattern, it is difficult to distinguish from ICC or metastatic adenocarcinomas. Based on scanning a panel of immunohistochemical markers, we propose that, for the diagnosis of HCC, Hep Par 1, CD34, and polyclonal CEA are first-line antibodies, and CK19 and MUC-1 are first-line antibodies for ICC[30,31].
UES is a unique hepatic malignant tumor that usually affects the pediatric population. To the best of our knowledge, only 70 cases of UES in adults have been reported worldwide[75,93]. Histologically, UES is characterized by a huge hemorrhagic mass and is composed of pleomorphic cells with eosinophilic cytoplasmic globules entrapped in a loose myxoid stroma[75]. Among 34 cases of UES in the EHBH series, 32.4% (n = 11) and 29.4% (n = 10) occurred in patients less than 12 and older than 50 years of age (range 5-70 years), respectively, and 32.4% (n = 11) had hepatitis B virus (HBV) infection, suggesting a possible causal link between chronic HBV infection and UES development.
The incidence of primary hepatic lymphoma (PHL, 0.09%, n = 23) was similar to that of UES and HB (0.1%). It has been reported that hepatitis C virus (HCV) plays a role in the pathogenesis of lymphoma, with an HCV prevalence rate of 9% to 42%, especially in Western countries[94]. In contrast, the prevalence of HCV in our patients with PHL was only 4.3% (1 of 23 cases), whereas 56.5% (13 of 23) were positive for HBV, and three of them underwent surgical resections for simultaneous coexistence of PHL with HCC as two independent masses in the liver. Thus, we hypothesize that HBV, as a kind of lymphotropic virus, may play an important pathogenic role in the development of PHL in China.
In summary, based on the large number of surgically resected PHSOLs in the EHBH series, we propose a comprehensive surgicopathological classification system that comprises more than 100 kinds of PHSOLs, with three basic types and six subtypes. Our classification system covers all the entities in the new histological classification system generated by the WHO, which included about 30 kinds of PHSOLs, except for microscopic cellular abnormalities[26]. We do not describe details concerning molecular genetics, diagnostic criteria, biological behaviors, treatment strategies, and clinical prognoses for each PHSOL, as they can be found in the given references. Although it is still possible that new types of PHSOLs will be discovered, we think that the above brief summary may provide useful information as a new classification system and current reference for clinicians and pathologists to understand the features of histological spectrum, as well as the differential diagnostic features, of PHSOLs.
Peer reviewers: Kuniya Tanaka, MD, PhD, Professor, Department of Gastroenterological Surgery, Yokohama City University, 3-9 Fukuura, Kanazawaku, Yokohama, Ktrj 112, Japan; Toshifumi Wakai, MD, PhD, Division of Digestive and General Surgery, Niigata University Graduate School of Medical and Dental Sciences, 1-757 Asahimachi-dori, Chuo-ku, Niigata City 951-8510, Japan
S- Editor Sun H L- Editor Stewart GJ E- Editor Zheng XM
| 1. | Cong WM, Wu MC. More emphasis on pathobiological features of hepatic tumors. Zhonghua Waike Zazhi. 2010;48:1121-1124. |
| 2. | Hirohashi S, Blum HE, Ishak KG, Deugnier Y, Kojiro M, Puig PL, Wanless IR, Fischer HP, Theise ND, Sakamoto M. Tumours of the Liver and Intrahepatic Bile Ducts. Pathology and Genetics of Tumours of the Digestive System. 3rd ed. Lyon: IARC Press 2000; 158-202. |
| 3. | Kondo F. Benign nodular hepatocellular lesions caused by abnormal hepatic circulation: etiological analysis and introduction of a new concept. J Gastroenterol Hepatol. 2001;16:1319-1328. |
| 4. | Tsuzuki T, Hoshino Y, Uchiyama T, Kitazima M, Mikata A. Compensatory hypertrophy of the lateral quadrant of the left hepatic lobe due to atrophy of the rest of the liver, appearing as a mass in the left upper quadrant of the abdomen: report of a case. Ann Surg. 1973;177:406-410. |
| 5. | Massaro M, Valencia MP, Guzman M, Mejia J. Accessory hepatic lobe mimicking an intra-abdominal tumor. J Comput Assist Tomogr. 2007;31:572-573. |
| 6. | Cong WM, Zhu SN. Diagnostic Surgical Pathology of Hepatobiliary Tumors. Shanghai: Shanghai Science and Technology Education 2002; . |
| 7. | Sharma S, Dean AG, Corn A, Kohli V, Wright HI, Sebastian A, Jabbour N. Ciliated hepatic foregut cyst: an increasingly diagnosed condition. Hepatobiliary Pancreat Dis Int. 2008;7:581-589. |
| 8. | Chiu B, Melin-Aldana H, Superina RA. Management of an epidermoid cyst of the intrahepatic ducts. J Pediatr Surg. 2005;40:e31-e33. |
| 9. | Huang WT, Chen WJ, Chen CL, Cheng YF, Wang JH, Eng HL. Endometrial cyst of the liver: a case report and review of the literature. J Clin Pathol. 2002;55:715-717. |
| 10. | Komori K, Hoshino K, Shirai J, Morikawa Y. Mesothelial cyst of the liver in a neonate. Pediatr Surg Int. 2008;24:463-465. |
| 11. | Czermak BV, Akhan O, Hiemetzberger R, Zelger B, Vogel W, Jaschke W, Rieger M, Kim SY, Lim JH. Echinococcosis of the liver. Abdom Imaging. 2008;33:133-143. |
| 12. | Trivedi PJ, Gupta P, Phillips-Hughes J, Ellis A. Biloma: an unusual complication in a patient with pancreatic cancer. World J Gastroenterol. 2009;15:5218-5220. |
| 13. | Machida T, Takahashi T, Itoh T, Hirayama M, Morita T, Horita S. Reactive lymphoid hyperplasia of the liver: a case report and review of literature. World J Gastroenterol. 2007;13:5403-5407. |
| 14. | Koea J, Taylor G, Miller M, Rodgers M, McCall J. Solitary necrotic nodule of the liver: a riddle that is difficult to answer. J Gastrointest Surg. 2003;7:627-630. |
| 15. | Wannesson L, Chigrinova E, Raditchkova M, Mazzucchelli L, Ghielmini M. Peliosis hepatis in cancer patients mimicking infection and metastases. Onkologie. 2009;32:54-56. |
| 16. | Khalid SK, Garcia-Tsao G. Hepatic vascular malformations in hereditary hemorrhagic telangiectasia. Semin Liver Dis. 2008;28:247-258. |
| 17. | Pungpapong S, Steers JL, Wallace MB, Krishna M, Keaveny AP. Hepatobiliary sarcoidosis mimicking Klatskin’s cholangiocarcinoma. Gastrointest Endosc. 2006;64:124-125. |
| 18. | Tamiolakis D, Venizelos J, Prassopoulos P, Simopoulos S, Bolioti S, Tsiapali M, Papadopoulos N. Intrahepatic extramedullary hematopoietic tumor mimicking metastatic carcinoma from a colonic primary. Onkologie. 2004;27:65-67. |
| 19. | Lublin M, Bartlett DL, Danforth DN, Kauffman H, Gallin JI, Malech HL, Shawker T, Choyke P, Kleiner DE, Schwartzentruber DJ. Hepatic abscess in patients with chronic granulomatous disease. Ann Surg. 2002;235:383-391. |
| 20. | Brookes MJ, Field M, Dawkins DM, Gearty J, Wilson P. Massive primary hepatic tuberculoma mimicking hepatocellular carcinoma in an immunocompetent host. MedGenMed. 2006;8:11. |
| 21. | Omar T, Cooper K. Botryomycosis of the liver. Histopathology. 1995;27:71-73. |
| 22. | Robertson SJ, Higgins RB, Powell C. Malacoplakia of liver: a case report. Hum Pathol. 1991;22:1294-1295. |
| 23. | Chun JM, Hwang YJ, Kim JY, Suh IS, Kim YI. Intrahepatic splenic tissue without medical history of splenic injury or splenectomy. Hepatogastroenterology. 2007;54:944-945. |
| 24. | Baba Y, Beppu T, Imai K, Masuda T, Iyama K, Sasano H, Baba H. A case of adrenal rest tumor of the liver: Radiological imaging and immunohistochemical study of steroidogenic enzymes. Hepatol Res. 2008;38:1154-1158. |
| 25. | Lamps LW. Hepatic granulomas, with an emphasis on infectious causes. Adv Anat Pathol. 2008;15:309-318. |
| 26. | Bosman FT, Carneiro F, Hruban RH, Theise ND, editors . WHO Classification of tumours of the digestive system. 4th ed. IARC: Lyon 2010; 196-261. |
| 27. | Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology. 2006;44:7-14. |
| 28. | Rebouissou S, Bioulac-Sage P, Zucman-Rossi J. Molecular pathogenesis of focal nodular hyperplasia and hepatocellular adenoma. J Hepatol. 2008;48:163-170. |
| 29. | Zhang SH, Cong WM, Wu MC. Focal nodular hyperplasia with concomitant hepatocellular carcinoma: a case report and clonal analysis. J Clin Pathol. 2004;57:556-559. |
| 30. | Cong W, Tan L, Zhang S, Xian Z, Wu W, Pan J, Zhang X. [Immunohistochemical spectrum in the detection and differentiation of intrahepatic neoplasms]. Zhonghua Zhongliu Za Zhi. 2002;24:553-556. |
| 31. | Dong H, Cong WL, Zhu ZZ, Wang B, Xian ZH, Yu H. [Evaluation of immunohistochemical markers for differential diagnosis of hepatocellular carcinoma from intrahepatic cholangiocarcinoma]. Zhonghua Zhongliu Za Zhi. 2008;30:702-705. |
| 32. | Wang HL, Anatelli F, Zhai QJ, Adley B, Chuang ST, Yang XJ. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med. 2008;132:1723-1728. |
| 33. | Greaves WO, Bhattacharya B. Hepatic adenomatosis. Arch Pathol Lab Med. 2008;132:1951-1955. |
| 34. | Tabibian JH, Lassman CR, Margolis DJ, Landaverde C, Busuttil RW, Durazo FA. Intraductal oncocytic papillary neoplasm of the liver: case and review of a rare variant. Ann Hepatol. 2008;7:168-173. |
| 35. | Varnholt H, Vauthey JN, Dal Cin P, Marsh Rde W, Bhathal PS, Hughes NR, Lauwers GY. Biliary adenofibroma: a rare neoplasm of bile duct origin with an indolent behavior. Am J Surg Pathol. 2003;27:693-698. |
| 36. | Zimmermann A, von der Brelie C, Berger B, Kappeler A, Candinas D. Primary perivascular epithelioid cell tumor of the liver not related to hepatic ligaments: hepatic PEComa as an emerging entity. Histol Histopathol. 2008;23:1185-1193. |
| 37. | Rojiani AM, Owen DA, Berry K, Woodhurst B, Anderson FH, Scudamore CH, Erb S. Hepatic hemangioblastoma. An unusual presentation in a patient with von Hippel-Lindau disease. Am J Surg Pathol. 1991;15:81-86. |
| 38. | Belli G, Ciciliano F, Lannelli A, Marano I. Hepatic resection for primary giant leiomyoma of the liver. HPB (Oxford). 2001;3:11-12. |
| 39. | Martí-Bonmatí L, Menor F, Vizcaino I, Vilar J. Lipoma of the liver: US, CT, and MRI appearance. Gastrointest Radiol. 1989;14:155-157. |
| 40. | Nishizaki T, Kanematsu T, Matsumata T, Yasunaga C, Kakizoe S, Sugimachi K. Myelolipoma of the liver. A case report. Cancer. 1989;63:930-934. |
| 41. | Lee WH, Kim TH, You SS, Choi SP, Min HJ, Kim HJ, Lee OJ, Ko GH. Benign schwannoma of the liver: a case report. J Korean Med Sci. 2008;23:727-730. |
| 42. | Malagari K, Drakopoulos S, Brountzos E, Sissopulos A, Efthimidadou A, Hadjiyiannakis E, Kelekis D. Plexiform neurofibroma of the liver: findings on mr imaging, angiography, and CT portography. AJR Am J Roentgenol. 2001;176:493-495. |
| 43. | Ghalib R, Howard T, Lowell J, Huettner P, Whelan A, Teefey S, Peters M, White H. Plexiform neurofibromatosis of the liver: case report and review of the literature. Hepatology. 1995;22:1154-1157. |
| 44. | Roman SA, Sosa JA. Functional paragangliomas presenting as primary liver tumors. South Med J. 2007;100:195-196. |
| 45. | Wu JS, Ahya SN, Reploeg MD, Singer GG, Brennan DC, Howard TK, Lowell JA. Pheochromocytoma presenting as a giant cystic tumor of the liver. Surgery. 2000;128:482-484. |
| 46. | Shibata C, Naito H, Funayama Y, Fukushima K, Takahashi K, Unno M, Sasaki I. Diagnosis and surgical treatment for primary liver gastrinoma: report of a case. Dig Dis Sci. 2006;51:1122-1125. |
| 47. | Lundstedt C, Linjawi T, Amin T. Liver VIPoma: report of two cases and literature review. Abdom Imaging. 1994;19:433-437. |
| 48. | Morisawa Y, Tanaka A, Yamamoto T, Uegaki S, Takamori Y, Ishii T, Kuyama Y, Fukusato T, Shiga J, Takikawa H. Primary hepatic somatostatinoma developed in a patient with von Recklinghausen’s disease. J Gastroenterol. 2006;41:389-391. |
| 49. | Certo M, Franca M, Gomes M, Machado R. Liver teratoma. Acta Gastroenterol Belg. 2008;71:275-279. |
| 50. | Flemming P, Becker T, Klempnauer J, Högemann D, Kreft A, Kreipe HH. Benign cystic mesothelioma of the liver. Am J Surg Pathol. 2002;26:1523-1527. |
| 51. | Bohra AK, Diamond T. Endometrioma of the liver. Int J Clin Pract. 2001;55:286-287. |
| 52. | Fried RH, Wardzala A, Willson RA, Sinanan MN, Marchioro TL, Haggitt R. Benign cartilaginous tumor (chondroma) of the liver. Gastroenterology. 1992;103:678-680. |
| 53. | Blumgart LH, Fong Y, Jarnagin WR. Hepatobiliary Cancer. London: Pmph Bc Decker 2001; . |
| 54. | Jaffe R. Liver involvement in the histiocytic disorders of childhood. Pediatr Dev Pathol. 2004;7:214-225. |
| 55. | Hill DA, Swanson PE, Anderson K, Covinsky MH, Finn LS, Ruchelli ED, Nascimento AG, Langer JC, Minkes RK, McAlister W. Desmoplastic nested spindle cell tumor of liver: report of four cases of a proposed new entity. Am J Surg Pathol. 2005;29:1-9. |
| 56. | Kaiserling E, Müller H. Neoplasm of hepatic stellate cells (spongiotic pericytoma): a new tumor entity in human liver. Pathol Res Pract. 2005;201:733-743. |
| 57. | Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, Scoggins CR, Martin RC, D’Angelica M, Staley CA, Choti MA. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol. 2009;16:640-648. |
| 58. | Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515-524. |
| 59. | Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G, Trillaud H. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481-489. |
| 60. | Nguyen TT, Gorman B, Shields D, Goodman Z. Malignant hepatic angiomyolipoma: report of a case and review of literature. Am J Surg Pathol. 2008;32:793-798. |
| 61. | Parfitt JR, Bella AJ, Izawa JI, Wehrli BM. Malignant neoplasm of perivascular epithelioid cells of the liver. Arch Pathol Lab Med. 2006;130:1219-1222. |
| 62. | Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544-1556. |
| 63. | Ali S, Russo MA, Margraf L. Biliary rhabdomyoscarcoma mimicking choledochal cyst. J Gastrointestin Liver Dis. 2009;18:95-97. |
| 64. | Ishak KG, Goodman ZD, Stocker JT. Tumors of the Liver and Intrahepatic Bile Ducts. Washington, DC: American Registry of Pathology 2001; . |
| 65. | Hozo I, Miric D, Bojic L, Giunio L, Lusic I, Culic V, Simunic M. Liver angiosarcoma and hemangiopericytoma after occupational exposure to vinyl chloride monomer. Environ Health Perspect. 2000;108:793-795. |
| 66. | Torres U, Hawkins WG, Antonescu CR, DeMatteo RP. Hepatic follicular dendritic cell sarcoma without Epstein-Barr virus expression. Arch Pathol Lab Med. 2005;129:1480-1483. |
| 67. | Demirhan B, Sökmensüer C, Karakayali H, Güngen Y, Doğan A, Haberal M. Primary extramedullary plasmacytoma of the liver. J Clin Pathol. 1997;50:74-76. |
| 68. | Tsiatis AC, Atkinson JB, Wright JK, Cates JM. Primary hepatic myxoid leiomyosarcoma: a case report and review of the literature. Ultrastruct Pathol. 2008;32:25-28. |
| 69. | Hawkins WG, Hoos A, Antonescu CR, Urist MJ, Leung DH, Gold JS, Woodruff JM, Lewis JJ, Brennan MF. Clinicopathologic analysis of patients with adult rhabdomyosarcoma. Cancer. 2001;91:794-803. |
| 70. | Nakahama M, Takanashi R, Yamazaki I, Machinami R. Primary fibrosarcoma of the liver. Immunohistochemical and electron microscopic studies. Acta Pathol Jpn. 1989;39:814-820. |
| 71. | Li YR, Akbari E, Tretiakova MS, Hart J, Akbari M, Urbanski SJ, Gao ZH. Primary hepatic malignant fibrous histiocytoma: clinicopathologic characteristics and prognostic value of ezrin expression. Am J Surg Pathol. 2008;32:1144-1158. |
| 72. | Kuo LM, Chou HS, Chan KM, Yu MC, Lee WC. A case of huge primary liposarcoma in the liver. World J Gastroenterol. 2006;12:1157-1159. |
| 73. | Fenwick SW, Wyatt JI, Toogood GJ, Lodge JP. Hepatic resection and transplantation for primary carcinoid tumors of the liver. Ann Surg. 2004;239:210-219. |
| 74. | Fiel MI, Schwartz M, Min AD, Sung MW, Thung SN. Malignant schwannoma of the liver in a patient without neurofibromatosis: a case report and review of the literature. Arch Pathol Lab Med. 1996;120:1145-1147. |
| 75. | Nishio J, Iwasaki H, Sakashita N, Haraoka S, Isayama T, Naito M, Miyayama H, Yamashita Y, Kikuchi M. Undifferentiated (embryonal) sarcoma of the liver in middle-aged adults: smooth muscle differentiation determined by immunohistochemistry and electron microscopy. Hum Pathol. 2003;34:246-252. |
| 76. | Nakasuka H, Okada S, Okusaka T, Ishii H, Ikeda M, Ito R, Kosakamoto H, Yoshimori M, Nakanishi Y, Sakamoto M. Undifferentiated carcinoma of the liver with neuroendocrine features: a case report. Jpn J Clin Oncol. 1998;28:401-404. |
| 77. | Si MW, Thorson JA, Lauwers GY, DalCin P, Furman J. Hepatocellular lymphoepithelioma-like carcinoma associated with epstein barr virus: a hitherto unrecognized entity. Diagn Mol Pathol. 2004;13:183-189. |
| 78. | Yuki N, Hijikata Y, Kato M, Kawahara K, Wakasa K. Squamous cell carcinoma as a rare entity of primary liver tumor with grave prognosis. Hepatol Res. 2006;36:322-327. |
| 79. | Theegarten D, Reinacher A, Graeven U, Philippou S. Mixed malignant germ cell tumour of the liver. Virchows Arch. 1998;433:93-96. |
| 80. | van der Hoef M, Niggli FK, Willi UV, Huisman TA. Solitary infantile choriocarcinoma of the liver: MRI findings. Pediatr Radiol. 2004;34:820-823. |
| 81. | Gilbert KL, Bergman S, Dodd LG, Volmar KE, Creager AJ. Cytomorphology of yolk sac tumor of the liver in fine-needle aspiration: a pediatric case. Diagn Cytopathol. 2006;34:421-423. |
| 82. | Cöl C. Immature teratoma in both mediastinum and liver of a 21-Year-old female patient. Acta Med Austriaca. 2003;30:26-28. |
| 83. | Yuri T, Danbara N, Shikata N, Fujimoto S, Nakano T, Sakaida N, Uemura Y, Tsubura A. Malignant rhabdoid tumor of the liver: case report and literature review. Pathol Int. 2004;54:623-629. |
| 84. | Kim DS, Lee SG, Jun SY, Kim KW, Ha TY, Kim KK. Primary malignant mesothelioma developed in liver. Hepatogastroenterology. 2008;55:1081-1084. |
| 85. | Srivastava A, Nielsen PG, Dal Cin P, Rosenberg AE. Monophasic synovial sarcoma of the liver. Arch Pathol Lab Med. 2005;129:1047-1049. |
| 86. | Tsuneyama K, Hoso M, Kono N, Kitagawa M, Masuda S, Matsuki N, Nakanuma Y. An unusual case of epithelial-myoepithelial carcinoma of the liver. Am J Surg Pathol. 1999;23:349-353. |
| 87. | Hu X, Forster J, Damjanov I. Primary malignant gastrointestinal stromal tumor of the liver. Arch Pathol Lab Med. 2003;127:1606-1608. |
| 88. | Govender D, Rughubar KN. Primary hepatic osteosarcoma: case report and literature review. Pathology. 1998;30:323-325. |
| 89. | Horie Y, Hori T, Hirayama C, Hashimoto K, Yumoto T, Tanikawa K. Osteoclast-like giant cell tumor of the liver. Acta Pathol Jpn. 1987;37:1327-1335. |
| 90. | Ordóñez NG. Desmoplastic small round cell tumor: I: a histopathologic study of 39 cases with emphasis on unusual histological patterns. Am J Surg Pathol. 1998;22:1303-1313. |
| 91. | Brodsky SV, Sandoval C, Sharma N, Yusuf Y, Facciuto ME, Humphrey M, Yeh YA, Braun A, Melamed M, Finegold MJ. Recurrent nested stromal epithelial tumor of the liver with extrahepatic metastasis: case report and review of literature. Pediatr Dev Pathol. 2008;11:469-473. |
| 92. | Heywood G, Burgart LJ, Nagorney DM. Ossifying malignant mixed epithelial and stromal tumor of the liver: a case report of a previously undescribed tumor. Cancer. 2002;94:1018-1022. |
| 93. | Lenze F, Birkfellner T, Lenz P, Hussein K, Länger F, Kreipe H, Domschke W. Undifferentiated embryonal sarcoma of the liver in adults. Cancer. 2008;112:2274-2282. |
| 94. | Bronowicki JP, Bineau C, Feugier P, Hermine O, Brousse N, Oberti F, Rousselet MC, Dharancy S, Gaulard P, Flejou JF. Primary lymphoma of the liver: clinical-pathological features and relationship with HCV infection in French patients. Hepatology. 2003;37:781-787. |