Published online May 7, 2011. doi: 10.3748/wjg.v17.i17.2229
Revised: September 25, 2010
Accepted: October 2, 2010
Published online: May 7, 2011
AIM: To investigate the effects of hyperbaric oxygenation (HBO) on regeneration of the biliary ductal system and postoperative cholestasis in hepatectomized rats.
METHODS: HBO was performed in Wistar rats daily starting 12 h after a 70% partial hepatectomy. Regenerated liver weight, serum parameters and the proliferating cell nuclear antigen labeling index of hepatocytes and biliary ductal cells were measured. Hepatocyte growth factor (HGF), c-Met and transforming growth factor (TGF) β-1 mRNA expression levels were analyzed by quantitative reverse transcription polymerase chain reaction.
RESULTS: HBO improved the postoperative serum levels of total bile acid but not transaminase levels. HBO promoted hepatocyte and biliary ductal cell proliferation. The hematoxylin and eosin-stained specimens revealed fewer ballooned hepatocytes and higher cell densities in the HBO group compared to the control group. HBO suppressed c-Met mRNA levels at 15 h but did not modulate HGF or TGF β-1 mRNA expression levels.
CONCLUSION: HBO promoted regeneration of biliary ductal cells and improved postoperative cholestasis after a partial hepatectomy.
- Citation: Idetsu A, Suehiro T, Okada K, Shimura T, Kuwano H. Hyperbaric oxygenation promotes regeneration of biliary cells and improves cholestasis in rats. World J Gastroenterol 2011; 17(17): 2229-2235
- URL: https://www.wjgnet.com/1007-9327/full/v17/i17/2229.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i17.2229
Extended hepatectomy and small graft liver transplantation can result in postoperative cholestasis and liver failure. Hepatectomized liver rapidly regenerates and its volume and function recover, but functional recovery lags behind quantitative recovery. Postoperative liver failure causes cholestasis; hence, it is important to improve cholestasis in order to reduce morbidity and mortality.
Hyperbaric oxygenation (HBO) has been used as a therapy in patients with carbon monoxide poisoning, decompression sickness and arterial gas embolism[1]. Effects of HBO that have been reported include upregulation of growth factors[2], down-regulation of inflammatory cytokines[3] and increased angiogenesis[4].
Studies have reported the effect of HBO on liver regeneration after partial hepatectomy, but few studies have reported its effect on biliary ductal regeneration and cholestasis. In this study, we investigated the effects of HBO on the biliary system and postoperative cholestasis after a partial hepatectomy.
All experiments were performed on 10-wk-old male Wistar rats (n = 78). Thirty-six rats were used in each group and preoperative data acquired from another 6 rats. Procedures were approved by the Review Committee on Animal Use of Gunma University, Maebashi, Japan. All the rats were housed under temperature- and light-controlled conditions with 12 h light and dark cycles. The rats were fed a standard rat diet and water as desired. Prior to the experiment, the rats were starved for 24 h with free access to water. Under ether anesthesia, a 70% partial hepatectomy was performed using the Higgins and Anderson technique[5]. After the transverse laparotomy, the median and left lobes were ligated and resected. All the rats were allowed free access to the standard diet and water after the surgery. The rats were sacrificed at various time points (12, 15, 24, 48, 72, and 96 h after the partial hepatectomy) by puncture of the inferior vena cava and exsanguination under ether anesthesia. The regenerated livers were removed and either fixed in 20% formalin or frozen and stored at -80°C.
The rats in the HBO group were placed in a hyperbaric chamber (Model KHO-200; Kawasaki Engineering Co., Ltd., Hyogo, Japan) pressurized to 2 ATA with 100% oxygen for 60 min. HBO was initiated 12 h after the end of surgery and performed once a day until postoperative day 4. HBO was not performed postoperatively in the control group.
The regenerated liver weight/% body weight ratio was calculated using the following equation: 100 × A/B, where A is the weight of the regenerated liver at the time of sacrifice and B is the rat body weight at the time of sacrifice.
The degree of hepatic injury and biliary function was assessed by determining the serum levels of alanine aminotransferase (ALT) and total bile acid (TBA) using standard laboratory methods.
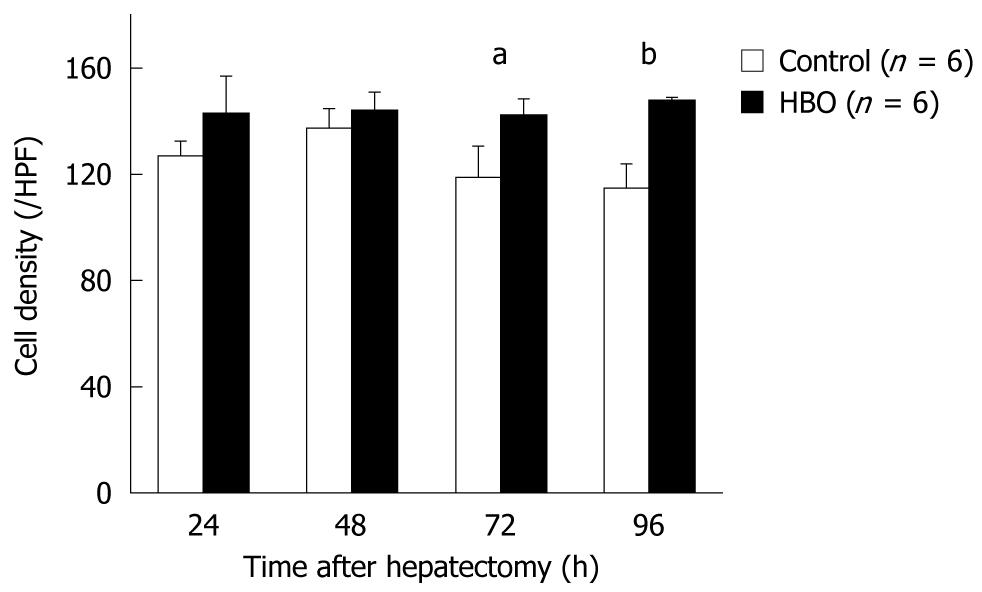
Formalin-fixed liver specimens were embedded in paraffin, and 3-μm sections were prepared. Hematoxylin and eosin (HE) staining was performed according to standard procedures. Hepatocytes were counted in random high power fields (HPFs) without a visible Glisson’s sheath or central vein (× 400). The cell density was expressed as the mean number of hepatocytes per HPF.
Proliferating cell nuclear antigen (PCNA) expression was immunohistochemically detected using a monoclonal antibody against PCNA [dilution: 1:400, horseradish peroxidase-labeled, DAKO, PC10, (Code No. M0879)]. PCNA-positive cells were counted in random HPFs (× 400). The PCNA-labeling index was expressed as the percentage of PCNA-positive cells/total number of cells. Hepatocytes and biliary ductal cells were counted.
Total RNA was extracted from freshly frozen liver using the RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The quantity of isolated RNA was measured using a ND-1000 spectrophotometer (NanoDrop Technologies, DE, USA). Template cDNA was synthesized from 13.5 μL of total RNA using an Omniscript reverse transcriptase kit (Qiagen), a random primer (hexadeoxyribonucleotide mixture) (TaKaRa, Shiga, Japan), and a ribonuclease inhibitor (porcine liver) (TaKaRa). Total RNA was reverse transcribed using 4 U of Omniscript reverse transcriptase in a reaction volume of 20 μL (60 min at 37°C, 5 min at 93°C, then placed on ice). The resultant cDNA samples were stored at -20°C until analysis.
Quantitative reverse transcription-polymerase chain reaction (PCR) analyses were performed using the ABI Prism 7000 Sequence detection system (Applied Biosystems, CA, USA). The standard reaction volume was 20 μL and contained 1 × SYBR Green PCR Master Mix (Applied Biosystems), 2.0 μL of cDNA template, and 0.5 μmol/L each of the forward and reverse primers. The initial PCR denaturation step was performed for 10 min at 95°C followed by 45 cycles of 15 s at 95°C (denaturation), 10 s at 60°C (annealing), and 30 s at 72°C (extension). All reactions were performed in duplicate. The data were adjusted using the mRNA level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA and expressed as a ratio of the GAPDH levels. The primers for hepatocyte growth factor (HGF) and transforming growth factor (TGF) β-1 were used as described in previously published assays[6], and the primers for c-Met and GAPDH were designed using Primer 3 (primer3_http://www.results.cgi) with sequences from Ensembl Genome Browser (http://www.ensembl.org/index.html) (Table 1).
| Gene | Primer sequence | Annealing temperature (°C) | |
| HGF | Sense | 5'-TTATGGGGAATGAGAAATGC | 60 |
| Antisense | 5'-TCGAACAAAAATACCAGGAC | ||
| c-Met (ENSRNOT00000009662) | Sense | 5'-CAGCGGCAATTCTAGACACA | 60 |
| Antisense | 5'-CTGAAGCTGCTTGTCACTCG | ||
| TGF β-1 | Sense | 5'-ATGACATGAACCGACCCTTC | 60 |
| Antisense | 5'-TGTGTTGGTTGTAGAGGGCA | ||
| GAPDH (ENSRNOT00000002652) | Sense | 5'-AGACTATGGATGGCCCCTCT | 60 |
| Antisense | 5'-GGTAGGAACACGGAAGACCA |
All values are expressed as mean ± SD. Statistical significance was determined using the post-hoc test of Bonferroni-Dunn. P < 0.05 was considered statistically significant.
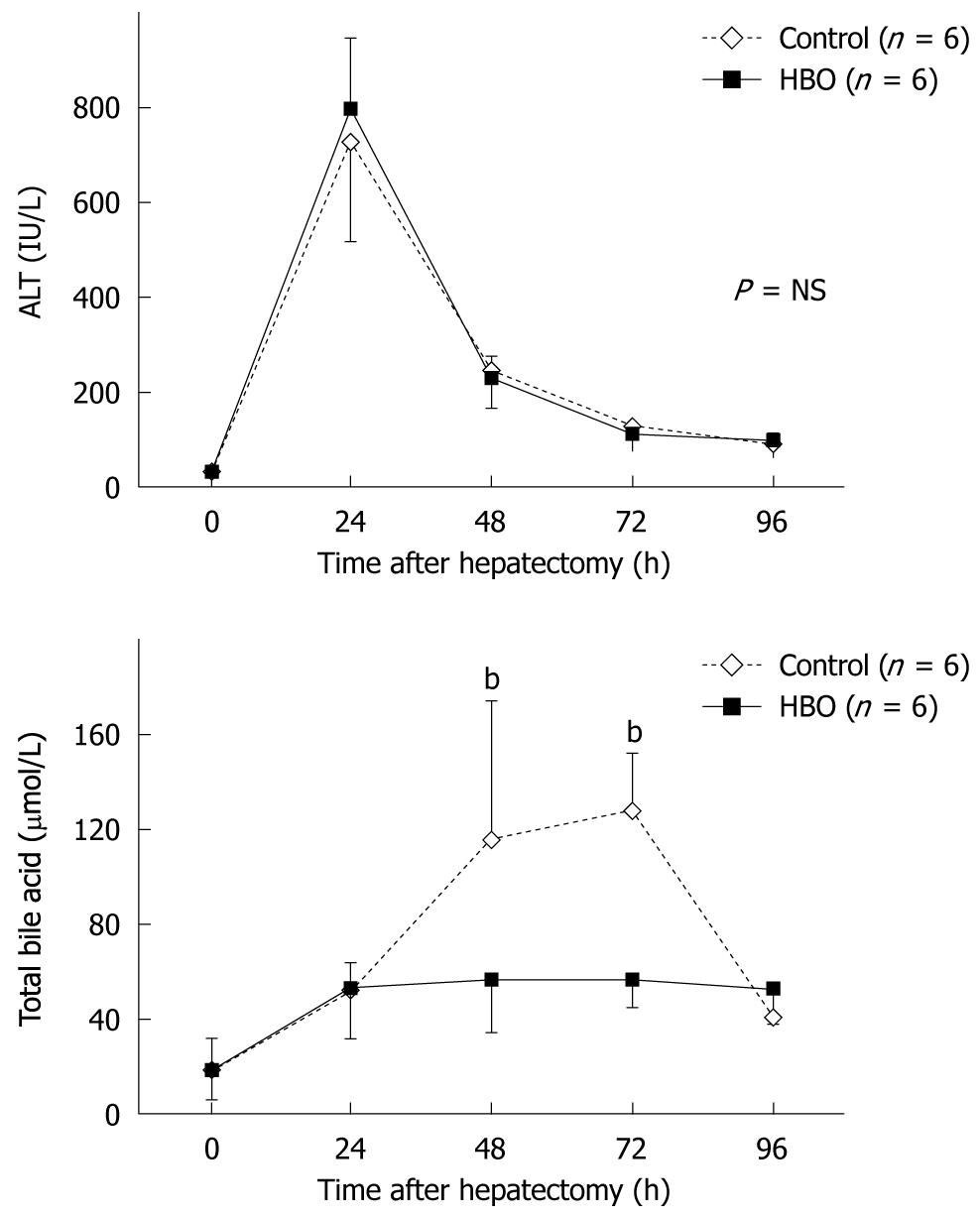
Blood parameters were measured at various time points. ALT levels peaked at 24 h after the partial hepatectomy in the control and HBO groups and then decreased rapidly (Figure 1). No significant differences were observed between the groups at any time point as the serum ALT level decreased. Serum TBA levels increased and peaked at 72 h (Figure 1); these were decreased by HBO. Significant differences were observed in TBA levels at 48 h and 72 h after the partial hepatectomy. These data indicate that HBO improved the postoperative biliary function.
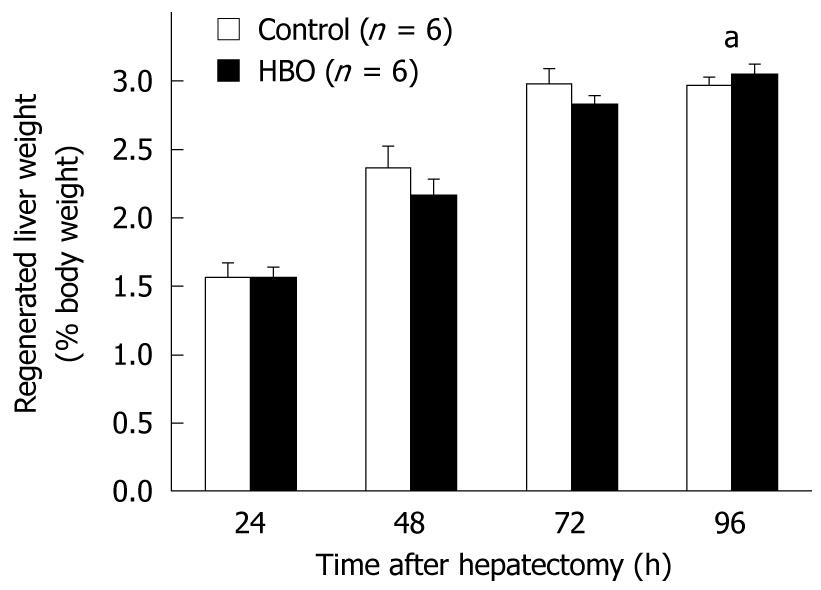
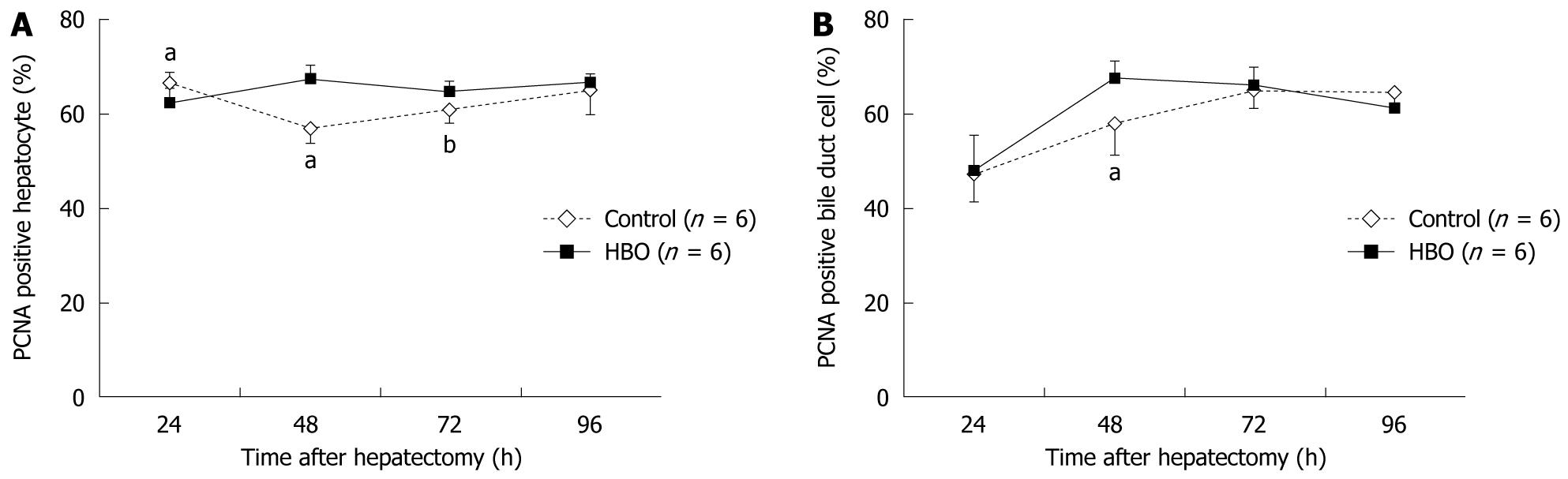
Data in Figure 2 reveal that the regenerated liver weight/% body weight increased daily after the partial hepatectomy. At 96 h, the regenerated liver weight ratio was higher in the HBO group than in the control group. The PCNA labeling index of the hepatocytes was higher at 48 and 72 h and lower at 24 h in the HBO group than in the control group (Figure 3A). The PCNA labeling index of the biliary ductal cells peaked at 48 h in the HBO group and was significantly higher in the HBO group than in the control group (Figure 3B). Combined, these data indicate that biliary ductal cell proliferation was greater in the HBO group than in the control group.
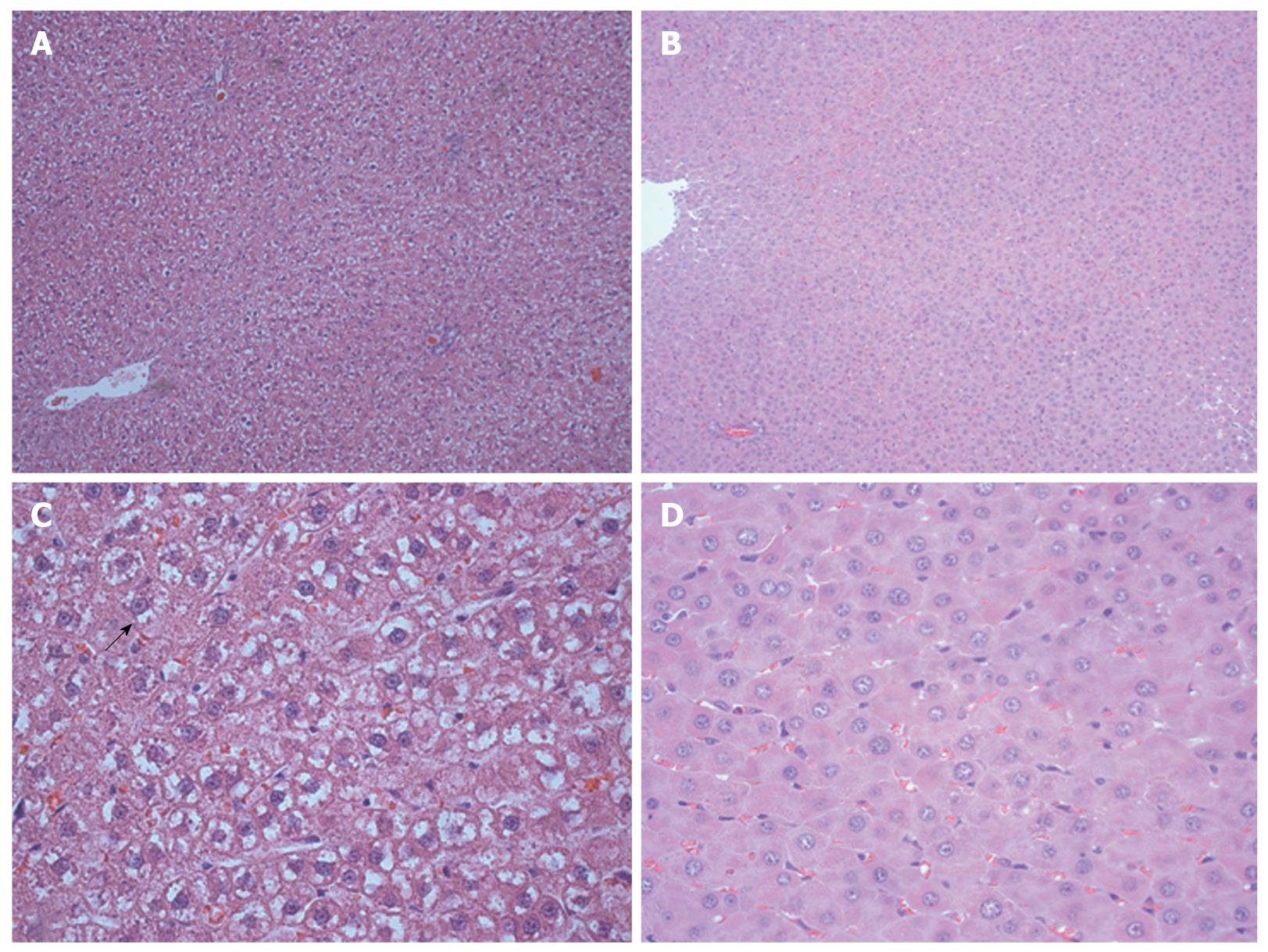
An edematous cytoplasm and ballooning hepatocytes were observed in the regenerated livers of the control group at 96 h after surgery; however, these findings were not observed in the HBO group (Figure 4). The cell density of the hepatocytes was higher in the HBO group at 72 and 96 h (Figure 5). These findings suggest that HBO inhibited cell edema and protected the hepatocytes during liver regeneration.
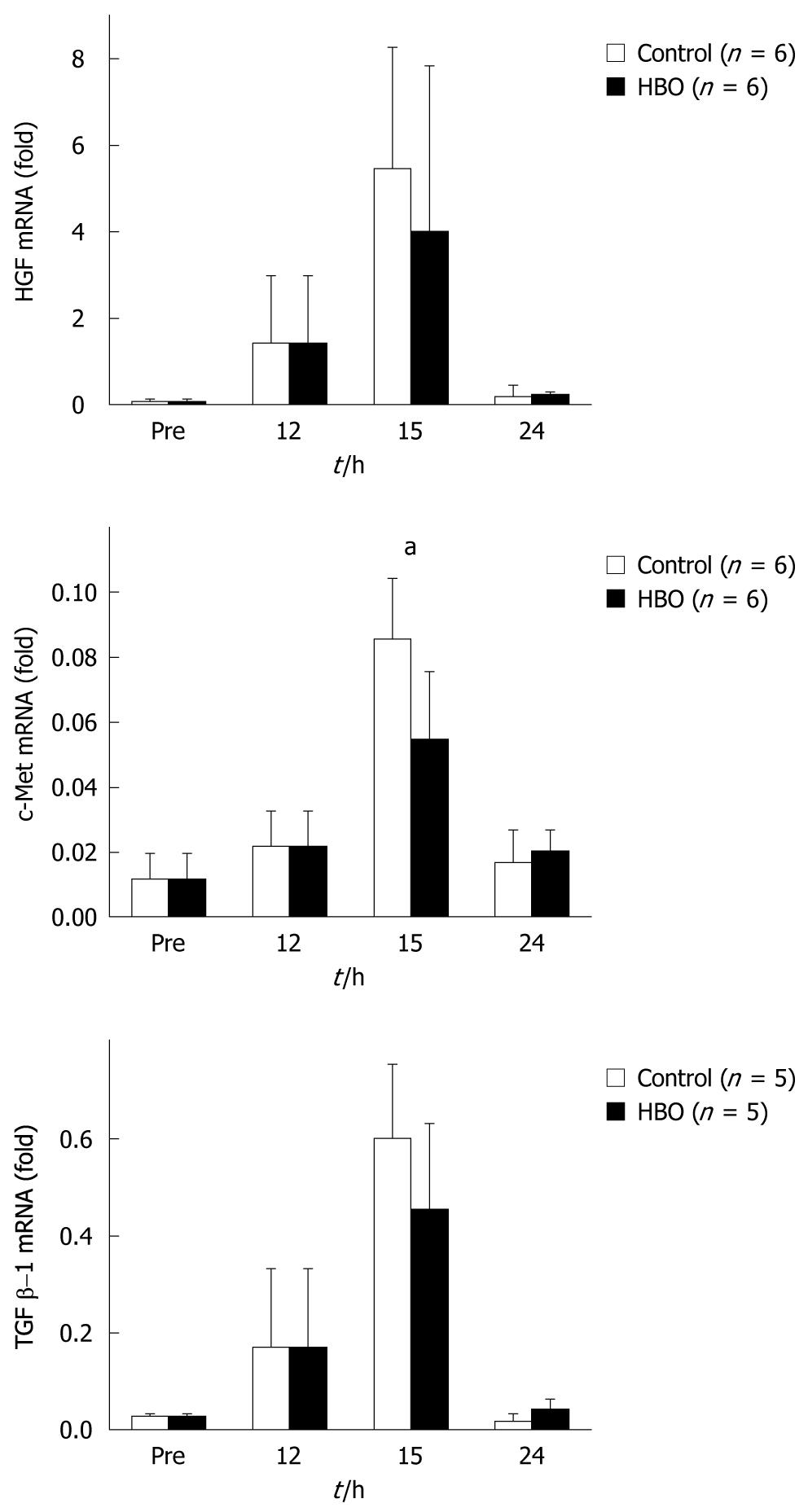
The expression of HGF and TGF β-1 mRNA peaked at 15 h in both groups and no significant differences in the mRNA levels of the HBO and control groups were observed. While the mRNA expression of c-Met increased and peaked at 15 h in both the groups, expression was significantly suppressed in the HBO group (Figure 6).
The goal of this study was to determine the effect of HBO on liver regeneration, particularly in biliary system restoration and improvement of postoperative cholestasis.
HBO involves the inspiration of a high concentration of oxygen in a pressure chamber at pressures higher than 1 ATA. During HBO, the partial pressure of oxygen increases, thereby increasing oxygen delivery throughout the body. The beneficial effects of HBO have been reported in ischemia-reperfusion injuries[6-8], acute liver failure after major hepatectomy[9], hepatic artery thrombosis after liver transplantation[10] and liver regeneration after portal vein embolization[11].
The hepatectomized liver enlarges to compensate for the mass of the resected lobes. In the early phase of liver regeneration, avascular clusters of hepatocytes are formed. Following hepatocyte proliferation, the vascular architecture and biliary ductal system are reconstructed in the regenerating liver. HBO promotes the proliferation of hepatocytes[11-13] and sinusoidal cells[14]. In the present study, data from the PCNA labeling index revealed that HBO promotes not only proliferation of hepatocytes but also biliary ductal cells. HBO probably improved bile drainage function during early phases of liver regeneration and decreased postoperative cholestasis.
During liver regeneration, hepatocytes exhibit morphologic changes such as cytoplasmic edema and ballooning. Ballooned hepatocytes are commonly observed in viral[15], drug-induced[16-18] and alcoholic[19,20] hepatic injuries and non-alcoholic steatohepatitis[21,22]. Hepatocytes are swollen in biopsies of orthotopic liver grafts after transplantation. Ballooning is associated with, but not directly caused by, bile retention. One of the pathogenetic factors of ballooned hepatocytes is ischemic damage. In a rat steatotic liver model, swollen hepatocytes caused abnormal microcirculation, manifested as reduced sinusoidal density[23]. The edematous state of hepatocytes can cause obstruction of the sinusoids and biliary ducts, secondary cholestasis and circulatory failure in the liver. HBO increases oxygen delivery and improves the ischemic state of the regenerated liver, breaking the cycle of hepatocyte ballooning and abnormal microcirculation. Our results suggest that HBO suppresses the edematous state of hepatocytes, accelerates bile drainage and improves postoperative cholestasis.
The present study revealed that HBO did not lower postoperative ALT levels. One study has suggested that HBO reduces serum ALT levels after a partial hepatectomy[14], while others have revealed no significant differences in ALT levels during liver regeneration[12,13,24]. The mechanisms of reduction of liver damage are mediated by various effects of HBO, which directly increases the oxygen concentration in the remnant liver and augments the expressions of HGF[11] and vascular endothelial growth factor (VEGF)[14].
HGF, known as the scatter factor, has mitogenic, motogenic, morpho-organogenic, angiogenic, and anti-apoptotic effects. HGF and its receptor c-Met are key factors for liver growth and function. TGF β-1 is an inhibitor of proliferation in hepatocyte cultures[25]. Data from the present study did not reveal significant differences in mRNA levels of HGF and TGF β-1 with HBO treatment after the partial hepatectomy. However, data did reveal that HBO suppressed c-Met mRNA expression at 15 h after the partial hepatectomy. HGF and c-Met promote liver regeneration, while TGF β-1 inhibits it[25]. A balance of these promoter and inhibitor levels is important for liver regeneration[26]. HBO modulated the expression of c-Met mRNA and might cause an improvement in cholestasis and liver edema.
In conclusion, data from this study suggests that HBO has beneficial effects on the regeneration of biliary ductal cells and postoperative cholestasis after a partial hepatectomy. HBO also improved cell edema of hepatocytes in the regenerating liver. HBO might be useful for improving resectability and for decreasing the frequency of postoperative complications, such as liver failure, after a major hepatectomy.
Extended hepatectomy and small graft liver transplantation can result in postoperative cholestasis and liver failure. Postoperative liver failure causes cholestasis; hence, it is important to improve cholestasis in order to reduce morbidity and mortality.
Hyperbaric oxygenation (HBO) has been used as a therapy in patients with carbon monoxide poisoning, decompression sickness and arterial gas embolism. Effects of HBO which have been reported include upregulation of growth factors, down-regulation of inflammatory cytokines and increased angiogenesis.
The beneficial effects of HBO have been reported in ischemia-reperfusion injuries, acute liver failure after major hepatectomy, hepatic artery thrombosis after liver transplantation and liver regeneration after portal vein embolization. Further to this, the study would suggest that HBO promotes regeneration of biliary cells and improves cholestasis in rats after a partial hepatectomy.
The study would suggest that HBO has beneficial effects on the regeneration of biliary cells and postoperative cholestasis after a partial hepatectomy. HBO also improved cell edema of hepatocytes in the regenerating liver. HBO might be useful for improving resectability and for decreasing the frequency of postoperative complications, such as liver failure after a major hepatectomy.
HBO involves the inspiration of a high concentration of oxygen in a pressure chamber at pressures higher than 1 ATA. During HBO, the partial pressure of oxygen increases, thereby increasing oxygen delivery throughout the body. In general, HBO has been used as a therapy in patients with carbon monoxide poisoning, decompression sickness and arterial gas embolism. Other beneficial effects of HBO in hepatic surgery and liver regeneration have been reported.
The authors investigated the effect of HBO administration on bile duct cellular regeneration and cholestasis in a 70% model of hepatectomy. This is an area of high significance as findings may have a potential impact on therapeutic strategies in liver failure and regeneration following major hepatectomy. In limitation, the conclusions are based on results up to 96 h. The regeneration cycle is usually complete by day 8 and it may be appropriate to extend the time interval for the results to be obtained to this stage, before an appropriate conclusion may be reached. It may very well be the optimum results evident are a transient phenomena during the regenerative cycle.
Peer reviewer: Christopher Christophi, Professor and Head of The University of Melbourne Department of Surgery, Austin Hospital. Melbourne, 145 Studley Road, Victoria 3084, Australia
S- Editor Sun H L- Editor Logan S E- Editor Zheng XM
| 1. | Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642-1648. |
| 2. | Kang TS, Gorti GK, Quan SY, Ho M, Koch RJ. Effect of hyperbaric oxygen on the growth factor profile of fibroblasts. Arch Facial Plast Surg. 2004;6:31-35. |
| 3. | Yamashita M, Yamashita M. Hyperbaric oxygen treatment attenuates cytokine induction after massive hemorrhage. Am J Physiol Endocrinol Metab. 2000;278:E811-E816. |
| 4. | Sheikh AY, Rollins MD, Hopf HW, Hunt TK. Hyperoxia improves microvascular perfusion in a murine wound model. Wound Repair Regen. 2005;13:303-308. |
| 5. | Higgins GM, Anderson RM: Experimental pathology of the liver. Arch Pathol. 1931;12:186–202. |
| 6. | Makino H, Shimizu H, Ito H, Kimura F, Ambiru S, Togawa A, Ohtsuka M, Yoshidome H, Kato A, Yoshitomi H. Changes in growth factor and cytokine expression in biliary obstructed rat liver and their relationship with delayed liver regeneration after partial hepatectomy. World J Gastroenterol. 2006;12:2053-2059. |
| 7. | Kihara K, Ueno S, Sakoda M, Aikou T. Effects of hyperbaric oxygen exposure on experimental hepatic ischemia reperfusion injury: relationship between its timing and neutrophil sequestration. Liver Transpl. 2005;11:1574-1580. |
| 8. | Ijichi H, Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Shimada M, Maehara Y. Effect of hyperbaric oxygen on cold storage of the liver in rats. Liver Int. 2006;26:248-253. |
| 9. | Ponikvar R, Buturović J, Cizman M, Mekjavić I, Kandus A, Premru V, Urbancic A, Zakotnik B, Bren A, Ivanovich P. Hyperbaric oxygenation, plasma exchange, and hemodialysis for treatment of acute liver failure in a 3-year-old child. Artif Organs. 1998;22:952-957. |
| 10. | Grover I, Conley L, Alzate G, Lavine J, Van Hoesen K, Khanna A. Hyperbaric oxygen therapy for hepatic artery thrombosis following liver transplantation: current concepts. Pediatr Transplant. 2006;10:234-239. |
| 11. | Uwagawa T, Unemura Y, Yamazaki Y. Hyperbaric oxygenation after portal vein emobilization for regeneration of the predicted remnant liver. J Surg Res. 2001;100:63-68. |
| 12. | Tolentino EC, Castro e Silva O, Zucoloto S, Souza ME, Gomes MC, Sankarankutty AK, Oliveira GR, Feres O. Effect of hyperbaric oxygen on liver regeneration in a rat model. Transplant Proc. 2006;38:1947-1952. |
| 13. | Nagamine K, Kubota T, Togo S, Nagashima Y, Mori M, Shimada H. Beneficial effect of hyperbaric oxygen therapy on liver regeneration after 90% hepatectomy in rats. Eur Surg Res. 2004;36:350-356. |
| 14. | Ijichi H, Taketomi A, Yoshizumi T, Uchiyama H, Yonemura Y, Soejima Y, Shimada M, Maehara Y. Hyperbaric oxygen induces vascular endothelial growth factor and reduces liver injury in regenerating rat liver after partial hepatectomy. J Hepatol. 2006;45:28-34. |
| 15. | Wang XC, Liu XM, Tan CZ, Liu R, Luo SL, Yue XH, Qin F, Bu ZR, Tian X, Song DY. Epidemic non-A, non-B hepatitis in Xinjiang. Clinical and pathologic observations. Chin Med J (Engl). 1990;103:890-898. |
| 16. | Nakajima T, Elovaara E, Okino T, Gelboin HV, Klockars M, Riihimäki V, Aoyama T, Vainio H. Different contributions of cytochrome P450 2E1 and P450 2B1/2 to chloroform hepatotoxicity in rat. Toxicol Appl Pharmacol. 1995;133:215-222. |
| 17. | Dwivedi Y, Rastogi R, Mehrotra R, Garg NK, Dhawan BN. Picroliv protects against aflatoxin B1 acute hepatotoxicity in rats. Pharmacol Res. 1993;27:189-199. |
| 18. | Pellinen P, Stenbäck F, Kojo A, Honkakoski P, Gelboin HV, Pasanen M. Regenerative changes in hepatic morphology and enhanced expression of CYP2B10 and CYP3A during daily administration of cocaine. Hepatology. 1996;23:515-523. |
| 19. | Koteish A, Yang S, Lin H, Huang X, Diehl AM. Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting Jun N-terminal kinase and caspase 3 activation. J Biol Chem. 2002;277:13037-13044. |
| 20. | Zhao M, Matter K, Laissue JA, Zimmermann A. Copper/zinc and manganese superoxide dismutases in alcoholic liver disease: immunohistochemical quantitation. Histol Histopathol. 1996;11:899-907. |
| 21. | Le TH, Caldwell SH, Redick JA, Sheppard BL, Davis CA, Arseneau KO, Iezzoni JC, Hespenheide EE, Al-Osaimi A, Peterson TC. The zonal distribution of megamitochondria with crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2004;39:1423-1429. |
| 22. | Ono M, Saibara T. Clinical features of nonalcoholic steatohepatitis in Japan: Evidence from the literature. J Gastroenterol. 2006;41:725-732. |
| 23. | Sun CK, Zhang XY, Zimmermann A, Davis G, Wheatley AM. Effect of ischemia-reperfusion injury on the microcirculation of the steatotic liver of the Zucker rat. Transplantation. 2001;72:1625-1631. |
| 24. | Ozdogan M, Ersoy E, Dundar K, Albayrak L, Devay S, Gundogdu H. Beneficial effect of hyperbaric oxygenation on liver regeneration in cirrhosis. J Surg Res. 2005;129:260-264. |
| 25. | Ross MA, Sander CM, Kleeb TB, Watkins SC, Stolz DB. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology. 2001;34:1135-1148. |
| 26. | Ninomiya M, Harada N, Shiotani S, Hiroshige S, Minagawa R, Soejima Y, Suehiro T, Nishizaki T, Shimada M, Sugimachi K. Hepatocyte growth factor and transforming growth factor beta1 contribute to regeneration of small-for-size liver graft immediately after transplantation. Transpl Int. 2003;16:814-819. |