Published online Apr 7, 2011. doi: 10.3748/wjg.v17.i13.1746
Revised: January 11, 2011
Accepted: January 18, 2011
Published online: April 7, 2011
AIM: To investigate the effect of carbachol on gastrointestinal function in a dog model of oral resuscitation for burn shock.
METHODS: Twenty Beagle dogs with intubation of the carotid artery, jugular vein and jejunum for 24 h were subjected to 35% total body surface area full-thickness burns, and were divided into three groups: no fluid resuscitation (NR, n = 10), in which animals did not receive fluid by any means in the first 24 h post-burn; oral fluid resuscitation (OR, n = 8), in which dogs were gavaged with glucose-electrolyte solution (GES) with volume and rate consistent with the Parkland formula; and oral fluid with carbachol group (OR/CAR, n = 8), in which dogs were gavaged with GES containing carbachol (20 μg/kg), with the same volume and rate as the OR group. Twenty-four hours after burns, all animals were given intravenous fluid replacement, and 72 h after injury, they received nutritional support. Hemodynamic and gastrointestinal parameters were measured serially with animals in conscious and cooperative state.
RESULTS: The mean arterial pressure, cardiac output and plasma volume dropped markedly, and gastrointestinal tissue perfusion was reduced obviously after the burn injury in all the three groups. Hemodynamic parameters and gastrointestinal tissue perfusion in the OR and OR/CAR groups were promoted to pre-injury level at 48 and 72 h, respectively, while hemodynamic parameters in the NR group did not return to pre-injury level till 72 h, and gastrointestinal tissue perfusion remained lower than pre-injury level until 120 h post-burn. CO2 of the gastric mucosa and intestinal mucosa blood flow of OR/CAR groups were 56.4 ± 4.7 mmHg and157.7 ± 17.7 blood perfusion units (BPU) at 24 h post-burn, respectively, which were significantly superior to those in the OR group (65.8 ± 5.8 mmHg and 127.7 ± 11.9 BPU, respectively, all P < 0.05). Gastric emptying and intestinal absorption rates of GES were significantly reduced to the lowest level (52.8% and 23.7% of pre-injury levels) in the OR group at about 2 and 4 h post-burn, and did not return to 80% of pre-injury level until 24 h. In the first 24 h post-burn, the rate of gastric emptying and intestinal water absorption were elevated by a mean 15.7% and 11.5%, respectively, in the OR/CAR group compared with the OR group. At 5 days, the mortality in the NR group was 30% (3/10), 12.5% in the OR group (1/8), and none in the OR/CAR group.
CONCLUSION: Carbachol had a beneficial effect on oral resuscitation of burn shock by promoting gastric emptying and intestinal absorption in our canine model.
- Citation: Hu S, Che JW, Tian YJ, Sheng ZY. Carbachol promotes gastrointestinal function during oral resuscitation of burn shock. World J Gastroenterol 2011; 17(13): 1746-1752
- URL: https://www.wjgnet.com/1007-9327/full/v17/i13/1746.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i13.1746
Rapid intravenous infusion of large quantity of fluids containing electrolytes and colloidal solutions remain the key measure to resuscitate hypovolemic shock as a result of a massive burn injury. This life-saving measure has unanimously been accepted worldwide. It has also been recognized that a delay in such replenishment could sometimes be fatal due to complications subsequent to delayed resuscitation of hypovolemic shock. Unfortunately, in certain cases, such as mass casualties in an incendiary bomb attack in battlefields, or a forest or prairie fire in regions with an austere environment with poor medical support and transportation facilities due to geographical barriers, not only there would be a shortage of medics to introduce an intravenous needle, but also the weight and bulk of the necessary intravenous fluids would make the treatment unrealistic. In such cases, it is our supposition that oral administration of fluids might be more practical, and it might be able to maintain the life of the victims till intravenous fluid replacement was available.
Oral fluid resuscitation has been reported with success in early clinical studies of burn care[1]. Thomas et al[2] have described oral fluid replacement in burn patients, and have concluded that oral resuscitation may have a slower initial onset of hemodynamic effectiveness, but after 3-4 h, it can be similarly effective. It is unanimously recognized that the main limiting factors for effective oral resuscitation of burn shock are diminution of gastric emptying capacity and intestinal absorption of fluid and electrolytes due to undermined gastrointestinal perfusion. It seems to be necessary to overcome these two hurdles before oral hydration can be successful for treatment of burn shock.
As enough data of oral resuscitation of burn shock could not be accumulated in clinical practice in ordinary situations, most investigations have been done with animals[3,4]. We found that the previous animal experiments have been limited by their short experimental duration of < 24 h post-burn, and performed under general anesthesia, which might interfere with the observation of gastric emptying and intestinal absorption of fluid and electrolytes. With these in mind, a canine model of burn shock was devised in which oral resuscitation was given in the first 24 h post-burn, followed by delayed intravenous fluid replacement, and the whole course of the experiment lasted for 120 h. Also, in this experiment, the effect of general anesthesia was eliminated, and carbachol, which is a cholinergic receptor agonist, was used in an attempt to shorten gastric emptying time and restore intestinal peristalsis.
All the experimental protocols were reviewed and approved by the Committee of Scientific Research of First Affiliated Hospital of General Hospital of PLA (Beijing, China).
Pure bred Beagle dogs (purchased from Experimental Animal Center of Academy of Military Medical Sciences of PLA, Beijing, China, License of qualification SCX 2005-0005), aged 16-20 mo, body weight 11-13 kg, were used. They were acclimatized in the animal house of our Research Laboratory for 2 wk before use. They were fasted for 24 h, and water was withheld for 4 h before the surgical preparation. Under anesthesia with 8 mg/kg ketamine (Gu-Tian Pharmacy, Fu Jian Province, China), the right carotid artery and jugular vein were individually cannulated for hemodynamic monitoring, collecting blood samples, and administration of drugs. Both cannula were led out through a subcutaneous passageway and fixed to the skin. A midline incision was made to open the peritoneal cavity to expose the proximal part of the jejunum, and small incisions were made on the jejunum 10, 20, and 50 cm distal to the Treitz ligament. A Silastic tube, 3 mm in diameter and 25 cm in length, was introduced into the jejunal lumen through each of the above openings. They were fixed with purse-string sutures, led out through a subcutaneous tunnel and fixed to the skin. A cystostomy was done for collecting urine.
Twenty-four hours after the above surgical procedures, an intravenous injection of 0.5 mL/kg propofol was given to all the animals to produce brief anesthesia for 10-15 min, which was long enough to eliminate pain during burn injury. Napalm (3%) was applied to the shaved neck and back of the dogs, and it was ignited for 30 s to produce a full-thickness burn that involved about 35% of the total body surface area (TBSA). The depth of the burn injury was verified by pathological examination.
The injured dogs were grouped into no fluid replacement (NR, n = 10), oral fluid replacement (OR, n = 8), and oral replacement of fluid with addition of carbachol (OR/CAR, n = 8). For NR, the animals received no fluid replacement or any other treatment. Dogs in the OR group received intragastric, pre-warmed glucose saline solution (each 1 L containing 59.83 mmol/L NaCl, 29.76 mmol/L NaHCO3, 20.18 mmol/L KCl, and 114.94 mmol/L glucose in distilled water), and the rate of gastric infusion was consistent with that of the Parkland formula[5] (4 mL/kg for each % TBSA burn, half of the total amount given in the first 8 h). In the OR/CAR group, 20 μg/kg carbachol (Sigma, St Louis, MO, USA) was added to the glucose-saline solution. Twenty-four hours after the burn injury, all the animals in the three groups were given intravenous fluid replenishment. Seventy-two hours after the burn injury, all the surviving animals were given 10% glucose with a mixture of 17 amino acids (Beijing Pharmaceutical, Beijing, China) and 30% fat emulsion (Hua Rui Pharmaceutical, Wu Xi, China). Also, intravenous Ringer’s solution was given to make up 100-150 mL of fluid per kg body weight and maintain blood potassium level > 3.5 mmol/L.
Picco-Plus (Pulsion, Germany) was used to determine mean arterial pressure (MAP) and cardiac output (CO). Plasma volume was determined with the indigo green dilution method[6]. At each time point for determination, 12.5 mg indocyanine green (ICG) (Dan Don Pharmaceutical, Liaoning, China) was intravenously given, and 3-mL blood samples were obtained at 1, 2 and 3 min after injection. The specimens were centrifuged at 1800 g at 4°C, and 1 mL plasma was obtained to determine the OD value of ICG with a 723N spectrophotometer (wave length, 820 nm), and the concentration of ICG was obtained from a plotted standard curve.
To determine CO2 partial pressure of the gastric mucosa (PgCO2), a gastric mucosa tonometer (Tonoca, Finland) was used. Intestinal mucosal blood flow (IMBF) was determined by passing a fiberoptic detector of a laser Doppler flow monitor (Peri Flus 5000 Master; Perimed, Jaarnfalla, Sweden) through the intestinal catheters, ensuring that the tips of the detectors were in contact with intestinal mucosa. The signals were transformed into blood perfusion units (BPU), which were input into a computer, and the PEIMED software package PSW 2.0 was used to plot the curves. Thirty seconds were spent for each measurement, and a stable curve that covered 10 s was taken for the calculation of the average.
The gastric emptying rate was determined by using phenol red (Sigma) as a signal, according to the Scarpignato principle[7]. Before the experiment, a standard curve was plotted with ODs of different dilutions of phenol red at 500 nm. To determine gastric emptying time, 2 mL phenol red at 100 mg/L was introduced into the stomach through an indwelling gastric tube. Two milliliters of gastric juice was obtained when the dye was well mixed with the gastric juice. The amount of the dye in the gastric content was determined by spectrophotometry. Thirty minutes later, another sample of gastric content was withdrawn and phenol red was again determined. With this process, the gastric emptying time was calculated.
Intestinal absorption rate was determined using a modified Cooper method[8]. Since phenol red is a large molecular dye, it is not absorbed by the intestinal mucosa. With the absorption of fluid, the concentration of the dye increases. Thus, intestinal absorption rate of fluid could be assessed. The method used in our experiment was as follows. Since three enteral tubes were implanted into the jejunum in equal distance, a known amount of fluid was introduced into the most proximal catheter (A) with a known velocity Va (mL/h) using an intelligent infusion pump (ZNB-XB; Xu-li Scientific Technology Co. Beijing, China), and then specimens of intestinal fluid (1 mL) were collected from two distal catheters, where fluid velocities were Vb and Vc. By measuring the concentrations of phenol red in various specimens, the intestinal absorption rate was calculated: Vb = Va × Ca/Cb, Vc = Vb × Cv/Cc, ΔV = Vb-Vc.
ΔV was the absorption rate in the segment of intestine between catheters B and C. By dividing the value by the length of the intestinal segment between B and C, the intestinal absorption rate of the oral feeding fluid could be calculated, and it was expressed as mL/h.m2, i.e. the amount of orally fed liquid absorbed by unit length of the intestine during unit time.
All data are presented as the mean ± SD. Statistical analysis was done using SPSS 11.0 statistical software for the F test. P < 0.05 was considered statistically significant.
In the NR group, three animals died at 12, 18 and 23 h after burn injury, with a 5 d mortality of 30.0% (3/10). In the OR group, one dog died 18 h after burn injury, with a 5 d mortality of 12.5% (1/8). In the OR/CAR group, no deaths occurred within 5 d of injury, with zero 5 d mortality.
As depicted in Table 1, MAP was lowered 2 h after burn injury in all three groups (all P < 0.05). It gradually rose after this period, and MAP in the OR and OR/CAR groups was higher than that in the NR group (P < 0.05) 4 h post-burn, and returned to pre-injury level 8 h after injury, but it was still lower than the pre-injury level at 72 and 120 h after injury in the NR group. CO and plasma volume (PV) were lowest at 24 h post-burn in the NR group, and increased later, but never to the level before burn injury. However, CO and PV in the OR and OR/CAR groups were higher than in the NR group at 4 and 8 h post-burn (all P < 0.05). CO and PV in the OR/CAR group were higher than in the OR group at 8 and 24 h post-burn (all P < 0.05). However, MAP showed no significant change between the OR/CAR and OR groups.
| Post-burn (h) | MAP (mmHg) | CO (L/min) | PV (mL/kg) | ||||||
| NR | OR | OR/CAR | NR | OR | OR/CAR | NR | OR | OR/CAR | |
| 0 | 135 ± 9.3 | 131 ± 18.7 | 128 ± 12.6 | 2.46 ± 0.13 | 2.43 ± 0.17 | 2.39 ± 0.19 | 49.6 ± 5.6 | 46.8 ± 4.2 | 48.3 ± 4.0 |
| 2 | 100 ± 7.2a | 94 ± 15.3a | 89 ± 10.0a | 1.28 ± 0.11a | 1.34 ± 0.1a | 1.30 ± 0.11a | 38.2 ± 3.8a | 40.3 ± 3.0a | 41.3 ± 3.1a |
| 4 | 108 ± 8.3a | 136 ± 18.9b | 130 ± 15.1b | 1.12 ± 0.10a | 1.54 ± 0.11ab | 1.48 ± 0.12ab | 32.8 ± 2.3a | 39.0 ± 3.8a | 40.8 ± 3.9a |
| 8 | 120 ± 14.3 | 125 ± 15.9 | 127 ± 14.3 | 1.20 ± 0.17a | 1.65 ± 0.12ab | 1.86 ± 0.14abc | 30.9 ± 3.4a | 36.2 ± 3.4ab | 42.2 ± 3.4abc |
| 24 | 124 ± 8.9 | 126 ± 8.8 | 129 ± 8.9 | 1.07 ± 0.17a | 1.94 ± 0.18ab | 2.16 ± 0.15abc | 30.6 ± 4.4a | 40.4 ± 3.0ab | 45.8 ± 3.6bc |
| 48 | 129 ± 8.7 | 135 ± 20.8 | 123 ± 15.8 | 1.88 ± 0.15a | 2.39 ± 0.23b | 2.49 ± 0.16b | 34.5 ± 2.4a | 43.0 ± 3.8b | 45.6 ± 3.6b |
| 72 | 115 ± 13.7a | 137 ± 11.0b | 135 ± 13.1b | 2.10 ± 0.13a | 2.34 ± 0.12b | 2.41 ± 0.20b | 35.8 ± 2.9a | 43.8 ± 3.4b | 45.8 ± 4.0b |
| 120 | 112 ± 11.4a | 134 ± 14.6b | 131 ± 14.2b | 2.15 ± 0.15a | 2.39 ± 0.15b | 2.41 ± 0.20b | 37.6 ± 3.5a | 44.7 ± 3.2b | 46.9 ± 3.4b |
As shown in Table 2, PgCO2 was rapidly elevated, and IMBF sharply decreased in all three groups. They were seen to elevate gradually afterwards. In the NR group, these two values were still worsen than that before injury at 120 h post-burn (all P < 0.05). However, in the OR and OR/CAR groups, they recovered to pre-burn levels at 72 h post-burn. From 2 h post-burn, PgCO2 in the OR group was lower than that in the NR group (P < 0.05), although it was higher than that in the OR/CAR group, but there was no significant difference (P > 0.05). IMBF was always higher in the OR and OR/CAR groups compared with the NR group (all P < 0.05), but the value was lower in the OR group compared with the OR/CAR group at 4, 8 and 24 h post-burn (all P < 0.05).
| Post-burn (h) | PgCO2 (mmHg) | IMBF (BPU) | ||||
| NR | OR | OR/CAR | NR | OR | OR/CAR | |
| 0 | 32.2 ± 3.7 | 33.2 ± 6.1 | 33.8 ± 6.8 | 203.8 ± 17.6 | 198.3 ± 11.9 | 207.3 ± 13.9 |
| 2 | 73.1 ± 7.7a | 60.8 ± 8.2ab | 57.4 ± 8.0ab | 74.2 ± 10.8a | 101.2 ± 12.2ab | 112.6 ± 10.2ab |
| 4 | 83.1 ± 6.5a | 74.0 ± 6.5ab | 70.0 ± 6.2ab | 71.5 ± 15.3a | 108.8 ± 12.2ab | 138.8 ± 14.1abc |
| 8 | 86.4 ± 8.6a | 69.2 ± 6.8ab | 68.5 ± 5.8ab | 77.8 ± 10.0a | 114.7 ± 12.0ab | 134.7 ± 13.9abc |
| 24 | 82.5 ± 7.6a | 65.8 ± 5.8ab | 56.4 ± 4.7a, b, | 79.2 ± 17.3a | 127.7 ± 11.9ab | 157.7 ± 17.7abc |
| 48 | 61.5 ± 8.2a | 56.0 ± 8.4a | 57.0 ± 6.4a | 146.8 ± 13.8a | 159.3 ± 19.1a | 179.3 ± 19.1ab |
| 72 | 56.8 ± 6.6a | 39.4 ± 8.9b | 35.4 ± 5.6b | 168.5 ± 9.7a | 180.7 ± 18.5 | 198.7 ± 16.5b |
| 120 | 45.8 ± 6.2a | 31.2 ± 5.0b | 34.2 ± 4.0b | 178.8 ± 16.5a | 203.5 ± 23.2b | 200.5 ± 18.2b |
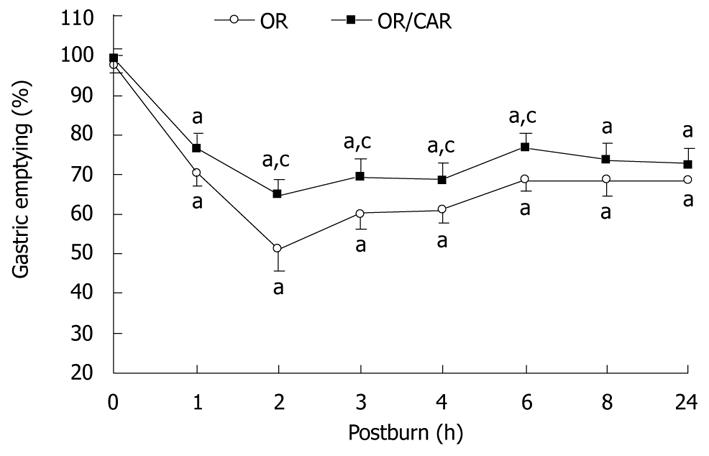
Figure 1 shows that, in the OR and OR/CAR groups, gastric emptying rate was lowered, especially in the former group, with 52.8% of the normal emptying rate at 2 h, and 70.5% at 24 h post-burn. In the OR/CAR group, it was also lowered, but reached 65.2% at 2 h, and 73.0% at 24 h post-burn, and the values were all significantly higher than those in the OR group at 2, 3, 4 and 6 h post-burn (all P < 0.05). Thus, it was estimated that approximately 15.7% more gastric content was expelled from the stomach in 24 h in the OR/CAR group as compared with the OR group.
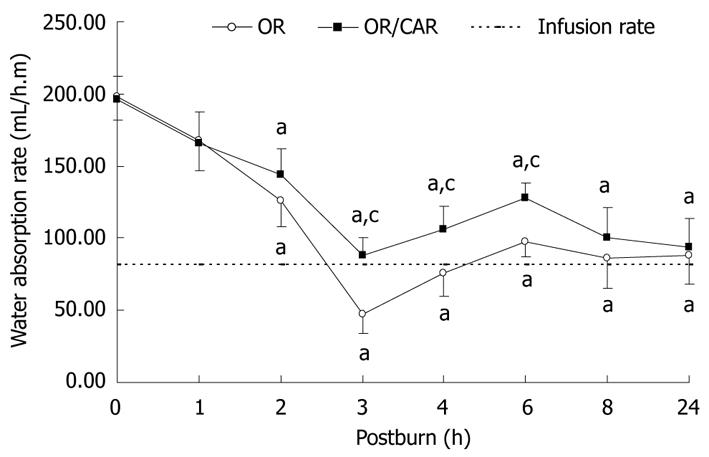
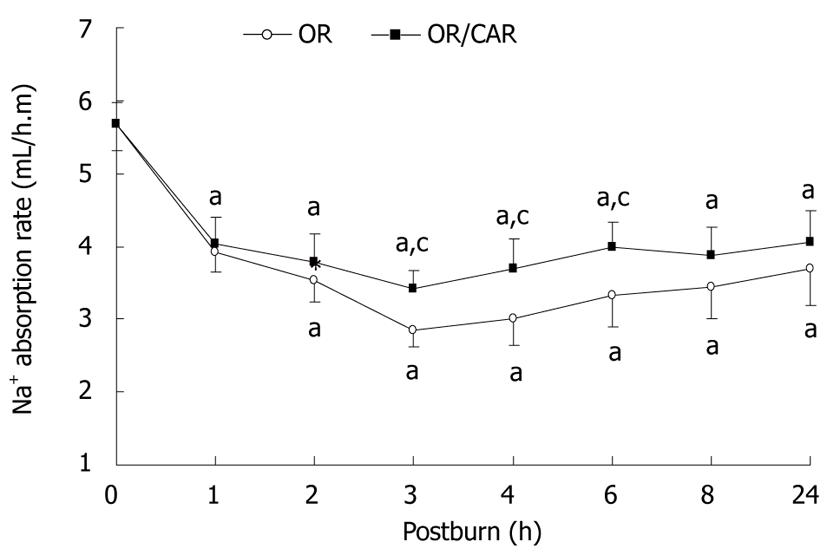
As shown in Figures 2 and 3, the rate of absorption of orally administered water and Na+ was lowest at 3 h post-burn in the OR group, and the values were 23.7% and 50.3% of pre-injury levels. These absorption rates were gradually increased to 44.4% and 65.1%, respectively, at 24 h post-burn. In the OR/CAR group, all these values were higher than those in the OR group at 3, 4 and 6 h post-burn (all P < 0.05). In the first 24 h post-burn, the rate of intestinal water absorption was elevated by 11.5% in the OR/CAR group. It was estimated that within 24 h after injury, the water absorption rate at 24 h in the OR and OR/CAR groups was 110.9 ± 17.1 mL/h.m and 127.8 ± 17.3 mL/h.m, respectively, and they were actually higher than the pre-requisite of the Parkland formula 82.1 ± 11.2 mL/h.m (P < 0.05).
Mass casualties from burn injury may occur in a region where the major problems facing medical personnel are the availability and probability of fluid replacement with sterile intravenous fluids. If we resuscitate a single burn victim weighing > 60 kg with a burn of 30% TBSA it would require > 5-7 kg of fluid for a medic to carry. In a very harsh environment with lack of decent medical support, resuscitation of burn shock is severely handicapped, and the life of those with extensive burn injury is jeopardized. One strategy for reducing such a hazard is to try to supplement liquid with sufficient electrolytes by mouth until intravenous infusion fluids are available[9]. Early in 1970, Monafo[10] reported a clinical trial of oral administration of hypertonic lactated saline solution to patients with burn injury of various extents, and he found that at least partial oral resuscitation of severe burns could be successful. However, oral resuscitation of burn shock has not been popular, because, ordinarily, intravenous resuscitation is almost always available, especially in cities, and even in many rural areas where medical facilities have been established. Nevertheless, in certain scenarios, such as fire disasters in cities after a strong earthquake, forest fires in mountainous terrain, or in combat zones after incendiary devices have exploded, when transportation is seriously lacking or hampered due to geographical barriers, and medical support is lacking, so that sterile intravenous fluids and trained personnel are not available, oral intake of fluids should be considered, in the hope that the victims can be tided over burn shock.
Sufficient data about oral resuscitation of burn shock can not be accumulated in clinical practice under normal circumstances, therefore, most investigations have been done with animals. In these experiments, animals were always under general anesthesia to prevent restlessness of the animals[1,3,4]. Thus, the results of oral hydration might not reflect the true states of fluid absorption because the anesthesia inevitably inhibits gastrointestinal motility and other functions. The present study was planned to measure all the physiological parameters in a conscious state. All the catheters for measuring physiological indexes were implanted 1 d before burn injury. Burn injury was produced under brief anesthesia to eliminate mental strain and pain. Thereafter, all the measurements were made with the animals in a fully conscious state. Pure bred Beagle dogs were used because they were tame, docile and cooperative. No restriction of the body was necessary during the whole course of the experiment, which rendered all the measurements complete without any restriction of the animals or general anesthesia. Thus, all the disturbances to gastrointestinal mobility or absorption were greatly alleviated. Therefore, there was no external interference to influence the measurements during the whole course of the experiment, which lasted for 120 h, which increased the reliability of the measurements.
The degree of perfusion of the gastrointestinal tract is at present considered as an important index of circulatory shock and tissue oxygen delivery. The determination of PCO2 of the gastric mucosa has been used to estimate the pH of the mucosa, and the change in pH of the mucosa reflects the condition of blood perfusion and oxygen delivery to the gastric tissue[11]. The procedure is untraumatic, and the catheter can also be used to give necessary fluids as well as a convenient tool for measuring gastric emptying rate. To determine blood perfusion of the intestinal mucosa by way of preformed fistulae has already been a standard method in monitoring the circulatory state of transplanted intestine[12]. In our experiment, a flexible fiberoptic detector of a laser Doppler flow monitor was passed through the preformed fistulae. Each reading took about 1 min only, so that it did not give any discomfort to the animal, and sequential measurements were assured. The experimental results showed that, in injured dogs without the benefit of fluid gavage, blood perfusion was rapidly diminished in the intestinal mucosa. It was also shown that this change was consistent with a decrease gastrointestinal mucosal perfusion, and its recovery lagged behind the improvement in hemodynamic parameters. Up to 120 h after injury, it was still lower than that before injury. These phenomena corroborated that which was found in human patients with hypodynamic shock.
When a solution with electrolytes and glucose is introduced into the stomach, the emptying rate of the liquid from the stomach depends on the pressure gradient between the stomach and duodenum. It also is influenced by the state of blood supply to the stomach and regulatory activity of the vagus nerve (cholinergic nerve) and humoral agents (motilin)[13]. Absorption of water through the intestinal mucosa depends on translocation of Na+ ions, while the latter process could only be realized with the presence and activity of Na+/K+-ATPase, which is localized in the basal layer of the intestinal mucosal epithelium. Therefore, it is obvious that the rate of absorption of water by the intestinal mucosa is under the influence of the intestinal blood flow, activity of Na+/K+-ATPase, and aquaporin (AQP)-1 expression, along with consumption of ATP. Thus, it is evident that with ischemia and hypoxia of the intestinal mucosa during hypovolemic shock, water absorption by the intestinal mucosa is hampered.
Heavy leakage of fluid from the circulation results in a sharp decrease in ATP. This shortage in ATP is further amplified by an increased demand created by feeding and absorption of water and electrolytes. Under such conditions, the presence of glucose, which can be metabolized into lactic acid, alanine and CO2 to produce ATP under anaerobic conditions, is essential. This additional ATP is helpful for the absorption potential of the intestinal mucosa. During the process of absorption, the transport of glucose is coupled with the transport of water molecules and Na+ ions, and two Na+ ions and 223 water molecules are absorbed. Therefore, addition of glucose to an electrolyte solution is an ideal liquid for oral replacement during hypovolemic shock[14,15].
Thomas et al[2] have described an experiment to study gastric emptying with oral replacement of fluid in hypovolemic shock. They gavaged glucose-electrolyte solution (GES) into the stomach of pigs with 40% TBSA burn injury, according to the Parkland formula. They showed that the gastric emptying volume increased with an increase in the volume of gavaged fluid. However, the volume of fluid passed into the duodenum was one half of the volume required by the Parkland formula, and hemodynamic parameters did not recover to the pre-injury level. Michell et al[4] have performed a study of duodenal infusion of glucose-electrolyte solution in pigs with 40% TBSA burns, and demonstrated that a total of 93% of infused solution was absorbed during the course of the 4 h experiment. However, these experiments were performed in animals under general anesthesia, and there was unavoidable impairment of gastrointestinal peristalsis and absorption ability. Therefore, the results could not be considered as reflecting the true condition of the gastrointestinal tract.
In our present study, we determined the gastric emptying and intestinal absorption rate of GES without the interfering effects of general anesthesia. We found that gastric emptying and intestinal absorption were significantly reduced to the lowest level (52.8% and 23.7% of pre-injury levels) in the OR group at about 2 and 4 h post-burn, respectively, and did not return to 80% of pre-injury level until 24 h. It was estimated that within 24 h after injury, the water absorption rate in the OR group was 110.9 ± 17.1 mL/h.m, and it was actually higher than the pre-requisite of the Parkland formula (82.1 ± 11.2 mL/h.m). This intestinal absorptive rate was similar to that of Michael et al[4]. The above results suggest that, in large animals (e.g. pigs or dogs), with < 40% TBSA burns, almost all GES infused according to the Parkland formula could be fully absorbed, although intestinal absorption was inhibited due to gut hypoperfusion. Therefore, we considered that gastric emptying is the main limitation to effective gastrointestinal resuscitation.
The results of using carbachol in our experiment clearly demonstrated that this drug could improve tissue blood perfusion of the gastrointestinal tract. Thus, it was helpful in expediting gastric emptying of its contents and improving intestinal absorption of water and electrolytes. Carbachol is a cholinergic receptor agonist. It stimulates peristalsis of the gastrointestinal tract by activating M cholinergic receptors, and also activates α7 subunits of cholinergic nicotinic receptors on macrophage and endothelial cells which leads to cellular deactivation and inhibition of cytokine release, thus attenuating the systemic or regional inflammatory response[16-18]. In addition, it is an antioxidant and inhibitor of apoptosis[19,20]. In our study, the addition of carbachol to the resuscitation fluid did improve blood perfusion of the gastrointestinal tissues, expedite gastric emptying, and improve intestinal absorption rate of water and electrolytes. These beneficial effects of carbachol may be attributable to the following mechanisms: (1) inhibition of release of proinflammatory cytokines alleviates the inflammatory reaction of the gastrointestinal tissue, thus resulting in reduced loss of AQP1[21,22]; (2) improvement in intestinal peristalsis and blood perfusion due to stimulation of M receptors facilitates intestinal absorption; and (3) promotion of activity of Na–/K Na+-ATPase, which is essential for absorption of water and especially Na+ by intestinal mucosa[23]. Our experiment showed that the gastric emptying time was promoted by 15.7%, and the intestinal absorption rate was increased by 11.5%.
In conclusion, our experiment has successfully reproduced a canine model of serious burn injury. In this model, we are able to study the effects of oral replenishment of GES for resuscitation of burn shock. The hemodynamic parameters, gastric emptying rate, and intestinal absorption of GES can be relatively accurately determined. The results of the experiment also show that burn shock can be ameliorated to a certain extent by administration of GES, with the addition of carbachol to enhance gastric emptying and intestinal absorption of fluids. Oral resuscitation for burn shock might be a surrogate measure where mass casualties from burn injury occur in an area where medical support is minimal and transportation is difficult. Carbachol may be beneficial because it is a cholinergic receptor agonist, and it possesses the function of enhancing gastric emptying and intestinal absorption of fluid when given by mouth. Therefore, in circumstances when intravenous replacement of fluids for resuscitation of burn shock is not feasible, oral feeding of GES, with addition of carbachol, may be possible.
Rapid intravenous infusion remains unanimously the key measure to resuscitate hypovolemic shock as a result of massive burn injury. Unfortunately, in armed conflicts or massive disasters (such as forest fire, earthquake, or terrorist attack) mass casualties occur in an austere environment with poor medical support and transportation facilities. In such cases, it is our supposition that oral or gastrointestinal administration of fluids might be more practical, and could have a positive effect in maintaining the life of the victims until intravenous replacement of fluids is available.
Gastrointestinal tract ischemia and hypoxia due to massive surgical stresses such as severe trauma, extensive burns and major surgery resulting in hypovolemic shock can lead to dysfunction of gastric emptying and intestinal absorption, followed by poor transportation and absorption of oral electrolytes and nutrients in the gastrointestinal tract. Improvement of mucosal blood perfusion, and enhancement of gastrointestinal tolerance to oral rehydration fluid and enteral nutrition are not only the foci of research in the surgical and critical care fields, but they are key factors for facilitation of oral resuscitation of hypovolemic shock.
A large animal model of severe burn injury was reproduced to investigate the feasibility of oral resuscitation of burn shock, without the disturbing effects of general anesthesia. The effects of carbachol, which is a cholinergic receptor agonist, on blood circulation, gastrointestinal perfusion, gastric emptying and intestinal absorption of fluid and electrolytes were investigated. The results indicated that oral resuscitation with the help of such a drug might be an ideal way in lieu of intravenous resuscitation for burn shock, especially in battlefields or other sites of mass casualties.
Orally administered fluid can be considered to be a simple and effective means of replacement of body fluid that is feasible for resuscitation of hypovolemic shock, especially when there is an extreme shortage of means of medical support in an austere environment such as battlefields and disasters.
The manuscript is very well written and the conclusions applicable.
Peer reviewer: Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Cancio LC, Kramer GC, Hoskins SL. Gastrointestinal fluid resuscitation of thermally injured patients. J Burn Care Res. 2006;27:561-569. |
| 2. | Thomas SJ, Kramer GC, Herndon DN. Burns: military options and tactical solutions. J Trauma. 2003;54:S207-S218. |
| 3. | Jiang KY, Li A, Yang ZC. The experimental investigation on oral rehydration of 30% TBSA superficial second degree burn in dogs. Zhonghua Zhengxing Shaoshang Waike Zazhi. 1988;4:288-291. |
| 4. | Michell MW, Oliveira HM, Kinsky MP, Vaid SU, Herndon DN, Kramer GC. Enteral resuscitation of burn shock using World Health Organization oral rehydration solution: a potential solution for mass casualty care. J Burn Care Res. 2006;27:819-825. |
| 6. | Sakka SG, Reinhart K, Meier-Hellmann A. Prognostic value of the indocyanine green plasma disappearance rate in critically ill patients. Chest. 2002;122:1715-1720. |
| 7. | Scarpignato C, Capovilla T, Bertaccini G. Action of caerulein on gastric emptying of the conscious rat. Arch Int Pharmacodyn Ther. 1980;246:286-294. |
| 8. | Cooper H, Levitan R, Fordtran JS, Ingelfinger FJ. A method for studying absorption of water and solute from the human small intestine. Gastroenterology. 1966;50:1-7. |
| 9. | Hu S. [The resuscitation strategies for hypovolemic shock under austere condition lacking intravenous fluid resuscitation facility]. Zhongguo Weizhongbing Jijiu Yixue. 2010;22:323-325. |
| 10. | Monafo WW. The treatment of burn shock by the intravenous and oral administration of hypertonic lactated saline solution. J Trauma. 1970;10:575-586. |
| 11. | Hu S, Sheng ZY. The effects of anisodamine and dobutamine on gut mucosal blood flow during gut ischemia/ reperfusion. World J Gastroenterol. 2002;8:555-557. |
| 12. | Oltean M, Herlenius G, Dindelegan G, Gäbel M, Mölne J, Nilsson O, Aneman A, Olausson M. Laser-Doppler flowmetry in the monitoring of the human intestinal allograft: a preliminary report. Transplant Proc. 2006;38:1723-1725. |
| 13. | Minami H, McCallum RW. The physiology and pathophysiology of gastric emptying in humans. Gastroenterology. 1984;86:1592-1610. |
| 14. | Kozar RA, Hu S, Hassoun HT, DeSoignie R, Moore FA. Specific intraluminal nutrients alter mucosal blood flow during gut ischemia/reperfusion. JPEN J Parenter Enteral Nutr. 2002;26:226-229. |
| 15. | Hu S, Sheng Z, Liu Q, Shi D, Jiang X, Sun D, Zhang R. [Effects of different enteral nutrients on gut absorptive capacity and energy metabolism during gut ischemia/reperfusion]. Zhonghua Yixue Zazhi. 2002;82:689-691. |
| 16. | Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384-388. |
| 17. | Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458-462. |
| 18. | Li YZ, Liu XH, Rong F, Hu S, Sheng ZY. Carbachol inhibits TNF-α-induced endothelial barrier dysfunction through alpha 7 nicotinic receptors. Acta Pharmacol Sin. 2010;31:1389-1394. |
| 19. | Hu S, Zou XF, Lü Y, Lu JY, Sheng ZY. [Effects of carbachol on apoptosis of intestinal epithelial cells after gut ischemia/reperfusion in rat]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007;19:463-466. |
| 20. | Che JW, Hu S, Geng SJ, Wu J, Wang L, Du Y, Tian YJ, Sheng ZY. Carbachol alleviates oxygen free radical injury in gut during enteral resuscitation of burn shock in rat. World Chin J Digestol. 2008;16:900–903. |
| 21. | Ishikawa Y, Eguchi T, Skowronski MT, Ishida H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem Biophys Res Commun. 1998;245:835-840. |
| 22. | Towne JE, Krane CM, Bachurski CJ, Menon AG. Tumor necrosis factor-alpha inhibits aquaporin 5 expression in mouse lung epithelial cells. J Biol Chem. 2001;276:18657-18664. |
| 23. | Bao C, Hu S, Zhou G, Tian Y, Wu Y, Sheng Z. Effect of carbachol on intestinal mucosal blood flow, activity of Na+-K+-ATPase, expression of aquaporin-1, and intestinal absorption rate during enteral resuscitation of burn shock in rats. J Burn Care Res. 2010;31:200-206. |