Published online Apr 28, 2011. doi: 10.3748/wjg.v17.i16.2143
Revised: November 13, 2010
Accepted: November 20, 2010
Published online: April 28, 2011
AIM: To evaluate the efficacy and safety of combination therapy with recombinant adenovirus p53 injection (rAdp53) and transcatheter hepatic arterial chemoembolization (TACE) for advanced hepatocellular carcinoma (HCC).
METHODS: A total of 82 patients with advanced HCC treated only with TACE served as control group. Another 68 patients with HCC treated with TACE in combination with recombinant adenovirus-p53 injection served as p53 treatment group. Patients were followed up for 12 mo. Safety and therapeutic effects were evaluated according to the improvement in clinical symptoms, leukocyte count, Karnofsky and RECIST criteria. Survival rate was calculated with Kaplan-Meier method.
RESULTS: The total effective rate was 58.3% for p53 treatment group, and 26.5% for control group (P < 0.05). The incidence of gastrointestinal symptoms was lower in p53 treatment group than in control group (P < 0.05). The 3-, 6- and 12-mo survival rates were significantly higher for p53 treatment group than for control group (P < 0.01). The combination treatment was well tolerated with such adverse events as fever (51.5%, P = 0.006) and pain of muscles and joints (13.2%, P = 0.003), which were significantly higher than the chemotherapy. Except for these minor adverse effects, no severe vector-related complications were identified. With respect to the efficacy, patients in p53 treatment group had less gastrointerestinal symptoms (P = 0.062), better improvement in tumor-related pain (P = 0.003), less downgrade of leukocyte counts (P = 0.003) and more upgrade of Karnofsky performance score (P = 0.029) than those in control group. The total effective rate (CR + PR) for p53 treatment group and control group was 58.3% and 26.5%, respectively, with distributions of different effect in two groups (P = 0.042). The survival rates were 89.71%, 76.13%, and 43.30% for p53 treatment group, and 68.15%, 36.98%, and 24.02% for control group, respectively, 3, 6 and 12 mo after treatment, suggesting that the survival rates are significantly higher for p53 treatment group than for control group (P = 0.0002).
CONCLUSION: The rAd-p53 gene therapy in combination with TACE is a safe and effective treatment modality for advanced HCC.
-
Citation: Guan YS, Liu Y, He Q, Li X, Yang L, Hu Y, La Z.
p53 gene therapy in combination with transcatheter arterial chemoembolization for HCC: One-year follow-up. World J Gastroenterol 2011; 17(16): 2143-2149 - URL: https://www.wjgnet.com/1007-9327/full/v17/i16/2143.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i16.2143
Gene therapy is a potentially new treatment modality for cancer patients and an engineered recombinant replication-defective adenovirus can express the tumor suppressor gene p53 (rAd-p53) with encouraging clinical responses[1-3]. rAd-p53 has been recently approved by the State Food and Drug Administration of China as the very first gene therapy product for head and neck squamous cell carcinoma (HNSCC)[4].
Hepatocellular carcinoma (HCC) is one of the major cancers in China with a poor prognosis due to its occult onset, rapid infiltrating growth and complicating liver cirrhosis. No effective treatment modality is available for it at present. Although transcatheter hepatic arterial chemoembolization (TACE) is currently one of the most popular treatment modalities for unresectable advanced HCC, the long-term survival rate of such patients remains low with a reported 5-year survival rate of 17%[5]. In this study, the safety and efficacy of rAd-p53 therapy in combination with TACE were examined in patients with advanced HCC.
rAd-p53 is a recombinant human serotype 5 adenovirus in which the E1 region is replaced by a human wild-type p53 expression cassette. The p53 gene is driven by a Rous sarcoma virus promoter with a bovine growth hormone poly (A) tail. The recombinant adenovirus is produced in human embryonic kidney 293 cells and manufactured by Shenzhen SiBionoGenTech Co. Ltd (Shenzhen, China) and marketed under the trade name of Gendince®. Before p53 gene therapy, a vial of rAd-p53 is taken out from a refrigerator in which the temperature is about -20°C. When thawed, the solution, diluted with 1 mL NS, is sucked into a 5-mL syringe for intra-tumor injection.
One hundred and fifty patients (83 men and 67 women) with advanced HCC were enrolled in this study from March to July 2004. Patients with Child C disease[6], tumor thrombus in the main portal trunk, or extrahepatic metastasis were excluded. These exclusion criteria were implemented to ensure at least a 3-mo life span in the enrolled patients so as to have enough time to follow up. All patients did not receive local ethanol injection, microwave coagulation, systemic chemotherapy or radiotherapy before and after TACE or gene therapy. All tumors were diagnosed according to pathologic examination, distinctive findings on computed tomography (CT), conventional angiography, magnetic resonance imaging (MRI), or serum tumor markers [alpha-fetoprotein (AFP) or ferritin]. The patients were divided into gene treatment group (n = 68) with a mean age of 43 years (range 20-72 years) and control group (n = 82) with a mean age of 45 years (range 18-75 years). No patient was classified as stage I or II while 91 patients were classified as stage III and 59 patients as stage IV according to the International Union against Cancer TNM classification[7].
Patients who gave their informed consent to receive Ad-p53 gene therapy served as gene treatment group, while those not willing to receive gene therapy served as control group. Patients in gene treatment group underwent rAd-p53 gene therapy and TACE while those in control group received only TACE. Although this was a retrospective nonrandomized study, no statistical difference was observed in baseline between the two groups. The characteristics of the two groups are illustrated in Table 1.
| Characteristics | Gene group(n = 68) | Control group(n = 82) | Statistic analysis |
| Age | 43.5 (20 - 72) | 45.7 (18 - 75) | NS |
| Sex (M/F) | 43/25 | 40/42 | NS |
| Child class A | 41 | 43 | NS |
| Child class B | 27 | 39 | NS |
| UICC TNM classification | |||
| Stage I and II | 0 | 0 | NS |
| Stage III | 31 (46.5%) | 60 (73.2%) | NS |
| Stage IV | 37 (54.4%) | 22 (26.8%) | NS |
| Size of main tumors | |||
| ≥ 5 cm | 53 (77.9%) | 61 (74.3%) | NS |
| < 5 cm | 15 (22.1%) | 21 (25.7%) | NS |
The patients in gene treatment group were placed in a supine, prone or lateral position on the CT scanning bed and asked to hold their breath after an inhalation. The slice for puncture was carefully determined, the puncture site on the surface of body as well as the needle-traveling depth and angle within the body were determined. The bed was moved to the slice and a marker for puncture was made on the body surface according to the laser beam emitted from the gantry. The bed was then moved out and the puncture site was sterilized. After local anesthesia, a 19-G needle was inserted into the puncture site according to the determined angle and depth as the operator asked the patient to hold his or her breath after an inhalation. Finally, another scan was performed to make sure that the tip of the needle was within the tumor, and the rAd-p53 gene was injected into the tumor in a multi-point fashion. Usually, this procedure is repeated according to the patient’s clinical condition and the interval between two procedures is about 1 wk. At each injection, 1-4 rAd-p53 injections are administered at a viral dose of 1-4 × 1012 VP (viral particles) according to the diameter of the lesion, and the intra-tumor injection usually lasts 1-2 min.
TACE was performed through the femoral artery using the Seldinger technique with local anesthesia. Arteriography of the celiac trunk and superior mesenteric artery was performed to visualize the arterial vascularization of liver and evaluate portal vein patency. An angiographic catheter was inserted into the right or left hepatic artery where the target tumor was located. TACE agents, involving embolic agent (Lipiodol) and anticancer drugs, were injected through the right or left hepatic artery. In both groups, the dose of Lipiodol, ranging 3-20 mL, was determined according to the tumor location, tumor size, number of tumors, and functional hepatic reserve. Anticancer drugs used were 5-Fluorouracil (800-1000 mg) and vinorelbine (30-40 mg). TACE was repeated according to the patient’s clinical condition at a 1-mo interval.
Clinical symptoms, leukocyte counts and Karnofsky index evaluation were recorded before and after treatment. After treatment, CT scan or MRI was performed every three months with or without contrast enhancement to evaluate the features of Lipiodol deposit and the therapeutic effect according to the response evaluation criteria for solid tumors[8]. If elevated tumor markers (AFP and ferritin), diminished Lipiodol, or enlarged lesions or new nodules were observed, the patients were readmitted for angiography and treatment. The starting point of survival analysis was regulated as the day of initial treatment. The Kaplan-Meier method was used to analyze the survival rates in the two groups.
Statistical analysis was performed to assess the baseline, leukocyte counts, Karnofsky index, clinical symptoms and survival curve between the two groups using the SPSS 11.0. P < 0.05 was considered statistically significant.
Two hundred and fifty-one p53 intra-tumor injections were performed for 83 lesions in 68 patients of gene treatment group. Of the 68 patients, 9 received one injection, 13 received two injections, 15 received three injections, 20 received four injections, 7 received five injections, 3 received six injections and 1 received seven injections. One hundred and ninety-two 2 (mean 2.82 procedures) and 167 (mean 2.03 procedures) procedures of TACE were performed in gene treatment and control groups, respectively. Arterial portal vein shunt (AVS), arterial hepatic vein shunt (APS) or/and portal vein involvement, signs that meant a high invasion and a poor prognosis were found in 27.9% (19/68) patients of gene treatment group and 36.6% (30/82) patients of control group, respectively, during the TACE. Although the patients with tumor thrombus in the main portal trunk were excluded, some of them developed vascular invasion because of tumor progression after they were enrolled in this study. No difference was observed in the incidence of malignancy signs such as AVS, APS or portal vein involvement between the two groups.
The clinical symptoms were carefully recorded after treatment (Table 2). Overall, rAd-p53 gene therapy in combination with TRCE was well tolerated. The most frequent adverse event occurred in patients receiving rAd-p53 gene therapy in combination with TACE was the flu-like symptom associated with fever. Of the 68 patients in gene treatment group, 35 (51.5%) had a fever at 38-39.5°C, usually occurred 3-10 h after p53 intra-tumor injection and decreased after physic cooling, and 9 (13.2%) had pain of muscles or joints which often faded away (Table 2). No other severe gene therapy-associated complications were encountered in this study.
The clinical symptoms were carefully recorded after treatment (Table 2). The patients in gene treatment group had less gastrointerestinal symptoms such as nausea, vomiting, abdominal pain or belling than those in control group. The palliative rate of mass-associated pain one week after treatment was 44.1% (30/68) for patients in gene treatment group, higher than that for those in control group.
Before and one week after treatment, the number of leukocytes was calculated (Table 3). Statistical analysis showed that the number of leukocytes was smaller in gene treatment group than in control group (P = 0.003).
| Group | Change degree (×109/L) | n (%) | ||
| < 4.0 | < 3.0 | < 2.0 | ||
| Gene group | 12 (25.0) | 4 (8.3) | 2 (4.2) | 18 (37.5) |
| Control group | 8 (13.3) | 20 (33.3) | 11 (18.3) | 39 (65.0) |
Karnofsky index was changed in gene treatment group one month after treatment (Table 4). Generally speaking, the patients in gene treatment group had a higher Karnofsky index than those in control group (P = 0.029).
| Group | Upgrade > 20 points | Upgrade > 10 points | No changes | Downgrade > 10 points | Total upgrade[n (%)] |
| Gene group | 14 | 28 | 18 | 8 | 42 (61.8) |
| Control group | 12 | 24 | 18 | 28 | 36 (43.9) |
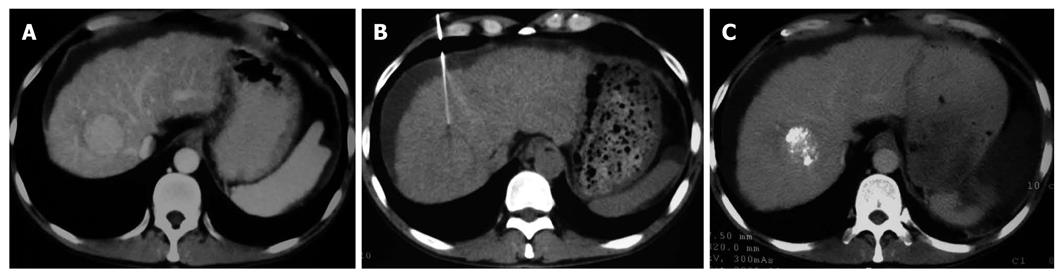
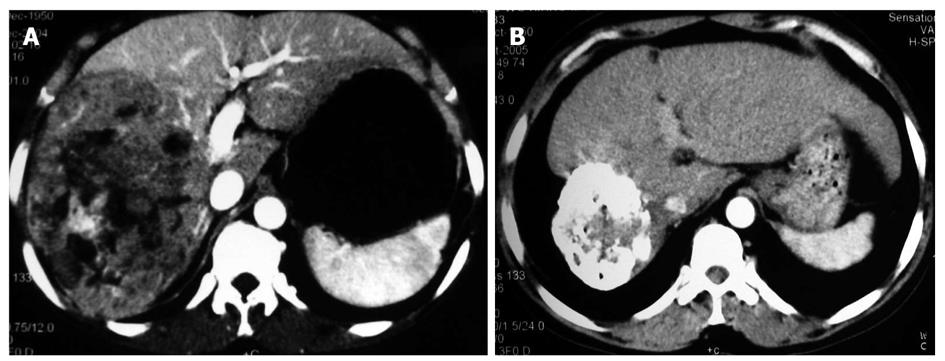
The therapeutic effect was evaluated following the response evaluation criteria for solid tumors after treatment. CR, PR, NC and PD in the two groups are listed in Table 5. The total effective rate (CR + PR) was 58.3% and 26.5% for the gene treatment group and control group, respectively (P < 0.05). Chi-square test showed that the distributions of therapeutic effect were statistically different (P = 0.042, Figures 1 and 2)
| Group | n | CR | PR | NC | PD | Effective rate (CR + PR) |
| Gene group | 68 | 0 | 46 | 15 | 7 | 67.60% |
| Control group | 82 | 0 | 42 | 27 | 13 | 51.20% |
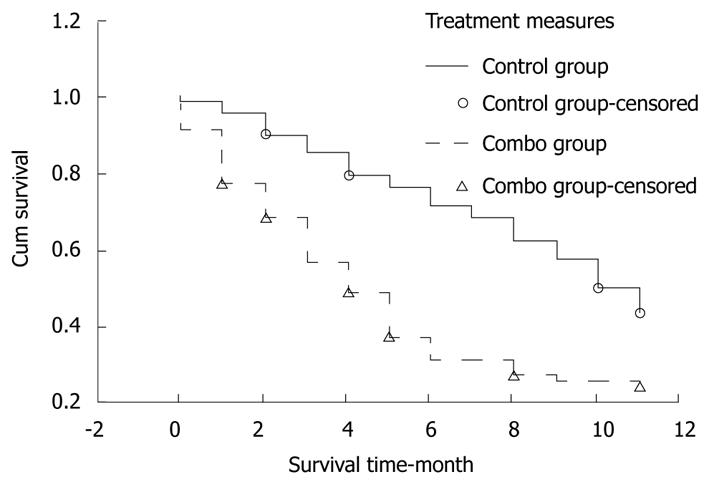
The patients were followed up for 12 mo. The number of withdrawal patients in gene treatment group and control group was 4 and 7, respectively. The survival rate was 89.71% (standard error 0.036), 76.13% (standard error 0.052), and 43.30% (standard error 0.061), respectively, for the patients in gene treatment group 3, 6, and 12 mo after treatment. The survival rate was 68.15% (standard error 0.051), 36.98% (standard error 0.054), and 24.02% (standard error 0.049), respectively, for those in control group 3, 6, and 12 mo after treatment. Log-rank test showed that the survival rate for the two groups was significantly different (P = 0.0002, Figure 3).
Hepatocellular carcinoma (HCC) is a highly malignant tumor with a very high morbidity and mortality. Since TACE was introduced as a palliative treatment of unresectable HCC, it has become one of the most common interventional therapies[9-12]. However, its therapeutic effect is also limited due to the lack of appropriate and reliable embolic agents, and the infiltrative or hypovascular nature, too large or small in size[13-15]. Another limitation of TACE is the need for repeated treatment, thus resulting in deterioration of liver function[16]. So, lots of efforts have been made to explore other new therapies in order to achieve the better efficacy of multiple treatments. PEI or RFA gene therapy in combination with TACE may improve the survival rate of HCC patients and decrease the risk of liver failure[17-19]. In this study, p53 gene therapy in combination with TACE could overcome the downside of TACE and improve the prognosis of HCC patients.
The p53 tumor suppressor gene is a gene guardian and loss of p53 is responsible for the lack of apoptotic signals in tumor cells and thus for their uncontrolled proliferation and recurrence[20]. Many human tumors carry mutations in the p53 gene[21,22] and mutant or absent p53 gene is associated with the resistance to radiotherapy and apoptosis-inducing chemotherapy[23]. It has been shown that p53 gene therapy in combination with radiotherapy or chemotherapy can control local tumor, suggesting that it is superior to either radiotherapy or chemotherapy alone[24,25]. It was reported that the incidence of p53 mutation is 61% in HCC[22]. Chen et al[26] also reported that mutations in the p53 gene are frequently detectable in recurrent HCC and the interval between surgical resection and recurrence of HCC is significantly longer in patients with the wild-type p53 gene than in those with mutant p53 gene mutations, strongly suggesting that the mutant p53 gene plays a role in pathogenesis of HCC. Jeng et al[27] demonstrated that the biological behavior of the mutant p53 gene is strongly related to the invasiveness of HCC and may also influence the postoperative course of HCC. Many scholars suggest that immunopositivity of the mutant p53 gene plays a role in predicting the prognosis of patients with HCC after resection[27-29].
The rAd-p53 gene has been approved in China under the trade name of Gendicine for the treatment of head and neck squamous cell carcinoma (HNSCC). In one of the trials[3], 75% tumors experienced complete regression following 8 wk of therapy involving 1 injection per week, which was significantly higher than that in control group, and combined chemotherapy and radiotherapy improved the treatment efficacy of over 3-fold. Although its recommended indications are limited in HNSCC according to the specification, good treatment efficacy can be achieved in HCC patients when rAd-p53 is used[30]. In the current study, Gendicine was used in treatment of HCC to evaluate its effect in order to provide some evidence for its off-table use in treatment of HCC.
As for the safety of rAd-p53 used in treatment of advanced HCC, just fever at 38-39.5°C was observed in our study, which was returned to normal after symptomatic treatment. In addition, some patients suffered from pain of muscles or joints and its cause is still controversial. However, no severe complications caused by Gendicine were observed. Although these adverse events have been observed in clinical practice, they can be well tolerated by most patients with no severe physical and mental harm.
The patients receiving p53 gene therapy had less severe post embolization syndrome than others after TACE. Gastrointestinal symptoms, such as nausea, vomiting and abdominal pain or belling, were less frequently observed in gene treatment group than in control group. The decreased number of leukocytes in gene treatment group was a pleasing phenomenon. However, its mechanism remains to be studied. The Karnofsky index was significantly higher, suggesting that the life quality of patients is largely improved in gene treatment group. It could be concluded that the rAd-p53 gene therapy could reduce the side effects of chemical drugs and Lipiodol embolization. Also, it was noticed that many patients in gene treatment group had a compact Lipiodol deposit manifested as a high homogenous density occupying the majority of tumor mass (Figures 1 and 2). Compact deposit means tumor necrosis. Further study is needed to observe whether p53 gene therapy is related to the better deposit of Lipiodol in lesions.
Theoretically, in-vitro p53 protein can bring about specific anti-tumor cells into effect in such ways as induction of apoptosis or necrosis, incentive of body immune response, regulation of cell cycle, etc. Two months after treatment, the distributions of therapeutic effect in the two groups were statistically different and the effective rate (CR + PR) was higher for p53 gene treatment group than for control group, suggesting that p53 gene therapy can enhance the efficacy of TACE, radiotherapy and chemotherapy.
Kaplan-Meier analysis showed that the survival rate was higher for gene treatment group than for control group. Because no other control study is available, the outcome of p53 gene therapy for such a large number of patients was not compared with that in other studies. The 1-year survival rate was lower in our study than in another study (67% vs 81%)[31], which may be attributed to the different baselines, in which our enrolled patients might have a larger lesion and a poorer liver function reserve.
Although it seems that the higher survival rate in gene treatment group may be attributed to the longer mean TACE time in patients of gene treatment group than in those of control group (2.82 vs 2.03), it was the clinical improvement after p53 gene therapy that made the patients in gene treatment group have more chance to receive repeated TACE. On the other hand, no difference was found in the incidence of malignancy DSA signs between the two groups. However, these signs appeared later with a lower incidence in gene treatment group than in control group, which is an interesting phenomena, and further study with a larger sample size is needed to confirm it.
Usually, the rAd-p53 gene begins to express p53 protein 3 h after intra-tumor injection, reaches its peak on day 3, and then gradually decreases according to the specification of Gendicine®. On day 5 after injection, the expression decreases to 30%. Because most of the chemotherapeutic drugs can affect DNA or RNA duplication or expression, cell cycle or nucleic acid metabolism would likewise affect the expression of p53 gene in tumor tissue. In this study, TACE was started 3-4 d after p53 injection when the p53 protein was highly expressed in tumor tissue, indicating that these anti-tumor drugs do not interfere with the expression of p53. However, the optimal interval remains to be further studied.
In conclusion, rAd-p53 gene therapy in combination with TACE is well tolerated and its anti-tumor efficacy is superior to that of TACE alone in terms of the survival rate and improved symptoms of HCC patients. Further clinical study with a large sample size is warranted to optimize the administration procedure and assess the impact of anti-p53 antibody on its therapeutic effect.
Hepatocellular carcinoma (HCC) is one of the major cancers in China with a poor prognosis due to its occult onset, rapid infiltrating growth and complicating liver cirrhosis. Although transcatheter arterial chemoembolization (TACE) has been used in treatment of HCC for years, its effect is often unsatisfactory.
Among the actively studied novel treatment modalities for HCC, the majority of experts hold that comprehensive or combination ones are most promising. In addition, gene therapy with p53 (rAd-p53) is a potentially new treatment modality for cancer.
TACE in combination of rAd-p53 injection has a synergistic effect on HCC and its strategy is gene addition. Tumor with mutant of the rAd-p53 gene is a better candidate for p53 therapy. However, this treatment is also effective in those with inactivated wild-type p53, a common condition in tumors. Injection of rAd-p53 can lead to apoptosis of tumor cells and TACE can result in necrosis of tumor tissue.
The results of this study demonstrate that TACE in combination with rAd-p53 with is well tolerated and its anti-tumor efficacy is superior to that of TACE alone with respect to the survival rate and improved symptoms. Further study with a large sample size would provide an alternative treatment modality for HCC.
p53 gene is a tumor suppressor gene which can prevent the formation of tumors. Mutations in p53 are found in most tumor types and contribute to complex molecular events leading to tumor formation. Recombinant adenovirus is one of the viral vectors which are commonly used to deliver genetic materials into cells. Gene therapy for diseases is to insert, alterate, or remove such materials in cells.
This is a well-designed study in which the authors analyzed the clinical effect of rAd-p53 injection and TACE on advanced HCC. The data show that the combination therapy is a safe and effective treatment modality for advanced HCC, and can significantly improve the survival rate of HCC patients.
Peer reviewer: Dr. Jun Qin, Baylor College of Medicine, One Baylor Plaza, Houston 77030, United States
S- Editor Sun H L- Editor Wang XL E- Editor Ma WH
| 1. | Robson T, Hirst DG. Transcriptional Targeting in Cancer Gene Therapy. J Biomed Biotechnol. 2003;2003:110-137. |
| 2. | Zhang SW, Xiao SW, Liu CQ, Sun Y, Su X, Li DM, Xu G, Cai Y, Zhu GY, Xu B. [Treatment of head and neck squamous cell carcinoma by recombinant adenovirus-p53 combined with radiotherapy: a phase II clinical trial of 42 cases]. Zhonghua YiXue ZaZhi. 2003;83:2023-2028. |
| 3. | Chen CB, Pan JJ, Xu LY. [Recombinant adenovirus p53 agent injection combined with radiotherapy in treatment of nasopharyngeal carcinoma: a phase II clinical trial]. Zhonghua YiXue ZaZhi. 2003;83:2033-2035. |
| 4. | Cancer gene therapy is first to be approved. Available from: URL:http://www.lucifer.com/pipermail/ectropy-chat/attachments/20030019/ee07cea0/attachment.htm . . |
| 5. | Ueno K, Miyazono N, Inoue H, Nishida H, Kanetsuki I, Nakajo M. Transcatheter arterial chemoembolization therapy using iodized oil for patients with unresectable hepatocellular carcinoma: evaluation of three kinds of regimens and analysis of prognostic factors. Cancer. 2000;88:1574-1581. |
| 6. | Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, Sato M, Yamanaka N, Shimamura Y, Ohto M. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699-704. |
| 7. | Sobin LH, Wittekind CH, editors. UICC TNM classification of malignant tumours. 5th ed. New York: John Wiley and Sons, 1997 . . |
| 8. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. |
| 9. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. |
| 10. | Li L, Wu PH, Li JQ, Zhang WZ, Lin HG, Zhang YQ. Segmental transcatheter arterial embolization for primary hepatocellular carcinoma. World J Gastroenterol. 1998;4:511-512. |
| 11. | Mizoe A, Yamaguchi J, Azuma T, Fujioka H, Furui J, Kanematsu T. Transcatheter arterial embolization for advanced hepatocellular carcinoma resulting in a curative resection: report of two cases. Hepatogastroenterology. 2000;47:1706-1710. |
| 12. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. |
| 13. | Qian J, Truebenbach J, Graepler F, Pereira P, Huppert P, Eul T, Wiemann G, Claussen C. Application of poly-lactide-co-glycolide-microspheres in the transarterial chemoembolization in an animal model of hepatocellular carcinoma. World J Gastroenterol. 2003;9:94-98. |
| 14. | Fan J, Ten GJ, He SC, Guo JH, Yang DP, Wang GY. Arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 1998;4:33-37. |
| 15. | Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S319-S328. |
| 16. | Ahrar K, Gupta S. Hepatic artery embolization for hepatocellular carcinoma: technique, patient selection, and outcomes. Surg Oncol Clin N Am. 2003;12:105-126. |
| 17. | Qian J, Feng GS, Vogl T. Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol. 2003;9:1885-1891. |
| 18. | Chen XM, Luo PF, Lin HH, Zhou ZJ, Shao PJ, Fu L, Li WK. [Long-term result of combination of transcatheter arterial chemoembolization and percutaneous ethanol injection for treatment of hepatocellular carcinoma]. Ai Zheng. 2004;23:829-832. |
| 19. | Guo WJ, Yu EX, Liu LM, Li J, Chen Z, Lin JH, Meng ZQ, Feng Y. Comparison between chemoembolization combined with radiotherapy and chemoembolization alone for large hepatocellular carcinoma. World J Gastroenterol. 2003;9:1697-1701. |
| 20. | Kouraklis G. Progress in cancer gene therapy. Acta Oncol. 1999;38:675-683. |
| 21. | Friedmann T. Gene therapy of cancer through restoration of tumor-suppressor functions? Cancer. 1992;70:1810-1817. |
| 22. | Hsia CC, Nakashima Y, Thorgeirsson SS, Harris CC, Minemura M, Momosaki S, Wang NJ, Tabor E. Correlation of immunohistochemical staining and mutations of p53 in human hepatocellular carcinoma. Oncol Rep. 2000;7:353-356. |
| 23. | Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, Housman DE, Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807-810. |
| 24. | Gjerset RA, Turla ST, Sobol RE, Scalise JJ, Mercola D, Collins H, Hopkins PJ. Use of wild-type p53 to achieve complete treatment sensitization of tumor cells expressing endogenous mutant p53. Mol Carcinog. 1995;14:275-285. |
| 25. | Nguyen DM, Spitz FR, Yen N, Cristiano RJ, Roth JA. Gene therapy for lung cancer: enhancement of tumor suppression by a combination of sequential systemic cisplatin and adenovirus-mediated p53 gene transfer. J Thorac Cardiovasc Surg. 1996;112:1372-1376; discussion 1376-1377. |
| 26. | Chen GG, Merchant JL, Lai PB, Ho RL, Hu X, Okada M, Huang SF, Chui AK, Law DJ, Li YG. Mutation of p53 in recurrent hepatocellular carcinoma and its association with the expression of ZBP-89. Am J Pathol. 2003;162:1823-1829. |
| 27. | Jeng KS, Sheen IS, Chen BF, Wu JY. Is the p53 gene mutation of prognostic value in hepatocellular carcinoma after resection? Arch Surg. 2000;135:1329-1333. |
| 28. | Qin LX, Tang ZY, Ma ZC, Wu ZQ, Zhou XD, Ye QH, Ji Y, Huang LW, Jia HL, Sun HC. P53 immunohistochemical scoring: an independent prognostic marker for patients after hepatocellular carcinoma resection. World J Gastroenterol. 2002;8:459-463. |
| 29. | Sheen IS, Jeng KS, Wu JY. Is p53 gene mutation an indicatior of the biological behaviors of recurrence of hepatocellular carcinoma? World J Gastroenterol. 2003;9:1202-1207. |
| 30. | Guan YS, Liu Y, Zhou XP, Li X, He Q, Sun L. p53 gene (Gendicine) and embolisation overcame recurrent hepatocellular carcinoma. Gut. 2005;54:1318-1319. |
| 31. | Kamada K, Nakanishi T, Kitamoto M, Aikata H, Kawakami Y, Ito K, Asahara T, Kajiyama G. Long-term prognosis of patients undergoing transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: comparison of cisplatin lipiodol suspension and doxorubicin hydrochloride emulsion. J Vasc Interv Radiol. 2001;12:847-854. |