INTRODUCTION
Treating chronic pain remains a significant clinical challenge, and in particular, current treatments for visceral pain conditions are still based mainly on empirical rather than mechanistic evidence. Search for a consistently effective pharmacological agent to treat these pain conditions remain elusive. Functional gastrointestinal (GI) pain, one of the most prevalent forms of chronic visceral pain, is commonly interpreted as a consequence of hypersensitivity of GI nociceptive pathways, either of the peripheral sensory nociceptors in the gut or of the central neurons[1,2]. Human and animal studies have identified several channels as pivotal for enhanced signal transmission along the pain axis, including voltage-gated sodium channels (VGSCs) Nav1.3, Nav1.7, Nav1.8, and Nav1.9, with the latter three preferentially expressed in peripheral sensory neurons[3-5]. VGSCs are integral membrane glycoproteins that are essential for generation and conduction of electrical impulses in excitable cells. Many of the most common neurological disorders, such as epilepsy, migraine, neurodegenerative diseases, and chronic pain involve abnormalities of neuronal excitability[6,7]. There is a growing body of evidence that implicates abnormal expression and function of VGSCs in these disorders[8-12]. Although there is an incomplete understanding of these channels and/or primary site of action at which these compounds exert their effects and significant side effects are often encountered, there is enough evidence emerging to give hope that a more refined approach may be achievable[13]. This article does not aim to provide an exhaustive list of potential targets, but to focus on some of the most recent advances in the field of pain research and medicine and to highlight where therapeutic strategies for relieving visceral pain may emerge in the near future.
BIOPHYSICAL AND MOLECULAR PROPERTIES OF VGSCS
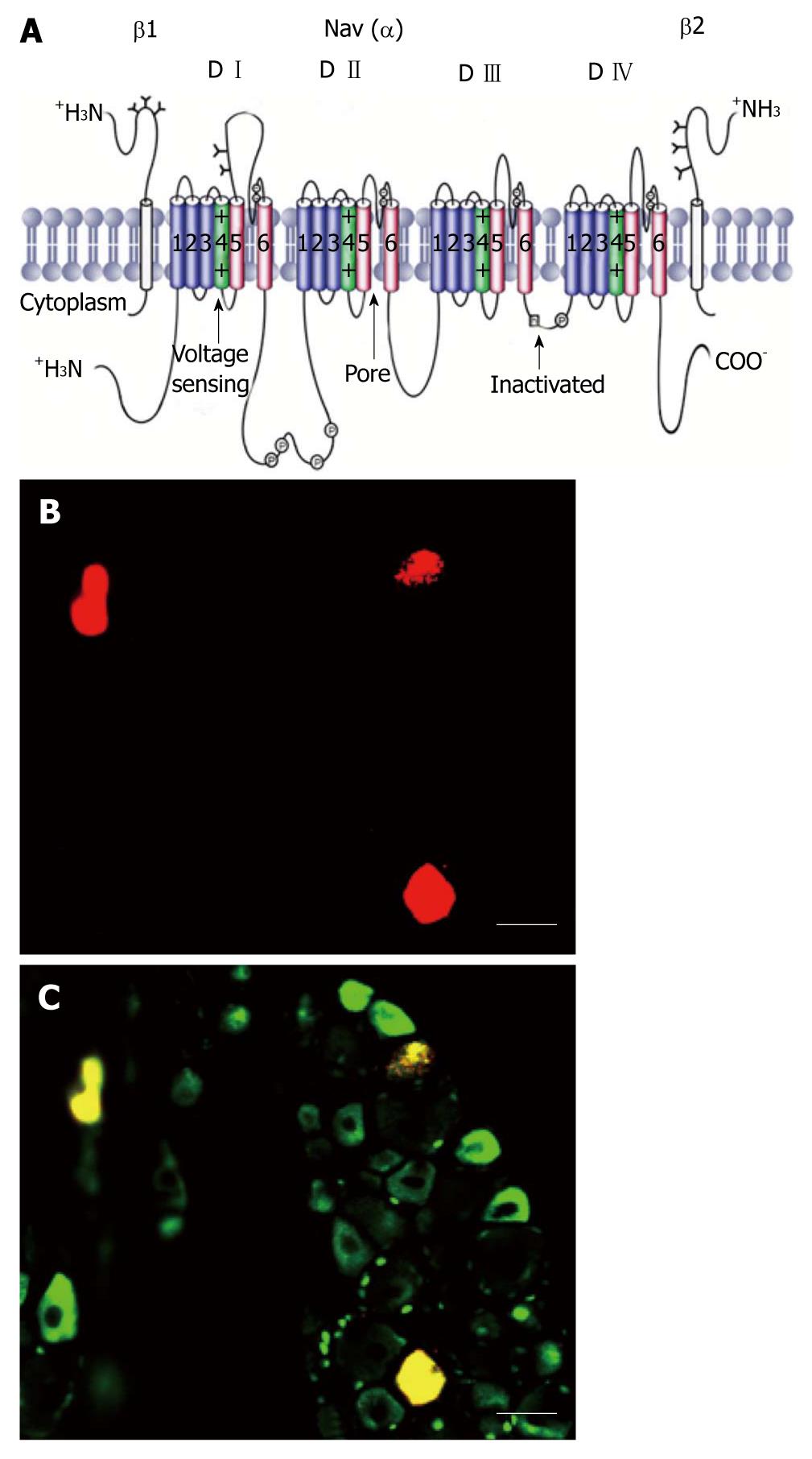
VGSCs are integral membrane glycoproteins that consist of a central α-subunit of 260 kDa associated with one or more auxiliary β-subunits of about 35 kDa (Figure 1A)[5,14]. Nine α-subunit subtypes have been cloned and functionally expressed. These subtypes are designated Nav1.1-Nav1.9 for the proteins and SCN1A-SCN5A and SCN8A-SCN11A for the genes; SCN6A/SCN7A codifies the related protein Nax. The VGSC α-subunit, which forms the ion-conducting pore and the channel gate for activation and inactivation, contains four domains (D I-D IV), each with six α-helical transmembrane segments labeled S1-S6 (Figure 1A). Pore loops between S5 and S6 (highlighted in red) in each of the four domains form the selectivity filter of the channel. Each pore loop contributes a single amino acid (aspartate from D I, glutamate from D II, lysine from D III, and alanine from D IV), which together form a narrow ring that is mainly responsible for conferring Na+ selectivity. The four S6 segments form the cytoplasmic end of the pore, which binds various types of therapeutically important pore-blocking compounds, including local anesthetics, and antiarrhythmic drugs. The S4 segments (highlighted in green) in each of the four domains contain regularly spaced, positively charged amino acid residues and serve as voltage-sensors, coupling membrane depolarization to channel activation. The intracellular loop between D III and D IV forms the fast-inactivation gate that occludes the cytoplasmic end of the pore when the channel inactivates. The C-terminal cytoplasmic domain is important for setting some of the properties of fast inactivation and contains binding sites for interacting proteins. In addition to fast inactivation, a distinct process called slow inactivation develops during prolonged depolarizing plateaus and during high frequency repetitive firing. The kinetics of onset and recovery of slow inactivation are about four orders of magnitude slower than those of fast inactivation. Slow inactivation does not depend on the fast inactivation gate formed by the intracellular loop between D III and D IV, but instead mainly involves rearrangements of the pore of the channel[15].
Figure 1 Schematic of a voltage-gated sodium channel polypeptide and Nav1.
8 positive colon specific dorsal root ganglion neurons. A: The transmembrane organization includes four domains (D I-D IV) joined by three intracellular loops (L1-L3) and the intracellular N and C termini. Cylinders represent probable α-helical segments. P in circles, sites of demonstrated protein phosphorylation by protein kinase A (PKA) or PKC; green, S4 voltage sensors; h in which square, inactivation particle in the inactivation gate loop (modified, with permission, from Catterall[14]); B: Colon specific dorsal root ganglion neurons labeled by Dil (red); C: Nav1.8 positive neurons (green). Colon specific Nav1.8 positive neurons are stained yellow. Bar = 50 μm for both B and C.
VGSCS IN PRIMARY SENSORY NEURONS INNERVATING VISCERAL ORGANS
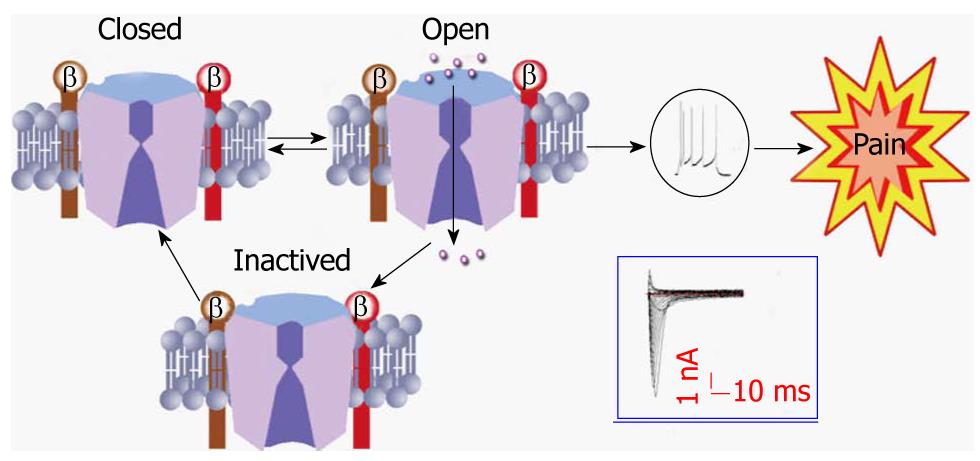
Primary sensory neurons, with their cell bodies located in the dorsal root ganglion (DRG), are the first link in the somatosensory pathway. They encode their messages in the form of a series of action potentials whose depolarizing upstroke is produced by sodium channels[3-5,16]. Most neuroscientists and neurologists are familiar with the textbook description of VGSC function (Figure 2). VGSCs contribute to the control of membrane excitability and underlie action potential generation. They are closed at resting membrane potentials characteristic of quiescent neurons. In response to membrane depolarization, they open within a few hundred microseconds (a process termed activation), resulting in an inward sodium ion (Na+) current, and then convert within a few milliseconds to a non-conducting inactivated state through a process called fast inactivation. Transient Na+ influx through thousands of rapidly opening and inactivating VGSCs results in the familiar transient macroscopic Na+ current detected in whole-cell voltage clamp studies[16]. This transient current gives rise to the depolarizing phase of the action potential in neurons, and eventually causes the sensation of pain (Figure 2).
Figure 2 A model showing the three states of the sodium channel.
Tissue damage/inflammation leads to activation of the sodium channel from a closed state. Once activated, sodium ions entered through the channel and evoke the action potential of the cell membrane, eventually producing the sensation of pain. Once the channel becomes inactivated, it must go to a closed state before being activated again. Action potentials and inward currents were recorded from a colon specific dorsal root ganglion neuron from a healthy rat.
Distinct distribution
Expression of the different subtypes of VGSCs is tissue- and/or cell-specific. The main subtypes expressed in adult brain neurons are Nav1.6. Nav1.2 in unmyelinated axons and in myelinated axons early in development (before being replaced by Nav1.6), and Nav1.1 in the neuronal somata. In rodents, Nav1.3 is expressed primarily in embryonic neurons; however, expression in the human central nervous system remains comparatively high into adulthood. Nav1.4 is the main subtype in adult skeletal muscle, whereas Nav1.5 is expressed in cardiac muscle, and it is also expressed in some neurons. In DRG neurons, Nav1.6 is the major sodium channel isoform at the nodes of Ranvier[17], is present in unmyelinated fibers in the sciatic nerve[18,19] and is preferentially expressed by TrkC (tyrosine receptor kinase C) neurons. Nav1.7, Nav1.8, and Nav1.9 are found in peripheral primary sensory afferents. They are more abundant in C-fiber neurons[16,20]. Small diameter DRG neurons can be broadly classified into isolectin B4 (IB4)(+) and IB4(-) neurons. James Brock had reviewed sensory and biophysical properties differences between IB4(+) and IB4(-) neurons[21]. Our preliminary experiments showed that most colon specific DRG neurons are Nav1.8 positive (Figure 1B and C).
Developmentally regulated expression
Expression of the different subtypes is developmentally regulated in DRG neurons[22-24]. Nav1.3 is overexpressed at embryonic day E1, peaks at E17, is reduced by postnatal day P15, and is not detectable by P30. Nav1.6 is upregulated from E17 only in large neurons. Expression of both Nav1.8 and Nav1.9 increases with age, beginning at E15 and E17, respectively, and reaching adult levels by P7. Their distribution is restricted mainly to those subpopulations of primary sensory neurons in developing and adult DRGs that give rise to unmyelinated C-fibers (neurofilament 200 negative). Nav1.8 is expressed in a higher proportion of neuronal profiles than Nav1.9 at all stages during development, as in the adult[24]. It is worthy of note that nerve injury would evoke expression of some of these channels that are not present in the sensory nerve system in the adult, and may contribute to abnormal pain sensation[20,25,26].
Expression in glial cells
Na-G, a distinct type of “glial” Na channel expressed at high levels in Schwann cells in vivo[27,28], was thought to play a role in regulation of cytoplasmic Na+ homoeostasis. However, recent studies show that action potential-like events have been recorded in glial precursor cells, astrocytes and a subset of oligodendrocyte precursor cells. More recently, Frieboes reported de novo expression of Nav1.8 in endoneurial Schwann cells following injury[10]. This is surprising since Nav1.8 expression is thought to be restricted to sensory neurons. No similar studies have been reported under pathophysiological conditions in visceral organs. Nevertheless, these data indicate a sodium channel in glial cells is linked to the development of neuropathic pain. Further investigation of the underlying molecular basis of VGSCs in glial cells and neural-glial interaction could yield promising targets for treatment of chronic pain.
VGSCS AND CHRONIC VISCERAL PAIN
There are relatively few published studies on roles of sodium channels in visceral pain and a uniform theme has not emerged. Saito et al[29] demonstrated a mutation in the cardiac sodium channel gene SCN5A in interstitial cells of Cajal (ICC) in irritable bowel syndrome (IBS) patients, suggesting SCN5A as a candidate gene in the pathophysiology of IBS. Additionally, Verne et al[30] showed there was a reduction in abdominal pain in IBS patients receiving intrarectal lidocaine, indicating a possible normalization of abnormal sodium channels in IBS patients. Yiangou et al[31] carried out an immunohistochemistry study on biopsies from patients with idiopathic rectal hypersensitivity which showed that the nerve fibers immunoreactive to the sodium channel Nav1.7 were significantly increased in this group compared with controls. Collectively, these clinical data suggest that voltage-gated sodium channels appear to play an important role in visceral pain[5,9].
Since no pharmacological tools to separate sodium currents are available, it is hard to relate the channel current to its molecular subtype. However, tetrodotoxin (TTX) is the most used drug to separate the TTX-S (sensitive) and TTX-R (resistant) sodium channel currents. Whole-cell patch-clamp recording of fast blue-labeled bladder afferent neurons demonstrated that the majority (70%) of bladder neurons that were small in size had capsaicin-sensitive TTX-R sodium channels[32,33]. More than 80% of gastric DRG neurons displayed TTX-R action potentials[34]. These data demonstrated that visceral sensory neurons expressed the TTX-R sodium channels that are thought to contribute to pain processing.
Sensitization of VGSCs
Several animal models have been used to determine the role for VGSCs under visceral pain conditions. These included the trinitrobenzenesulphonic acid (TNBS)-induced colitis, painful chronic pancreatitis, and acetic acid-induced gastric ulcers. TNBS-induced colitis significantly enhanced neuronal excitability of colon specific DRG neurons, which is associated with a significant potentiation of slow inactivating TTX-R Na+ current density in mice[35] and in guinea pigs[36]. Changes in expression of Nav1.7, 1.8 and 1.9 protein and mRNA during TNBS colitis were measured using Western blotting and quantitative polymerase chain reaction. On day 7 of colitis, there was a 3-fold increase in Nav1.8 protein in ganglia from T9-13, but Nav1.7 and 1.9 levels remained unchanged. Surprisingly, there was no corresponding change in the Nav1.8 α-subunit mRNA levels[37], indicating that the TNBS specifically evoked an increase in the numbers of Nav1.8 channels, which contributed to an increased current. In a rat model of gastric hyperalgesia, Gebhart and colleagues reported that ingestion of iodoacetamide increased the peak TTX-R sodium current which was associated with a left-shifted voltage-dependent activation in DRG neurons[38]. In a rat model of painful chronic pancreatitis, however, no change in sodium peak current density was observed in pancreas-specific DRG neurons (Xu et al[39], unpublished data) although there was an alteration in voltage-gated potassium channel function in the same model. Although the data from different models are complicated and somehow controversial, they point out the fact that changes in function and expression of VGSCs are organ or/and disease specific.
As mentioned previously, no pharmacological tools to separate TTX-R currents are available. However, researchers have employed antisense oligodeoxynucleotide or Nav1.8 and Nav1.9 null mice to evaluate their current roles in animal models of visceral hypersensitivity. Inhibition of expression of Nav1.8 (PN3/SNS) sodium channels by an antisense oligodeoxynucleotide injected intrathecally suppressed bladder hyperactivity and c-fos expression in the spinal cord induced by chemical irritation of the urinary bladder in rats[32]. In Nippostrongylus brasiliensis infection-induced transient jejunitis, hyperexcitability was absent in Nav1.8-/- mice[40]. Further experiments showed that Nav1.8-null mice displayed normal nociceptive behavior provoked by acute noxious stimulation of abdominal viscera. Nav1.8-null mice also showed weak pain and no referred hyperalgesia to intracolonic capsaicin and mustard oil[41]. In a model of cyclophosphamide-induced bladder cystitis, Nav1.8 null mice showed normal responses. These data suggest that Nav1.8 plays an essential role in mediating spontaneous activity in sensitized nociceptors. However, the role for Nav1.9 is inconsistent. Hillsley et al[40] reported that Nav1.9–/– mice maintained hyperexcitability in Nippostrongylus brasiliensis infection-induced transient jejunitis. On the other hand, Martinez reported that R-848 (the toll-like receptor 7 activator)-induced hypersensitivity was blunt in Nav1.9-null mice although their normal pain responses were similar to those of wild-type mice[42]. Hence, they suggested that Nav1.9 channels do not significantly contribute to normal visceral pain responses to acute colonic mechanical stimulation but may be important for the development of inflammation-related visceral hyperalgesic responses. This idea was further supported by a recent report that peripheral nerve recordings from pelvic afferents in Nav1.9-null mice revealed a lack of sensitization to intravesicularly applied prostaglandin (PG) E2[43]. Again, this points out that the role for Nav1.9 may be organ and/or disease specific.
Modulation of VGSCs
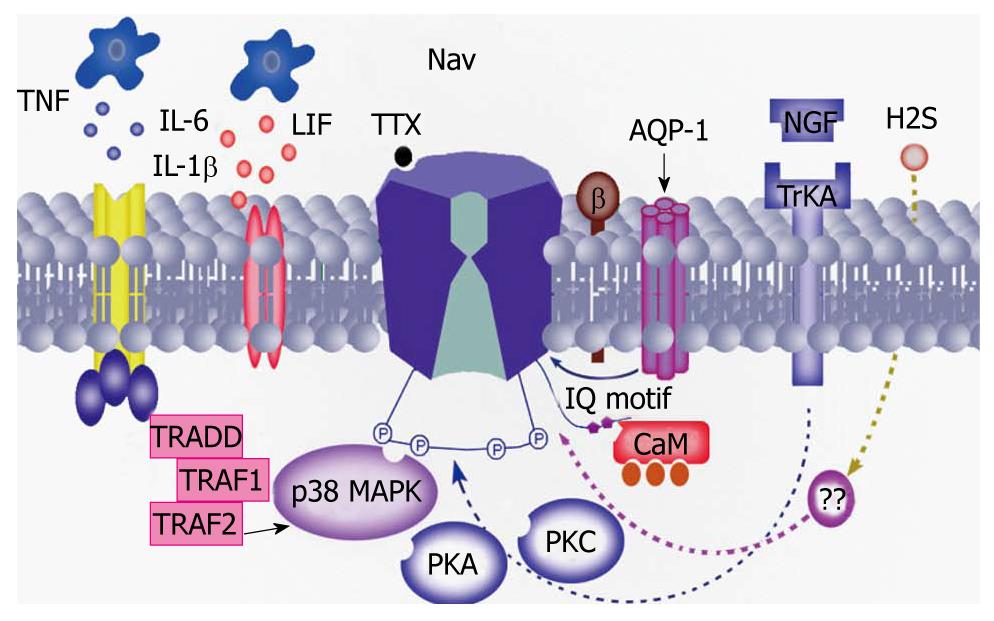
There is growing evidence to support the hypothesis that inflammatory mediators are involved in the modulation of VGSCs function and expression under pathophysiological states (Figure 3). Among these are well-studied nerve growth factor (NGF) and PGE2. Blockade of NGF and tyrosine kinase receptors inhibits peripheral mechanical sensitivity accompanying cystitis in rats[44]. NGF continuously infused into the intrathecal space at the L6-S1 level of the spinal cord for 1 or 2 wk using osmotic pumps (0.5 μL/h) significantly lowered the threshold for spike activation. In addition, the number of TTX-R action potentials during 600 ms depolarizing pulses was significantly and time-dependently increased[33]. Another inflammatory messenger PGE2 induced a rapid (< 15 s) increase in TTX-R INa that was associated with a hyperpolarizing shift in the conductance-voltage curve (3.4 ± 0.7 mV), an increase in the rate of inactivation (4.21 ± 0.7 ms at 0 mV), and no change in steady-state availability. These results do suggest that modulation of TTX-R INa by NGF and PGE2 in colonic afferents is an underlying mechanism of hyperalgesia associated with inflammation of the colon, and that this current constitutes a novel target for therapeutic relief of visceral inflammatory pain[45-47]. Other modulators include, but are not limited to, aquaporins (AQP-1), interleukin (IL)-1β and gaseous modulators/transmitters. AQP-1 may directly interact with Nav1.8 channels in DRG neurons[48], while IL-1β may act via a p38 mitogen-activated protein kinase pathway[49]. Hydrogen sulfide (H2S), as an endogenous novel third gaseous modulator/transmitter, might directly or indirectly modulate the function of VGSCs since it enhanced excitability of primary sensory neurons, and its endogenous generating enzyme was upregulated under visceral pain conditions[50].
Figure 3 Possible mechanisms underlying the modulation of voltage-gated sodium channel activity.
Inflammatory mediators such as nerve growth factor (NGF), prostaglandin (PG) E2, interleukin (IL)-1β may sensitize sodium channels by different intracellular signal transduction pathways such as protein kinase A (PKA), protein kinase C (PKC), mitogen-activated protein kinase and calmodulin (CaM). In addition, a recent study showed that aquaporin (AQP)-1 directly interacted with the sodium channel, thus contributing to the perception of pain[48]. TTX: Tetrodotoxin; LIF: leukemia inhibitory factor; Trk: Tyrosine receptor kinase; TNF: Tumor necrosis factor; TRAF: Tumor necrosis factor receptor-associated factor; TRADD: TNF receptor-associated death domain protein.
The regulation of VGSCs by intracellular protein kinases is another important mechanism for maintenance of persistent behavioral hypernociception. Direct phosphorylation of Nav1.7 by pERK1/2 and modulation of Nav1.8 by p38 regulated the gating properties of these channels[49,51]. In addition, functional regulation of the Nav1.8 by PKA and PKCepsilon in the primary sensory neuron is important for the development of the peripheral pro-nociceptive state induced by repetitive inflammatory stimuli and for the maintenance of behavioral persistent hypernociception[52]. Since the Nav1.8 C-terminus carries a conserved calmodulin-binding isoleucine-glutamine motif, it is reasonable to speculate that these two proteins can interact in vivo. Indeed, calmodulin demonstrated a regulation of Nav1.8 currents[53]. Unfortunately, our understanding of regulation of VGSCs in visceral pain conditions lags behind our knowledge of mechanisms of somatic pain. Future experiments are warranted to determine whether and how Nav1.8 channels are modulated by inflammatory and stress mediators in the peripheral nerve system.
MANAGEMENT OF VISCERAL PAIN WITH VGSC BLOCKERS
Treating chronic pain remains a significant clinical challenge, and in particular current treatment options for visceral pain are very limited and marginally effective. Na+ channel blockade is one of the most powerful and best-proven analgesic principles, beginning with the use of local anesthetics for sensory blockade and then with the discovery that Nav-blocking anticonvulsants also have benefit as pain therapy clinically[5,13,16,54-57]. Based on their mechanism of action, sodium channel blockers are divided into the following categories: (1) Extracellular blockers, which bind to and occlude the extracellular pore of the channel, e.g. TTX and saxitoxin; (2) Intracellular blockers, which block from the intracellular side of the channel, including local anesthetics, class I antiarrhythmic agents, and some anticonvulsants; and (3) Unknown mechanisms of inhibition of the sodium current in ventricular cells of guinea pigs by e.g. caffeine[58], or A-803467, a specific blocker of the Nav1.8 channel (SCN10A)[59].
Among the neuronal Na+ channel subtypes, Nav1.8 is believed to be of importance for certain visceral pain states, and Nav1.8-preferring channel blockers should be able to relieve pain without causing severe effects (due to the restricted expression of this channel type). The compounds are lidocaine, mexiletine, benzocaine, and ambroxol, which are clinically used to treat pain after local or systemic administration. Of these compounds, ambroxol has been reported to effectively suppress pain symptoms in animal models of chronic, neuropathic and inflammatory pain[60]. The analgesic effects of ambroxol by either systemic administration to animals, or by topical application in humans can be explained by ambroxol-induced blockade of ion channels in peripheral neurons. However, this has not yet been used to treat visceral pain. Pregabalin effectively inhibits TNBS-induced chronic colonic allodynia in the rat[61]. Pregabalin increased distension sensory thresholds to normal levels in IBS patients with rectal hypersensitivity[62]. Although downregulation of the α2δ calcium channel subunit may be one of the mechanisms underlying the analgesic effect of pregablin[63], the precise mechanism of pregabalin action is not fully understood. Nevertheless, these findings support the idea that pharmacological substances that modify the function of VGSCs would be potentially useful in treating pain conditions[11]. Further experiments are needed to provide more evidence for physicians to treat visceral pain with these pharmacological substances.
Numerous natural toxins have evolved to target sodium channels, either by blocking current through the pore or by modifying channel gating. Among well-studied toxins, the peptide conotoxins from marine snail venoms and δ-atracotoxin and scorpion venom toxins (e.g. birtoxin) constitute another promising source of such modulators. These peptide toxins are of considerable interest not only as probes for investigating the functioning of ion channels and receptors but also as potential therapeutics for neurological disorders, including neuropathic pain and epilepsy[64,65]. There are three classes of conopeptides that modulate VGSCs: the pore-blocking μ-conotoxins, the δ-conotoxins which delay or inhibit VGSC inactivation, and the μO-conotoxins which inhibit VGSC Na+ conductance independent of the TTX binding site. Some of these toxins have been evaluated in animal studies and in preliminary clinical studies, but others are highly novel and may develop into potential drugs for the treatment of pain states by engineering a selective µ-conopeptide that has high affinity and efficacy.
It is noted that until now none of the known neurotoxins or small molecule VGSC inhibitors/modulators is highly selective for a specific VGSC subtype. However, the recent discovery of a genetic link in inherited pain syndromes has advanced our understanding of the contribution of sodium channels to pain in humans and given us hope that it is possible to generate subunit specific blockers. “Gain-of-function” mutations in SCN9A, the gene which encodes Nav1.7, have been linked to two human-inherited pain syndromes, inherited erythromelalgia and paroxysmal extreme pain disorder, while “loss-of-function” mutations in SCN9A have been linked to complete insensitivity to pain[12,66]. Inhibition of expression of Nav1.8 (PN3/SNS) sodium channels by an antisense oligodeoxynucleotide[32] or by Nav1.8 knockdown[40,41] would be useful tools for treatment of chronic visceral pain in the future.
CONCLUSION
An emerging theme that unifies many supposedly diverse functional GI disorders is altered neuronal excitability, caused by abnormal expression and function of membrane ion channels in organ-specific primary sensory neurons. VGSCs as the main determinants of intrinsic neuronal excitability are particularly appealing targets for pharmacological intervention. Other ion channels such as vanilloid receptor, ATP receptors or potassium channels, which are certainly involved in pain processing, have not been discussed in this review. Nevertheless, the advent of new experimental medicine techniques presents us with an opportunity to test the effectiveness of novel medicines in great detail. The ability to perform hypothesis-driven research with tool compounds in targeted patients with demonstrable visceral hypersensitivity is a stimulating prospect. As VGSCs are expressed in the glial cells, future studies should be directed toward understanding whether glia-neuron interaction is involved in the peripheral sensitization of nociceptors under visceral pain conditions. Since Na+ channel subtypes are expressed in a regionally and temporally specific pattern and current VGSC blockers offer little discrimination between various VGSC subtypes, the development of selective blockers could increase their clinical usefulness. For example, a drug that selectively inhibits the Nav1.8 VGSC, which appears to be crucially and specifically involved in visceral nociception, would presumably act as a novel and powerful analgesic, with few side effects in most indications.
Peer reviewer: Dan L Dumitrascu, Professor, President, Romanian Society of Neurogastroenterology, 2nd Medical Department University of Medicine and Pharmacy Iuliu Hatieganu Cluj, Romania
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH