Published online May 7, 2011. doi: 10.3748/wjg.v17.i17.2241
Revised: March 24, 2011
Accepted: March 31, 2011
Published online: May 7, 2011
AIM: To determine the optimal dosage and mechanism of Ginkgolide B (BN52021) on severe acute pancreatitis (SAP) of rats.
METHODS: Seventy male Wistar rats were randomly divided into seven groups (10 for each group). Sham-operation group (SO), SAP model group (SAP), dimethyl sulfoxide (DMSO) contrast group (DMSO), and groups treated with 2.5 mg/kg BN52021 (BN1), 5 mg/kg BN52021 (BN2), 10 mg/kg BN52021 (BN3), and 20 μg/kg Sandostatin (SS). The SAP model was established in Wistar rats by injecting 5% sodium taurocholate retrogradely into the common bilio-pancreatic duct. The rats of SO, DMSO and BN52021 were injected with 0.9% NaCl, 0.5% DMSO and BN52021 through femoral vein 15 min after the operation. The SS group was injected with Sandostatin subcutaneously. All rats were anaesthetized at 6 h after operation, and venous blood was collected to determine the levels of serum amylase and phospholipase A2 (PLA2), and pancreas tissue was harvested and stained.
RESULTS: There was no significant difference between the SAP and DMSO groups in serum amylase level, PLA2, ascites and pathologic score, but significant difference was found in SAP/DMSO groups compared with those in SO group (P < 0.05) and the levels of serum amylase, PLA2, ascites, and pathologic score were lower in the BN1, BN2, BN3 and SS groups than in the SAP and DMSO groups (P < 0.05). However, among BN1, BN2, BN3 and SS groups, BN2 had the best effect in decreasing the levels of serum amylase and PLA2 (P < 0.05). Expression of platelet activating factor (PAF) receptor (PAFR) mRNA and protein showed no significant difference between the SAP and DMSO groups, or among BN1, BN2, BN3 and SS groups, but there was remarkable difference between SAP/DMSO group and SO group (P < 0.05), and expression of PAFR mRNA and protein was higher in the BN1, BN2, BN3 and SS groups than in the SAP and DMSO groups (P < 0.05). PAFR expression was observed in the nucleus and cytoplasm of pancreatic islet cells in Wistar rats by immunohistochemistry.
CONCLUSION: By iv injection, 5 mg/kg of BN52021 is the optimal dosage for SAP rats. BN52021 may inhibit the interaction/binding of PAF with PAFR.
- Citation: Ji RL, Xia SH, Di Y, Xu W. Mechanism and dose-effect of Ginkgolide B on severe acute pancreatitis of rats. World J Gastroenterol 2011; 17(17): 2241-2247
- URL: https://www.wjgnet.com/1007-9327/full/v17/i17/2241.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i17.2241
Platelet activating factor (PAF) is a biologically active phospholipid mediator, playing its role by binding to PAF receptor (PAFR) which is a unique G-protein-coupled seven transmembrane receptor, and it activates multiple intracellular signaling pathways[1]. Flickinger et al[2] revealed specific localization of PAFR in the pancreatic vascular endothelium, but not in other pancreatic cell types. Recent studies demonstrated that PAF played an important role in the pathological progress of severe acute pancreatitis (SAP)[3].
Ginkgolide B (code: BN52021) is one of the four Ginkgolide constituents (Ginkgolide A, B, C and J), which are present in the whole extract of Ginkgo biloba leavies. It has been found that BN52021 possesses activity as antagonists of the action of PAF. It can inhibit PAF-induced cascade effect in inflammatory reactions. For example, it can reduce the portal vein pressure of liver cirrhosis, exhibit an anti-shock effect, and exert protective functions on experimental asthma[4,5].
SAP is dangerous to life. It can cause multiple organ system failure (MOSF) and if the patients do not receive effective treatment in time, they may die. But up till now, there has been no effective drug for this disease. Recent studies have shown that BN52021 has best effect on experimental SAP[6,7], but there is no report on its optimal dosage, and its mechanism has not been fully understood. Especially as an extract of Ginkgo biloba, it is hypotoxic, and can be used in clinical treatment of SAP. We investigated the optimal dosage of BN52021 and its mechanism on SAP.
BN52021 and sodium taurocholate were purchased from Sigma (St. Louis, MO, USA), amylase kit from Beijing Kemei Reagent Co. (Beijing, China), trizol and diethyl pyrocarbonate (DEPC) from Invitrogen (Carlsbad, CA, USA), RT kit from MBI Fermentas (Ontario, Canada), DNTPs and RNA enzyme inhibitor from TaKaRa Dalian Co., Ltd. (Dalian, China), DNA Tag enzyme from Promega (Madison, WI, USA), primary antibody of PAF-R rabbit-anti-rat serum and the enhanced chemiluminescence system from Santa Cruz Biotechnology (Santa Cruz, CA, USA), secondary antibody of sheep-anti-rabbit from Beijing Dingguo Biotechnology Co., Ltd. (Beijing, China), β-actin from Beijing Zhongshan Biotechnology Co., Ltd. (Beijing, China), DYY-12 electrophoresis system and electric trans-blot SD from Beijing Liuyi Instrument Factory (Beijing, China), Type-2720 polymerase chain reaction (PCR) apparatus from ABI (Foster, CA, USA), and gel scanning and imaging system and vertical electrophoresis system from Bio-Rad (Hercules, CA, USA).
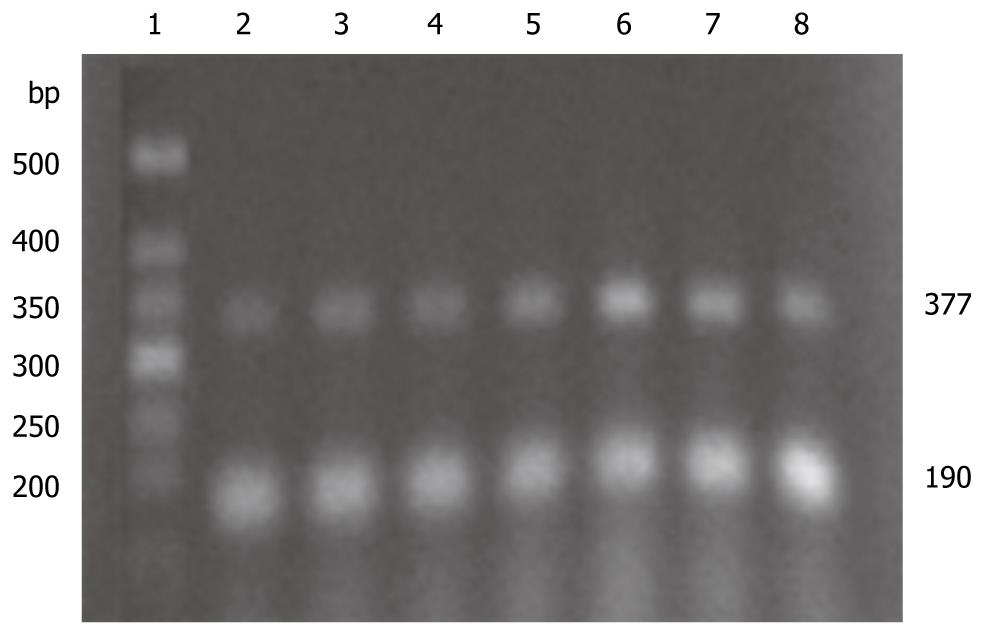
PAF-R and β-actin primer series are provided by Invitrogen (Carlsbad, CA, USA): PAF-R: forward primer (F): 5'-CCGCTGTGGATTGTCTATTA-3', reverse primer (R): 5'-AGGAGG TGATGAAGATGTGG-3' (377 bp)[8]; β-actin: F: 5'-TCC TAGCACCATGAAGATC-3', R: 5'-AAACGCAGCTCAGTAACAG-3' (190 bp)[9].
Seventy Wistar male rats (Laboratory Animal Center of PLA, Academy of Military Medical Sciences, Beijing, China), aged 6-8 wk, and weighing 200-220 g (Grade II, Certificate SCXK 2002-001) are used for this study. All rats were maintained in an environment of controlled temperature (22-25°C), humidity (55%-58%), and lighting (12 h light/12 h dark), with free access to tap water and regular chow diet. Rats were divided randomly into 7 groups: Sham-operation group (SO group, n = 10), SAP model group (SAP group, n = 10), DMSO contrast group (DMSO group, n = 10), and treatment groups of 2.5 mg/kg BN52021 (BN1 group, n = 10), 5.0 mg/kg BN52021 (BN2 group, n = 10), 10.0 mg/kg BN52021 (BN3 group, n = 10), and 20.0 μg/kg Sandostatin (SS group, n = 10)[10]. SAP model was established according to Aho et al[10]. All rats were weighed, marked and fasted for 24 h before operation. The rats were anesthetized by abdominal injection of 0.4% pentobarbital sodium (40 mg/kg), and fixed in dorsal decubitus. A 2-cm cut was made at the center of the upper belly, entering the abdominal cavity to look for rat’s duodenum and pancreaticobiliary duct. The hepatic end of the pancreaticobiliary duct was clipped with a non-invasive vascular clip, and pancreaticobiliary duct retrograde centesis was performed with an obtuse needle through duodenum seromuscular layer. Then 5% sodium taurocholate (0.1 mL/100 g) was injected in the retrograde direction of pancreaticobiliary duct with a micro-syringe at an injection rate of 0.20 mL/min. After injection, the port of pancreaticobiliary duct entering duodenum was clipped with a non-invasive vascular clip and observed for 10 min. When no active bleeding was confirmed in the abdominal cavity, the abdomen was closed in two layers and the incision was covered with sterile gauze. Same treatment was given to the SO group but without injections into the duct. Fifteen min after the model was completed, the rats in SO and SAP groups were injected with physiological saline (0.9% NaCl) through femoral vein at a dose of 5ml/kg; the rats of DMSO group were injected with DMSO (0.5% DMSO) at a dose of 5ml/kg; the BN1, BN2, and BN3 groups were rapidly injected with BN52021 at doses of 2.5, 5.0 and 10.0 mg/kg, dissolved with 0.5% DMSO; and SS group was injected with Sandostatin subcutaneously at a dose of 20.0 μg/kg[11].
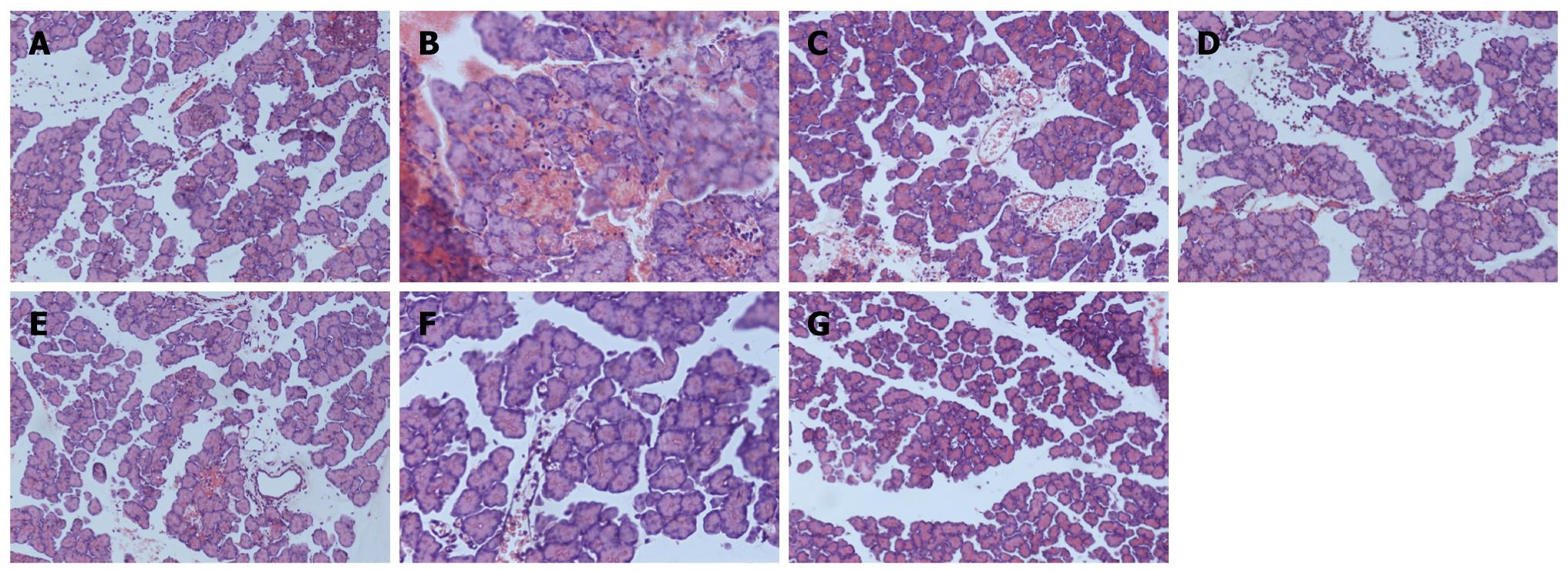
Rats in each group were anaesthetized at 6 h after first injection of Sodium taurocholate, because the T1/2 of BN52021 is 5.23 h[12], venous blood was collected from right atrium and was centrifuged for 10 min at 3000 g/min after a 10-min water bath at 37°C. The supernatant was placed into a sterilized EP tube, and stored in refrigerator at -20°C for serum amylase determination. Portions of pancreatic tissues were placed in liquid nitrogen overnight and frozen in refrigerator at -80°C, meanwhile, another portion of pancreatic tissues was fixed with 40 g/L neutral buffer formaldehyde, embedded in paraffin wax, cut into slices and stained with HE for pathological observation and scoring. Pneumonic tissues were fixed with 40 g/L neutral buffer formaldehyde, embedded in paraffin wax, and cut into slices for immunohistochemical studies.
Serum amylase and PLA2 were detected with fully automatic biochemical apparatus, amylase and PLA2 kits. Pancreatic tissue samples were observed pathologically and scored according to the reference[13].
After total RNA from pancreatic tissue in each group was extracted, its integrality was checked with agarose electrophoresis, and its concentration and purity were determined with an UV spectrophotometer, and the concentration was calculated. RNA 5 μg and Oligo DT15 1.25 μg were placed into a water bath at 70°C for 5 min, rapidly put into ice for 5 min, and centrifuged just for 15 s. 5 × M-MLV reverse transcription buffer 5 mL, DNTPS 0.05 μmol, RNA enzyme inhibitor 40 U, M-MLV and reverse transcriptase 1 μL were added, and diluted to 25 μL with deionized water treated by DEPC. The whole mixture was maintained at 42°C for 60 min. Reverse transcription was conducted and reverse transcriptase was deactivated at 95°C for 5 min. The mixture was maintained at 4°C for 5 min and preserved at -20°C. Fifty microliters PCR reaction system consists of 3 μL reverse transcription product, 0.01 μmol DNTPS, 5 μL 10 × PCR buffer, 0.075 mol MgCl2 (Magnesium Chloride), 50 pmol of each PAF-R specific sense strand and anti-sense strand, and 50 pmol of each β-actin sense strand and anti-sense strand. Reaction conditions were as follows: pre-denaturizing at 94°C for 3 min, denaturizing at 94°C for 45 s, annealing at 58°C for 45 s, and extending at 72°C for 45 s, 38 cycles in total; extending at 72°C for 7 min, and preserving at 4°C. The PCR product was included in the PAF-R gene sequence of rat spleen (gi:470384), which was performed by Beijing Boya Biotechnology Co., Ltd. The PCR product was analyzed by 2% agarose gel electrophoresis, ethidium bromide staining, and a gel imaging system scanning. Gray level of PCR product was treated by PAF-R and β-actin was analyzed using the Quantity-One Software, and the change of PAF-R mRNA expression was evaluated semi-quantitatively.
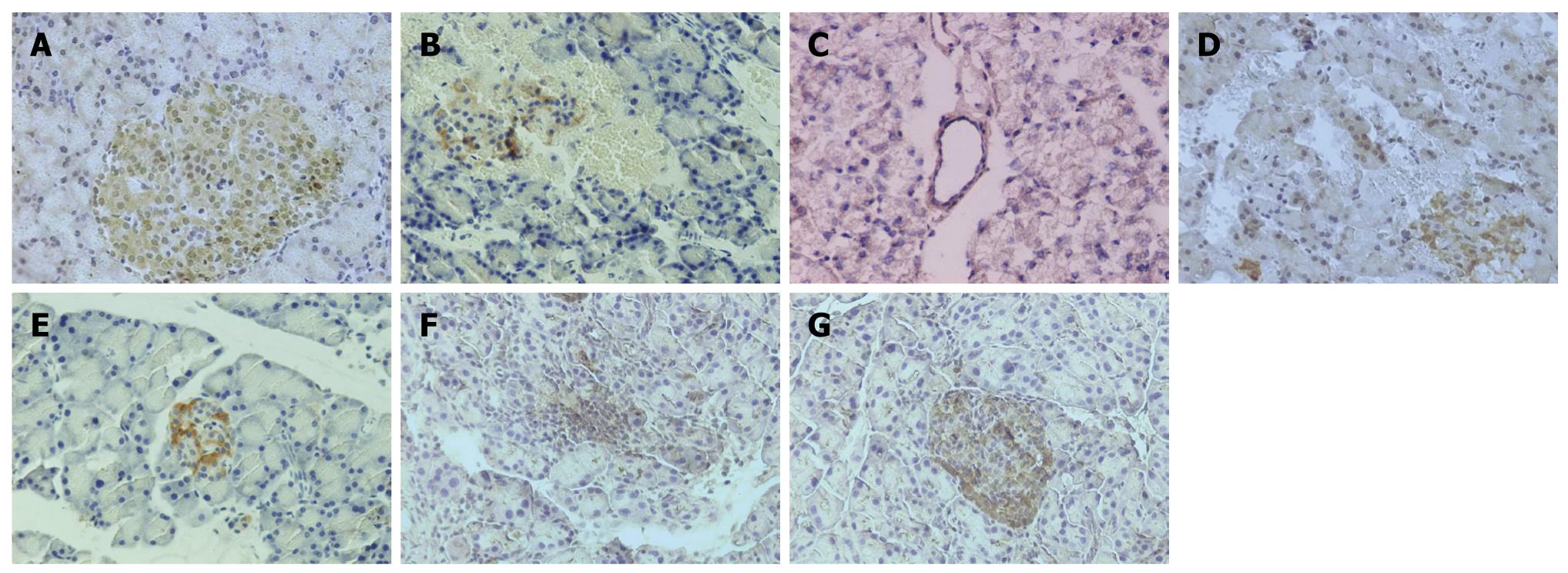
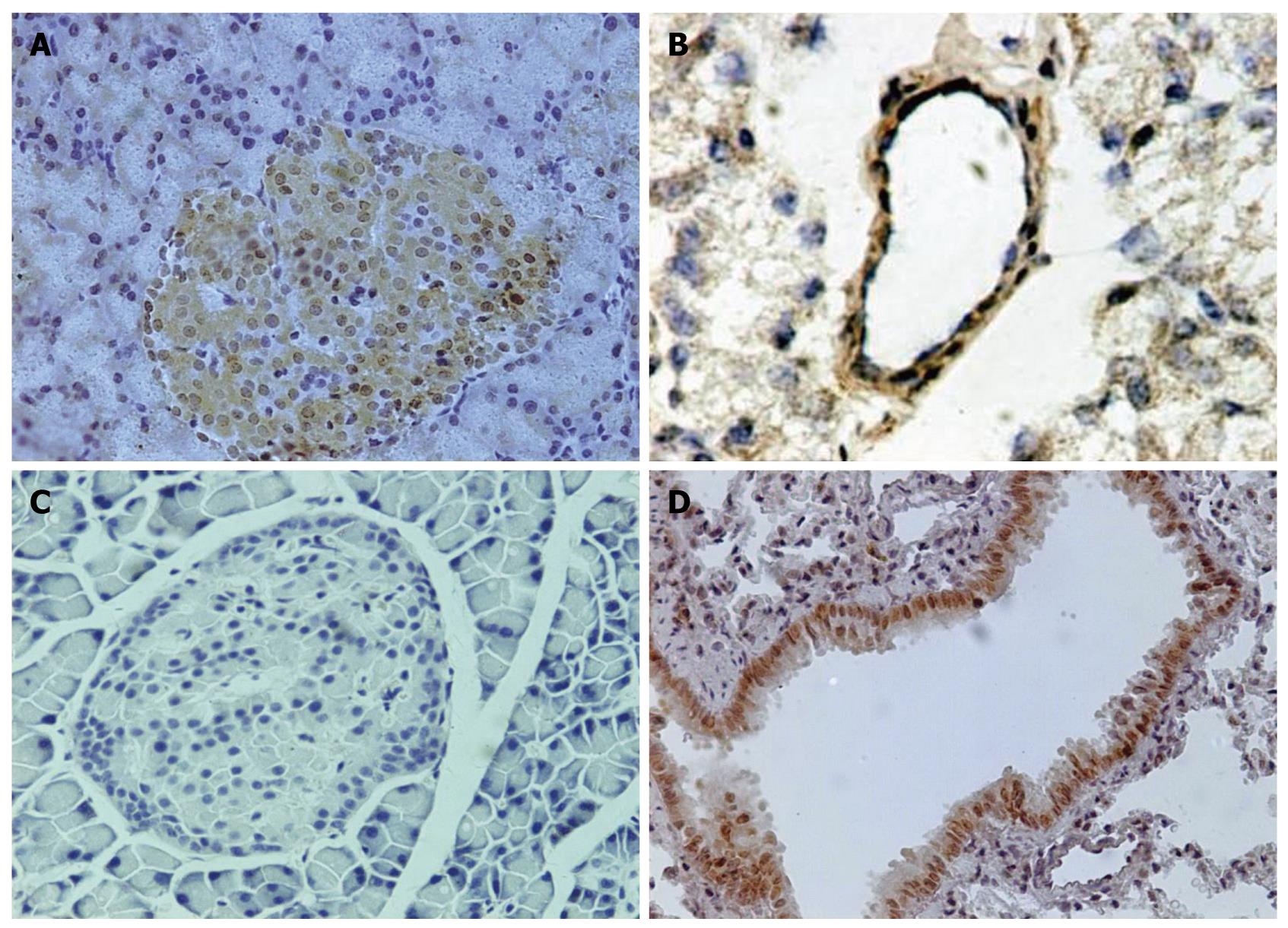
Paraffin slices of pancreas (5 μm) were made, mounted on slides and treated with paraformaldehyde-lysine-periodate solution. The slides were immersed in 100% dimethylbenzene for 15 min, 100% ethanol for 10 min, 95% ethanol for 5 min, 80% ethanol for 5 min, 70% ethanol for 5min, and washed with phosphate-buffered saline (PBS). The slides were immersed in citrate solution for 15 min in microwave at high temperature, cooled at room temperature, washed for 3 times with PBS, incubated with the rabbit polyclonal antibody against a recombinant protein corresponding to amino acids at the amino terminus of PAFR (1:100) for 18 h at 4°C, then incubated with goat anti-rabbit IgG-HRP polymer (1:500) for 0.5 h at 4°C, washed with PBS after incubation, and then incubated with DABH2O2 solution for color development, and washed with lotic water. The slices were counterstained with hematoxylin and mounted with coverslips. The slices were observed and analyzed, and the quantity of PAFR protein expression was determined according to the density of positive cells. Some frozen slices of pancreatic tissues and paraffin slices of lung tissues were made to observe the location of PAFR in pancreatic and lung tissues by immunohistochemistry.
The experiments were repeated for 3 times and their average values served as the final values; all values were expressed as mean ± SD. Data were processed with SPSS 11.5 Statistical Software, and LSD and Dunnett’s post-hoc method were used for comparison of ANOVA. Statistical significance was established at P < 0.05.
There were no significant differences in the serum levels of amylase and PLA2, quantity of ascites and pathological scores between SAP and DMSO groups. It was higher in SAP/DMSO group than in SO group (P < 0.05), and lower in BN1, BN2, BN3 and SS group than in SAP/DMSO group (P < 0.05). However, BN2 had the best effect in decreasing the serum level of amylase and PLA2 among BN1, BN2, BN3 and SS groups (P < 0.05) (Table 1, Figure 1).
| Group | Amylase (U/L) | PLA2 (μmol/min per mililiter) | Ascites (mL) | Pathological scores |
| SO | 1923 ± 527ac | 0.0434 ± 0.019ac | 0.0ac | 0ac |
| SAP | 6448 ± 240c | 0.2990 ± 0.0225c | 5.4 ± 1.3c | 9.15 ± 0.55c |
| DMSO | 6070 ± 464c | 0.2728 ± 0.4461c | 5.0 ± 2.0c | 9.10 ± 0.65c |
| BN1 | 4744 ± 1031ac | 0.2320 ± 0.0890ac | 2.4 ± 0.9a | 6.65 ± 0.42a |
| BN2 | 3452 ± 548a | 0.1049 ± 0.0528a | 1.6 ± 0.5a | 5.55 ± 0.36a |
| BN3 | 4777 ± 168ac | 0.1069 ± 0.0440a | 2.6 ± 0.9a | 5.90 ± 0.19a |
| SS | 4646 ± 675ac | 0.1632 ± 0.0523ac | 2.4 ± 0.5a | 5.70 ± 0.30a |
There was no significant difference in expressions of PAFR mRNA and PAFR protein between SAP and DMSO groups, neither among BN1, BN2, BN3 and SS groups. Expression of PAFR mRNA and its proteins in pancreatic tissues was lower in SAP/DMSO group than in SO group (P < 0.05), and higher in BN1, BN2, BN3 and SS groups than in SAP/DMSO group (P < 0.05) (Table 2, Figures 2 and 3).
PAFR was located in pancreatic microvascular endothelial cells and bronchia epithelial cells. It was first found in the nuclei of pancreatic islet cells and cytoplasm of Wistar rats (Figure 4).
Amylase, PLA2, score of pathologic changes of pancreas and quantity of ascites were reduced after administration of BN52021 at 2.510 mg/kg, and the pancreas PAFR mRNA or protein was protected. PAFR expression was found in the pancreas’ islet, which has not been reported by others up till now. The best effect was shown at 5 mg/kg among BN groups. BN52021 at 5 and 10 mg/kg showed equivalent effects on SAP, and 10 mg/kg was less effective such as in serum amylase. This may be caused by the dose range between 10 and 5 mg/kg.
PLA2 is pivotal in the pathogenesis of SAP. There are two types of PLA2: cytosolic phospholipase A2 (cPLA2) and secretory PLA2 (sPLA2).There is a cross-talk between sPLA2 and cPLA2. sPLA2 activates a signaling cascade, including activation of cPLA2, arachidonic acid release, PAF production, and induction of COX-2[14]. PLA2 can decompose the membrane to release PAF. It is known that PAF is an initiative factor in the pathogenesis of SAP, and the cascade exacerbates the course of pancreatitis by activating the PLA2 in membrane. BN52021 antagonizes PAF by restraining the activation of PLA2 and this is the first report about the relation between BN52021 and PLA2 in SAP.
Quantity of ascites showed the severity of pathological changes of pancreas. When pancreas is involved in inflammation, hemorrhage and putrescence, it would irritate peritoneum and peritoneal permeability was increased, resulting in the release of ascites. PLA2 was closely correlated with the release of ascites[15]. The results indicated that BN52021 can reduce the PLA2 in plasma and decrease the quantity of ascites, and that BN52021 may interact definitely on other inflammatory factors to reduce peritoneal permeability.
The level or regulation of PAFR mRNA and PAFR protein has not been clearly studied. In our experiments, expression of PAFR in pancreas was up-regulated in the initial 3 h in SAP, but down-regulated after 6 h[16]. The reason for this may be in the first 3 h, pancreas could release more inflammatory factors to induce the up-regulation of PAFR, such as tumor necrosis factor α[17] and interferon-β, but cellular membrane was destroyed completely along with severe damages occurring in the pancreas after 6 h. In addition, protein kinase C excess activation was reported to decrease expression of PAFR[18], followed by a down-regulation of mRNA. BN52021 protected the membrane so that it maintained the quantity of PAFR. There was significant difference between SAP and BN52021 groups. The ratio of IOD of PAFR to β-actin was gradually improved in 2.5 and 10 mg/kg BN52021 groups, although there was no significant difference among BN52021 groups, and remarkable difference was found among SO group, 5 mg/kg SAP group and 5 mg/kg BN group.
It was firstly reported that PAFR was located in pancreas’ islet, and it is important to study the mechanism of pancreas inflammation. BN52021 exerted its protective effects in patients with diabetes and pancreatitis, but the active mechanism is not clear. When inflammation in pancreas happened, PAFR was up-regulated, and the cells released more PAF, which damaged the pancreas. As BN52021 competitively inhibited the combination of PAF and PAFR and released less PAF, the pancreas was protected. It meant that BN52021 achieved its effects on SAP not only by reducing the quantity of PAFR, but also by inhibiting the combination of PAF and PAFR and maintaining the integrity of the cells of pancreas.
In conclusion, 5 mg/kg BN52021 is the optimal dose in treating SAP rats, which can reduce the amylase and PLA2 in serum and the quantity of ascites. It prevents the pancreas from severe damage induced by sodium taurocholate. Compared with Sandostatin, a classical drug for SAP, BN52021 exerts the best effects in treating SAP rats. This paper also showed that BN52021 achieves therapeutic effects in SAP rats not only by reducing the quantity of PAFR, but also by inhibiting the combination of PAF and PAFR and maintaining the integrity of the cells of pancreas. Because of its hypotoxicity as an extract of Ginkgo bibola, it will be of significance in clinical application. More experiments are needed for the extensive application of BN52021 in clinic.
Platelet activating factor (PAF) is a family of phosphorylcholine esters with diverse potent physiological effects. It is closely associated with the cell membrane and is found in a variety of cells. Numerous cell types and tissues have been shown to synthesize and release PAF upon stimulation and at the same time to exhibit biological responses to it. Flickinger et al found the specific localization of PAF receptor (PAFR) in the pancreatic vascular endothelium, but not in other pancreatic cell types.
Recent studies demonstrated that PAF played an important role in the pathological progress of severe acute pancreatitis (SAP). It has been found that BN52021 possesses activity as antagonists of the action of PAF. However, there is no report on its optimal dosage. Although BN52021 has shown best effects on treating SAP, its mechanism has not been elucidated in details. This paper investigated the optimal dosage of BN52021 and its mechanism on SAP.
Recent reports have highlighted the importance of PAF in SAP and have shown that BN52021 has best effect on experimental SAP. This is the first study to report the optimal dosage of BN52021. The authors discovered that there is PAFR expression in the pancreas’ islet, which has not been reported by others up to now. The in vitro studies suggest that this protein may be the cause of the repression in E-cadherin observed in this cancer.
By understanding how PAF is induced and by blocking its expression, this study may represent a future strategy for SAP treatment with BN52021.
PAF is a biologically active phospholipid mediator, playing its role by binding to platelet activating factor receptor (PAFR) which is a unique G-protein-coupled seven transmembrane receptor, and it activates multiple intracellular signaling pathways, including the nucleal PAFR. Ginkgolide B (code:BN52021) is one of the four Ginkgolide constituents (Ginkgolide A, B, C and J), which are present in the whole extract of Ginkgo biloba leavies.
The authors examined the level of serum amylase and PLA2, quantity of ascites, and pathological scores, the expression of PAFR mRNA and its proteins in pancreatic tissues and location of PAFR in pancreatic and lung tissues. It revealed that PAFR expressed in the pancreas’ islet and the best effect on SAP was shown in the 5 mg/kg group. This paper also showed that BN52021 achieves the therapeutic action on SAP rats not only by reducing the quantity of PAFR, but also by inhibiting the combination of PAF and PAFR and maintaining the integrity of the cells of pancreas. The results are interesting and may represent a molecular mechanism of BN52021 on SAP.
Peer reviewers: Eugene P Ceppa, MD, Department of Surgery, DUMC 3443, Durham, NC 27710, United States; Giuseppe Brisinda, MD, Department of Surgery, Catholic School of Medicine University Hospital Agostino Gemelli, Largo Agostino Gemelli 8, Rome, 00168, Italy
S- Editor Sun H L- Editor Ma JY E- Editor Zheng XM
| 1. | Marrache AM, Gobeil F Jr, Bernier SG, Stankova J, Rola-Pleszczynski M, Choufani S, Bkaily G, Bourdeau A, Sirois MG, Vazquez-Tello A. Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J Immunol. 2002;169:6474-6481. |
| 2. | Flickinger BD, Olson MS. Localization of the platelet-activating factor receptor to rat pancreatic microvascular endothelial cells. Am J Pathol. 1999;154:1353-1358. |
| 3. | Liu LR, Xia SH. Role of platelet-activating factor in the pathogenesis of acute pancreatitis. World J Gastroenterol. 2006;12:539-545. |
| 4. | Yang Y, Nemoto EM, Harvey SA, Subbotin VM, Gandhi CR. Increased hepatic platelet activating factor (PAF) and PAF receptors in carbon tetrachloride induced liver cirrhosis. Gut. 2004;53:877-883. |
| 5. | Langley SM, Chai PJ, Jaggers JJ, Ungerleider RM. Platelet-activating factor receptor antagonism improves cerebral recovery after circulatory arrest. Ann Thorac Surg. 1999;68:1578-1584; discussion 1585. |
| 6. | Bedirli A, Gokahmetoglu S, Sakrak O, Soyuer I, Ince O, Sozuer E. Beneficial effects of recombinant platelet-activating factor acetylhydrolase and BN 52021 on bacterial translocation in cerulein-induced pancreatitis. Eur Surg Res. 2004;36:136-141. |
| 7. | Xia SH, Fang DC, Hu CX, Bi HY, Yang YZ, Di Y. Effect of BN52021 on NFkappa-Bp65 expression in pancreatic tissues of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:882-888. |
| 8. | Diserbo M, Cand F, Ziade M, Verdetti J. Stimulation of platelet-activating factor (PAF) receptors increases inositol phosphate production and cytosolic free Ca2+ concentrations in N1E-115 neuroblastoma cells. Cell Calcium. 1995;17:442-452. |
| 9. | Collins LC, Roberts AM. Effects of platelet-activating factor on arteriolar and venular tone in rat trachea. Microvasc Res. 1997;53:63-72. |
| 10. | Aho HJ, Ahola RA, Tolvanen AM, Nevalainen TJ. Experimental pancreatitis in the rat. Changes in pulmonary phospholipids during sodium taurocholate-induced acute pancreatitis. Res Exp Med (Berl). 1983;182:79-84. |
| 11. | Gong Z, Yuan Y, Lou K, Tu S, Zhai Z, Xu J. Mechanisms of Chinese herb emodin and somatostatin analogs on pancreatic regeneration in acute pancreatitis in rats. Pancreas. 2002;25:154-160. |
| 12. | Fourtillan JB, Brisson AM, Girault J, Ingrand I, Decourt JP, Drieu K, Jouenne P, Biber A. [Pharmacokinetic properties of Bilobalide and Ginkgolides A and B in healthy subjects after intravenous and oral administration of Ginkgo biloba extract (EGb 761)]. Therapie. 1995;50:137-144. |
| 13. | Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56. |
| 14. | Kolko M, Rodriguez de Turco EB, Diemer NH, Bazan NG. Neuronal damage by secretory phospholipase A2: modulation by cytosolic phospholipase A2, platelet-activating factor, and cyclooxygenase-2 in neuronal cells in culture. Neurosci Lett. 2003;338:164-168. |
| 15. | Tomita Y, Kuwabara K, Furue S, Tanaka K, Yamada K, Ueno M, Ono T, Maruyama T, Ajiki T, Onoyama H. Effect of a selective inhibitor of secretory phospholipase A2, S-5920/LY315920Na, on experimental acute pancreatitis in rats. J Pharmacol Sci. 2004;96:144-154. |
| 16. | Xia SH, Hu CX, Zhao ZL, Xia GD, Di Y. Significance of platelet activating factor receptor expression in pancreatic tissues of rats with severe acute pancreatitis and effects of BN52021. World J Gastroenterol. 2007;13:2992-2998. |
| 17. | Dagenais P, Thivierge M, Stankova J, Rola-Pleszczynski M. Modulation of platelet-activating factor receptor (PAFR) gene expression via NF kappa B in MonoMac-1 cells. Inflamm Res. 1997;46 Suppl 2:S161-S162. |