Published online Apr 7, 2011. doi: 10.3748/wjg.v17.i13.1739
Revised: January 17, 2011
Accepted: January 22, 2011
Published online: April 7, 2011
AIM: To evaluate the benefit and effectiveness of MR-arterioportography (MR-AP) to achieve the highest sensitivity for detection and evaluation of hepatocellular carcinoma (HCC).
METHODS: Twenty liver cirrhosis patients with suspected HCC were included before transarterial chemoembolization. In all patients double-enhanced Magnetic resonance imaging (MRI) was performed. A bolus of 10 mL Magnevist® was injected through a selectively placed catheter in the superior mesenteric artery and MRI of the liver was performed in arterioportographic phase. Two independent readers evaluated number, size and localization of detected lesions. Diagnostic quality was determined using a 4-point scale. Differences were analyzed for significance using a t-test. Interobserver variability was calculated.
RESULTS: In all 20 patients (100%), MR-AP was feasible. Diagnostic quality was, in all cases, between 1 and 2 for both modalities and readers. MR-AP detected significantly more lesions than double-enhanced MRI (102.5 vs 61, respectively, P < 0.0024). The inter-observer variability was 0.881 for MRI and 0.903 for MR-AP.
CONCLUSION: Our study confirmed that the MR-AP as an additional modality for detection of HCC is beneficial, as significantly more lesions were detected compared to MRI with liver-specific contrast.
- Citation: Rennert J, Jung EM, Schreyer AG, Hoffstetter P, Heiss P, Feuerbach S, Zorger N. MR-arterioportography: A new technical approach for detection of liver lesions. World J Gastroenterol 2011; 17(13): 1739-1745
- URL: https://www.wjgnet.com/1007-9327/full/v17/i13/1739.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i13.1739
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver and often develops in patients with underlying liver cirrhosis due to excessive alcohol intake, chronic hepatitis or primary biliary cirrhosis.
In the treatment of hepatocellular carcinoma, surgical resection is considered the only potentially curative therapy. However, technical improvements in hepatic surgery have extended the indications for surgery remarkably, and also regional therapeutic procedures such as transcatheter arterial chemoembolization (TACE)[1-3] and radiofrequency ablation (RFA)[1,4,5] have proved to be very successful. A prolonged time of survival following diagnosis is noted. Therefore, the pre-operative or pre-interventional workup of patients with suspected liver malignancy is even more important, especially concerning the evaluation and characterization of focal or diffuse lesions in the cirrhotic liver. Magnetic resonance imaging (MRI) has been used to improve identification of focal hepatic masses in a cirrhotic liver.
Dynamic MRI after a bolus injection of gadopentetate dimeglumine has been accepted as a valuable method for the detection and characterization of liver tumors[6-9]. Studies have shown that superparamagnetic iron oxide-enhanced magnetic resonance imaging (SPIO-MRI) increases sensitivity[4,10].
In order to determine the treatment of choice for HCC, studies have shown that examinations by both computed tomography angiography (CTA) and computed tomography arterioportography (CT-AP) are indispensable because of the high sensitivity of CT-AP in detecting hepatic lesions and the capability of CTA to characterize them[11,12]. However, in contrast to its high sensitivity in detecting lesions, the specificity of CT-AP for characterizing intrahepatic lesions is low. Tumor-mimicking benign perfusion abnormalities and benign lesions (e.g. hemangiomas, arterio-venous shunts) have led to a reported incidence of false-positive lesions between 9% and 63% in primary and secondary liver lesions[13,14].
Despite advances in CT or MRI, ultrasound (US) with or without application of contrast agents also plays a key role in the diagnostic algorithm of HCC due to its low cost, availability and non-invasiveness.
Until now, there have hardly been any studies comparing the effectiveness of MR-arterioportography (MR-AP) and contrast-enhanced MRI for diagnosis of malignant liver lesions. Thus, the purpose of this study was to combine the advantages of modern contrast-enhanced MRI with the technique of arterioportography to achieve the highest sensitivity for diagnosis of malignant liver lesions in patients suffering from HCC.
Approval for this study was obtained from the institutional review board in conformity with the Declaration of Helsinki. Before the procedures were conducted, written informed consent was obtained from each patient for MRI, MR-AP and angiography after the nature of the procedure was fully explained.
During the period from February 2005 to September 2007, 20 patients [18 men, 2 women, age range from 47 to 76 years (mean age, 62 years)] with symptoms suggestive of primary malignant hepatic tumors were referred to our department. As HCC is commonly associated with liver cirrhosis, all patients had deteriorated liver but a tolerable renal function, and the cardiovascular status was stable. Twelve of our 20 patients suffered from alcohol toxic liver cirrhosis. In 4 out of 20 patients the underlying disease was chronic hepatitis (2 patients with chronic hepatitis B, 2 patients with chronic hepatitis C). In 4 out of 20 patients, the fundamental disease could not be elicited. Concerning the severity of cirrhosis, 8 out of 20 patients were classified as Child Pugh score A, 7 out of 20 patients as Child Pugh score B and 5 out of 20 patients as Child Pugh score C.
The existence of malignant hepatic tumors was confirmed using multislice MRI and CT. MR-AP was performed to evaluate the tumor extent in order to suggest interventional therapy, surgery or chemotherapy.
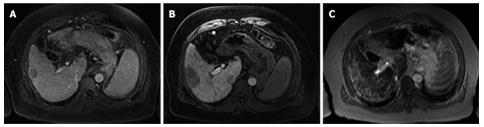
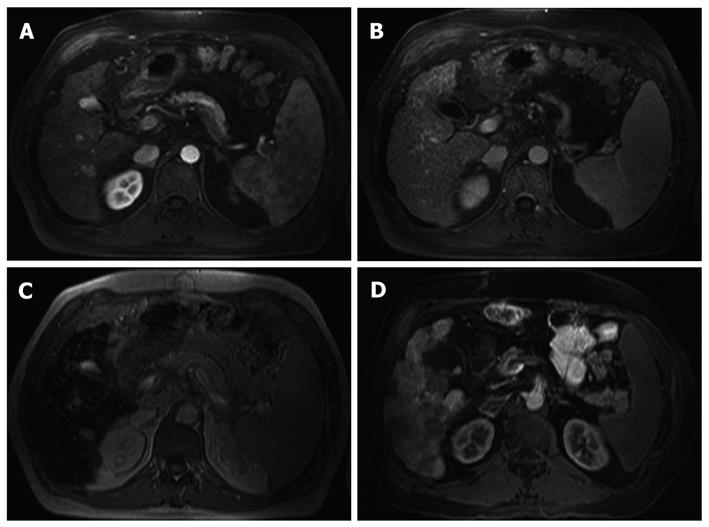
Diagnosis of HCC was histologically confirmed in 15 out of 19 patients. In one patient (patient No. 16), existence of a malignant hepatic lesion was excluded histologically following liver transplantation.
In 13 out of 20 patients the α-fetoprotein (AFP) level was elevated, ranging from 16.4-2513 ng/mL (mean 488.1 ng/mL). In 7 patients (including patient No. 16) AFP levels were within a normal range.
Angiography: Before TACE and for MR-arterioportography, the femoral common artery was punctured under local anesthesia using the Seldinger technique and a 5-French angiographic catheter (Cobra, Cook Medical, USA) was positioned in the proximal superior mesenteric artery. A diagnostic angiography was performed to visualize the portal vein and to exclude shunts which could involve contrasting via the portal vein.
MRI: MRI was performed on a 1.5-T whole-body scanner (Magnetom Sonata, Siemens Medical Solutions, Germany) equipped with a high-performance gradient (Quantum) system (maximum gradient strength, 30 mT/m; slew rate, 125 T/ms). A combination of the standard body phased-array coil with spine array coils was used for signal reception.
MR-AP standard protocol (Table 1): For MR-AP, 10 mL gadopentetate dimeglumine was injected through the catheter placed in the superior mesenteric artery at a rate of 2 mL/s with a power injector (Medrad Spectris MR Injector, USA).
| Scans | Plane | TE | TR | Flip angle |
| Unenhanced | ||||
| T2-weighted TRUFI | Coronal | 1.9 | 3.8 | 71° |
| T2-weighted TRUFI | Transversal | 1.88 | 3.76 | 71° |
| T1-weighted VIBE | Transversal | 2.02 | 4.78 | 10° |
| Enhanced | ||||
| T1-weighted FLASH FS CE | Transversal | 4.76 | 123 | 70° |
| T1-weighted VIBE dynamic1 | Transversal | 2.02 | 4.78 | 10° |
MRI standard protocol (Table 2): For MRI, 0.2 mmol/kg body weight gadopentetate dimeglumine was injected intravenously at a rate of 2 mL/s with a power injector (Medrad Spectris MR Injector). T1-weighted VIBE transversal Dynamic scans were acquired 20, 40, and 120 s after application of gadopentetate dimeglumine. T2-star-weighted Flash 2D scans and T2-weighted TSE FS scans were obtained after application of 1.4 mL Ferucarbotran (Resovist®, Bayer Schering Pharma AG, Germany).
| Scans | Plane | TE | TR | Flip angle |
| Unenhanced | ||||
| T1-weighted VIBE | Transversal | 1.59 | 4.37 | 10° |
| T2-star-weighted FLASH 2D | Transversal | 10.00 | 169.00 | 90° |
| T2-weighted TSE FS | Transversal | 105.00 | 2740.00 | 170° |
| T1-weighted FLASH opp | Transversal | 2.71 | 100.00 | 70° |
| T1-weighted FLASH in | Transversal | 4.76 | 87.00 | 60° |
| T2-weighted HASTE | Transversal | 85.00 | 1000.00 | 150° |
| T2-weighted TRUFI | Coronal | 1.83 | 3.65 | 71° |
| T1-weighted VIBE | Transversal | 1.55 | 4.81 | 10° |
| Enhanced | ||||
| T1-weighted VIBE dynamic1 | Transversal | 1.55 | 4.81 | 10° |
| T1-weighted FLASH FS | Transversal | 4.76 | 123.00 | 70° |
| T1-weighted FLASH FS | Coronal | 4.76 | 94.00 | 70° |
| T2-star-weighted FLASH 22 | Transversal | 10.00 | 69.00 | 90° |
| T2-weighted TSH FS2 | Transversal | 105.00 | 2740.00 | 170° |
In the retrospective reviewing procedure, all images of each technique were interpreted and evaluated independently by two observers with great experience in abdominal MRI. No clinical information or patient diagnosis was given to the observers. The images from each technique were interpreted in separate sessions in a randomized sequence. In the first session, the two observers reviewed a set of images that included both unenhanced and gadopentetate dimeglumine-enhanced, as well as Resovist-enhanced, images.
In the second session, each observer reviewed a set of images (MR-AP set) that included gadopentetate dimeglumine-enhanced images after injection via the superior mesenteric artery.
For characterization of liver lesions, all images of each examination were reviewed together using all the sequences available. Each observer recorded the number of suspected lesions noted, their size, and the segmental location. Furthermore, the image quality of MR and MR-arterioportography was documented on a four point scale: 1-excellent, 2-minor diagnostic limitations, 3-major diagnostic limitations, 4-non-diagnostic.
Statistical software (SPSS, version 14, Chicago, USA) was used for statistical analysis. We evaluated the differences with regard to number of lesions found using MR-arterioportography and MRI. Furthermore, the inter-observer differences in evaluation of MR-arterioportography and double-enhanced MRI were analyzed. Paired-samples t tests and χ2 test were used to compare. In paired-samples t tests, P < 0.05 indicated a statistically significant difference.
Twenty patients with liver cirrhosis underwent combined MR-arterioportography and SPIO-MRI examinations. No adverse reactions were experienced by any of the patients who received Gd-DTPA and SPIO.
In all 20 patients (100%), MR-arterioportography was feasible. The image quality in both modalities (MR-AP and MRI) was excellent (1-2 in MR-arterioportography, SD: 0.4865; 1-2 in MR, SD: 0.4292). In patient No. 8, a MR-AP evaluation was not suitable due to a diffuse infiltration of virtually all liver segments, thus the patient was excluded. Altogether, 102.5 hepatic lesions were detected using MR-AP, whereas only 61 lesions could be detected using MRI (Table 3). This difference is considered to be statistically significant (P < 0.0024).
| Patient | Age (yr) | MR-APnumber of lesions∑102/103 | Double-enhanced MRI number of lesions∑60/56 | ||
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | ||
| 1 | 62 | 9 | 9 | 5 | 4 |
| 2 | 47 | 7 | 8 | 2 | 2 |
| 3 | 70 | 4 | 5 | 2 | 2 |
| 4 | 74 | 7 | 8 | 4 | 3 |
| 5 | 51 | 3 | 3 | 3 | 3 |
| 6 | 76 | 1 | 2 | 1 | 1 |
| 7 | 70 | 6 | 6 | 9 | 8 |
| 81 | 63 | - | - | (7) | (7) |
| 9 | 70 | 4 | 4 | 2 | 2 |
| 10 | 63 | 9 | 8 | 2 | 2 |
| 11 | 51 | 9 | 8 | 6 | 5 |
| 12 | 63 | 10 | 11 | 3 | 2 |
| 13 | 57 | 6 | 6 | 2 | 2 |
| 14 | 67 | 5 | 4 | 4 | 5 |
| 15 | 51 | 4 | 3 | 3 | 2 |
| 16 | 53 | 0 | 0 | 1 | 1 |
| 17 | 51 | 2 | 2 | 2 | 2 |
| 18 | 54 | 5 | 6 | 3 | 3 |
| 19 | 57 | 9 | 8 | 4 | 4 |
| 20 | 73 | 2 | 2 | 3 | 3 |
The kappa analyses of two observers regarding the number of lesions detected by both modalities showed substantial to excellent agreement (κ = 0.903, 95% confidence interval: 0.844 to 0.962 for MR-AP; and κ = 0.881, 95% confidence interval: 0.795 to 0.966 for MRI). In particular, κ values with MR-AP imaging indicated excellent agreement.
The lesions found in all patients ranged in size from 7-120 mm (mean 28.24 mm) for MRI and from 4-120 mm (mean 24.62 mm) for MR-AP. This difference is not considered to be statistically significant. In MRI, 16 lesions (26.2%) were 10 mm or less in diameter. In MR-AP, 30 lesions (29.3%) were 10 mm or less in diameter.
In a total of 15 out of 19 patients, more lesions were detected by MR-AP compared to MRI with liver-specific contrast. According to the characteristic features displayed in MR-AP, all lesions were classified as malignant. Thus, as a consequence of the finding of these additional lesions, further treatment (TACE, RFA or surgery) was performed upon the patients.
In all but one patient (patient No. 16), the lesions found displayed a characteristic MRI signal, including arterial enhancement to a greater or lesser extent, with late wash out and an absent accumulation of Ferucarbotran (Resovist®). Thus, these lesions were classified as malignant. In addition, these lesions also showed characteristic features in MR-AP, such as absent enhancement of gadopentetate dimeglumine with profound demarcation compared to healthy liver tissue. Hence, the lesions were also classified as malignant.
Diagnosis of HCCs was histologically confirmed in 15 out of 19 patients. In patient No. 16, one lesion was found in segment 8 of the liver which showed a portal venous enhancement with accumulation of Ferucarbotran and no traceability in MR-AP. The lesion therefore was classified as benign, i.e. regenerated nodule that was confirmed histologically following liver transplantation (Figures 1 and 2).
Before decisions are made as to hepatic resection of hepatocellular carcinoma or interventional treatment such as TACE, percutaneous ethanol injection therapy and radiofrequency ablation (RFA), accurate information regarding the number and localization of lesions is essential.
Because of its high sensitivity for detecting lesions, CT-AP is one of the most reliable tools for detection of liver lesions. The rationale of CT-AP is for contrast material to be delivered directly to the liver through the portal vein before it can return to the hepatic artery from the systemic circulation, to optimize the detection of tumor lesions that do not have portal vein flow and appear as hypodense nodules. The aim of our study was to combine the advantages of arterioportal contrast and MRI to detect liver lesions in patients with HCC. MRI with liver-specific contrast agents is currently the imaging modality of choice. Most studies that have directly compared MRI with CT-AP in patients with HCC or metastases reported no significant differences in sensitivity[15-17], but in some studies a higher specificity for MRI is reported[13].
Preoperative or pre-interventional workup of patients with suspected liver malignancy is even more important, especially concerning the evaluation and characterization of a focal lesion or diffuse infiltration of the cirrhotic liver.
Previous studies have shown the usefulness of ferumoxide-enhanced MR imaging for the detection of hepatic tumors of different histological types[18,19]. After intravenous injection, the SPIO-particles are cleared by macrophages and can be identified histologically in Kupffer’s cells of the liver. Poorly differentiated hepatic tumors lack Kupffer’s cells; therefore, the T2 relaxation does not change after the administration of SPIO causing an increased lesion-to-liver contrast.
Studies of patients with hepatic metastases have shown that ferumoxide-enhanced MR imaging is more sensitive than unenhanced MR imaging[20,21] or contrast-enhanced CT[22,23], and at least as accurate as CT during arterioportography[10].
Regarding the detection of HCCs, it has been reported that the sensitivity of combined CT during arterioportography and CT hepatic arteriography is 89%-95%[24,25] in comparison to 80.4% on contrast-enhanced CT alone[26]. Furthermore, the sensitivity of MR sequences with ferumoxide enhancement was reported to be 78%-92%[18,19,24], and that of MR-AP 94%-97%[27,28].
Diffusion-weighted imaging (DWI) is a MRI technique that provides imaging of diffusion in biological tissues. Recent technical developments have reduced the image deformation associated with this technique and have increased the signal to-noise ratio, thus making DWI of the body feasible[29], especially for detection of liver malignancies[30]. Studies have reported a higher sensitivity of DWI for detection of small hepatic metastases compared to SPIO-enhanced T2-weighted images and breath-hold T2-weighted images[31,32], particularly due to a profound signal intensity of the HCC in the cirrhotic liver. In comparison, cysts and hemangiomas, the most frequently found benign lesions of the liver, typically show low or absent signal intensities[33].
Our study demonstrated that MR-arterioportography as an alternative method for detection of HCC is not only feasible but showed a significantly larger number of lesions than ferumoxide-enhanced MR imaging, especially in patients with hepatic cirrhosis.
Also, the lesion’s size seems to play a crucial role. Out of the 41 lesions that were exclusively found using MR-AP, 21 were 10 mm or less in diameter. This confirms the high sensitivity of MR-AP, especially regarding smaller lesions. However, a definite differentiation among HCC nodules, regenerative dysplastic nodules or, for example, small arterio-venous shunts that are very common in patients with hepatocellular carcinoma is not possible.
One limitation of the study is that a histological confirmation of HCC or benign lesions could only be obtained in 16 out of 20 patients. In the other 4 patients, further treatment was based upon the combination of typical image morphology and elevated alpha fetoprotein. Another limitation is the relatively small number of patients that were examined in this study. Prospective studies are necessary in order to ascertain the diagnostic reliability of MR-AP compared to established methods.
In summary, our study confirmed that the use of MR-arterioportography as an additional modality for detection of hepatocellular carcinoma is useful. Using this technique, significantly more lesions could be detected in comparison to MRI with liver-specific contrast agent.
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver and often develops in patients with underlying liver cirrhosis. In the treatment of HCC, surgical resection is considered the only potentially curative therapy; however, other regional therapeutic procedures such as transcatheter arterial embolization or radiofrequency ablations have proved to be very successful. Thus, the pre-interventional evaluation of patients with suspected liver malignancy is even more important, especially concerning the evaluation and characterization of focal or diffuse lesions in the cirrhotic liver.
MR imaging with liver-specific contrast agents has been used to improve identification of focal hepatic masses in a cirrhotic liver. Also, studies have shown that examinations by computed tomography arterioportography (CT-AP) have a very high sensitivity for detection of hepatic lesions compared to a relatively low specificity. In this study, the benefit and effectiveness of MR-arterioportography (MR-AP) in achieving the highest sensitivity for detection and evaluation of HCC was evaluated.
Until now, there have hardly been any studies comparing the effectiveness of MR-AP and contrast-enhanced MRI for diagnosis of malignant liver lesions. This study confirmed that MR-arterioportography as an additional modality for detection of HCC is truly beneficial and may lead to change in treatment in many patients.
The results showed that using the MR-AP approach, significantly more lesions could be detected in comparison to MRI with liver-specific contrast agent. Thus, it might play an important role for future strategy of therapeutic interventions.
MR-AP is an MRI procedure where the contrast agent is injected through a catheter placed in the superior mesenteric artery.
It is well-written and is novel work.
Peer reviewers: Anuj Mishra, MD, Professor, Department of Radiology, National Organ Transplant Program, Central Hospital, Tripoli, PO Box 7913, Libya; Sri P Misra, Professor, Gastroenterology, Moti Lal Nehru Medical College, Allahabad 211001, India
S- Editor Sun H L- Editor Logan S E- Editor Ma WH
| 1. | Hamm B, Thoeni RF, Gould RG, Bernardino ME, Lüning M, Saini S, Mahfouz AE, Taupitz M, Wolf KJ. Focal liver lesions: characterization with nonenhanced and dynamic contrast material-enhanced MR imaging. Radiology. 1994;190:417-423. |
| 2. | Yamashita Y, Fan ZM, Yamamoto H, Matsukawa T, Yoshimatsu S, Miyazaki T, Sumi M, Harada M, Takahashi M. Spin-echo and dynamic gadolinium-enhanced FLASH MR imaging of hepatocellular carcinoma: correlation with histopathologic findings. J Magn Reson Imaging. 1994;4:83-90. |
| 3. | Yamashita Y, Hatanaka Y, Yamamoto H, Arakawa A, Matsukawa T, Miyazaki T, Takahashi M. Differential diagnosis of focal liver lesions: role of spin-echo and contrast-enhanced dynamic MR imaging. Radiology. 1994;193:59-65. |
| 4. | Semelka RC, Shoenut JP, Kroeker MA, Greenberg HM, Simm FC, Minuk GY, Kroeker RM, Micflikier AB. Focal liver disease: comparison of dynamic contrast-enhanced CT and T2-weighted fat-suppressed, FLASH, and dynamic gadolinium-enhanced MR imaging at 1.5 T. Radiology. 1992;184:687-694. |
| 5. | Shibata T, Murakami T, Ogata N. Percutaneous microwave coagulation therapy for patients with primary and metastatic hepatic tumors during interruption of hepatic blood flow. Cancer. 2000;88:302-311. |
| 6. | Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381-391. |
| 7. | Ito K, Honjo K, Fujita T, Matsui M, Awaya H, Matsumoto T, Matsunaga N, Nakanishi T. Therapeutic efficacy of transcatheter arterial chemoembolization for hepatocellular carcinoma: MRI and pathology. J Comput Assist Tomogr. 1995;19:198-203. |
| 8. | Kamada K, Nakanishi T, Kitamoto M, Aikata H, Kawakami Y, Ito K, Asahara T, Kajiyama G. Long-term prognosis of patients undergoing transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: comparison of cisplatin lipiodol suspension and doxorubicin hydrochloride emulsion. J Vasc Interv Radiol. 2001;12:847-854. |
| 9. | Seki T, Tamai T, Nakagawa T, Imamura M, Nishimura A, Yamashiki N, Ikeda K, Inoue K. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. 2000;89:1245-1251. |
| 10. | Senéterre E, Taourel P, Bouvier Y, Pradel J, Van Beers B, Daures JP, Pringot J, Mathieu D, Bruel JM. Detection of hepatic metastases: ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology. 1996;200:785-792. |
| 11. | Kim SR, Ando K, Mita K, Fuki S, Ikawa H, Kanbara Y, Imoto S, Matsuoka T, Hayashi Y, Kudo M. Superiority of CT arterioportal angiography to contrast-enhanced CT and MRI in the diagnosis of hepatocellular carcinoma in nodules smaller than 2 cm. Oncology. 2007;72 Suppl 1:58-66. |
| 12. | Kim SR, Imoto S, Ikawa H, Ando K, Mita K, Fuki S, Sakamoto M, Kanbara Y, Matsuoka T, Kudo M. Well-to moderately-differentiated HCC manifesting hyperattenuation on both CT during arteriography and arterial portography. World J Gastroenterol. 2007;13:5775-5778. |
| 13. | Kondo H, Kanematsu M, Hoshi H, Murakami T, Kim T, Hori M, Matsuo M, Nakamura H. Preoperative detection of malignant hepatic tumors: comparison of combined methods of MR imaging with combined methods of CT. AJR Am J Roentgenol. 2000;174:947-954. |
| 14. | Soyer P, Bluemke DA, Hruban RH, Sitzmann JV, Fishman EK. Hepatic metastases from colorectal cancer: detection and false-positive findings with helical CT during arterial portography. Radiology. 1994;193:71-74. |
| 15. | Kanematsu M, Hoshi H, Murakami T, Inaba Y, Kim T, Yamada T, Kato M, Yokoyama R, Nakamura H. Detection of hepatocellular carcinoma in patients with cirrhosis: MR imaging versus angiographically assisted helical CT. AJR Am J Roentgenol. 1997;169:1507-1515. |
| 16. | Noguchi Y, Murakami T, Kim T, Hori M, Osuga K, Kawata S, Kumano S, Okada A, Sugiura T, Nakamura H. Detection of hepatocellular carcinoma: comparison of dynamic MR imaging with dynamic double arterial phase helical CT. AJR Am J Roentgenol. 2003;180:455-460. |
| 17. | Semelka RC, Cance WG, Marcos HB, Mauro MA. Liver metastases: comparison of current MR techniques and spiral CT during arterial portography for detection in 20 surgically staged cases. Radiology. 1999;213:86-91. |
| 18. | Tang Y, Yamashita Y, Arakawa A, Namimoto T, Mitsuzaki K, Abe Y, Katahira K, Takahashi M. Detection of hepatocellular carcinoma arising in cirrhotic livers: comparison of gadolinium- and ferumoxides-enhanced MR imaging. AJR Am J Roentgenol. 1999;172:1547-1554. |
| 19. | Yamamoto H, Yamashita Y, Yoshimatsu S, Baba Y, Hatanaka Y, Murakami R, Nishiharu T, Takahashi M, Higashida Y, Moribe N. Hepatocellular carcinoma in cirrhotic livers: detection with unenhanced and iron oxide-enhanced MR imaging. Radiology. 1995;195:106-112. |
| 20. | Bellin MF, Zaim S, Auberton E, Sarfati G, Duron JJ, Khayat D, Grellet J. Liver metastases: safety and efficacy of detection with superparamagnetic iron oxide in MR imaging. Radiology. 1994;193:657-663. |
| 21. | Fretz CJ, Elizondo G, Weissleder R, Hahn PF, Stark DD, Ferrucci JT Jr. Superparamagnetic iron oxide-enhanced MR imaging: pulse sequence optimization for detection of liver cancer. Radiology. 1989;172:393-397. |
| 22. | Hagspiel KD, Neidl KF, Eichenberger AC, Weder W, Marincek B. Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced and unenhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology. 1995;196:471-478. |
| 23. | Ward J, Naik KS, Guthrie JA, Wilson D, Robinson PJ. Hepatic lesion detection: comparison of MR imaging after the administration of superparamagnetic iron oxide with dual-phase CT by using alternative-free response receiver operating characteristic analysis. Radiology. 1999;210:459-466. |
| 24. | Choi D, Kim S, Lim J, Lee W, Jang H, Lee S, Lim H. Preop erative detection of hepatocellular carcinoma: ferumoxides-enhanced mr imaging versus combined helical CT during arterial portography and CT hepatic arteriography. AJR Am J Roentgenol. 2001;176:475-482. |
| 25. | Murakami T, Oi H, Hori M, Kim T, Takahashi S, Tomoda K, Narumi Y, Nakamura H. Helical CT during arterial portography and hepatic arteriography for detecting hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 1997;169:131-135. |
| 26. | Dai Y, Chen MH, Fan ZH, Yan K, Yin SS, Zhang XP. Diagnosis of small hepatic nodules detected by surveillance ultrasound in patients with cirrhosis: Comparison between contrast-enhanced ultrasound and contrast-enhanced helical computed tomography. Hepatol Res. 2008;38:281-290. |
| 27. | Hosch WP, Schmidt SM, Plaza S, Dechow C, Schmidt J, Ley S, Kauffmann GW, Hansmann J. Comparison of CT during arterial portography and MR during arterial portography in the detection of liver metastases. AJR Am J Roentgenol. 2006;186:1502-1511. |
| 28. | Yu JS, Kim KW, Lee JT, Yoo HS. MR imaging during arterial portography for assessment of hepatocellular carcinoma: comparison with CT during arterial portography. AJR Am J Roentgenol. 1998;170:1501-1506. |
| 29. | Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22:275-282. |
| 30. | Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393-398. |
| 31. | Nasu K, Kuroki Y, Nawano S, Kuroki S, Tsukamoto T, Yamamoto S, Motoori K, Ueda T. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006;239:122-130. |
| 32. | Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812-822. |
| 33. | Koyama T, Tamai K, Togashi K. Current status of body MR imaging: fast MR imaging and diffusion-weighted imaging. Int J Clin Oncol. 2006;11:278-85. |