Published online Apr 7, 2011. doi: 10.3748/wjg.v17.i13.1718
Revised: December 21, 2010
Accepted: December 28, 2010
Published online: April 7, 2011
AIM: To investigate expression of Bcl-2 and Bax in gastric ischemia-reperfusion (GI-R) and involvement of extracellular signal-regulated kinase (ERK) 1/2 activation.
METHODS: The GI-R model was established by ligature of the celiac artery for 30 min and reperfusion in Sprague-Dawley rats. Rats were assigned to groups in accordance with their evaluation period: control, 0, 0.5, 1, 3, 6, 24, 48, and 72 h. Expression and distribution of Bcl-2 and Bax proteins were analyzed by immunohistochemistry and western blotting in gastric tissue samples after sacrifice.
RESULTS: Compared with controls, the percentage of positive cells and protein levels of Bcl-2 decreased in the early phases of reperfusion, reached its minimum at 1 h (P < 0.05); it then increased, reaching its peak at 24 h of reperfusion (P < 0.05). The pattern of Bax expression was opposite to that of Bcl-2. Bax expression increased after reperfusion, with its peak at 1 h of reperfusion (P < 0.05), and then it decreased gradually to a minimum at 24 h after reperfusion (P < 0.05). On the other hand, inhibition of activation of ERK1/2 induced by PD98059, a specific upstream MEK inhibitor, had significant effects on Bcl-2 and Bax in GI-R. Compared with GI-R treatment only at 3 h of reperfusion, PD98059 reduced the number of Bcl-2 positive cells (0.58% of R3h group, P < 0.05) and Bcl-2 protein level (74% of R3h group, P < 0.05) but increased the number of Bax-positive cells (1.33-fold vs R3h group, P < 0.05) and Bax protein level (1.35-fold of R3h group, P < 0.05).
CONCLUSION: These results indicated that the Bcl-2 and Bax played a pivotal role in the gastric mucosal I-R injury and repair by activation of ERK1/2.
- Citation: Qiao WL, Wang GM, Shi Y, Wu JX, Qi YJ, Zhang JF, Sun H, Yan CD. Differential expression of Bcl-2 and Bax during gastric ischemia-reperfusion of rats. World J Gastroenterol 2011; 17(13): 1718-1724
- URL: https://www.wjgnet.com/1007-9327/full/v17/i13/1718.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i13.1718
Current research into gastric ischemia-reperfusion (GI-R) has focused on its pathogenic and underlying molecular mechanism[1-8]. GI-R injury and repair is related to the changes in gastric mucosal cellular apoptosis and proliferation induced by GI-R in rats. In our previous experiments, we have explored the time course of gastric mucosal apoptosis and proliferation induced by GI-R, and the role of the extracellular signal-regulated kinase 1 and 2 (ERK1/2) signaling pathway in GI-R-induced gastric mucosal injury and repair[7]. We have found that serious gastric mucosal damage occurs rapidly at the early stage of reperfusion and is closely related to the suppression of ERK1/2 activation. The activity of ERK1/2 increases as the time of reperfusion is extended, and the activated ERK1/2 might inhibit apoptosis and promote proliferation in gastric mucosal cells. However, the precise mechanisms by which activated ERK1/2 accomplishes gastric mucosal apoptosis and proliferation are unknown.
The balance between apoptosis and cellular proliferation is a key in gastric injury and repair, and is regulated by several genes, including p53 and members of the Bcl-2 family such as Bax and Bcl-2[9-11]. The Bax gene is a proliferative suppressor gene that encodes Bax protein that promotes apoptosis. On the other hand, bcl gene encodes Bcl-2 protein that blocks wild type p53-mediated apoptosis, and heterodimers with Bax, antagonizing the function of Bax[12].
Therefore, it is conceivable that increased cellular apoptosis and proliferation, because of altered expression of the regulating proteins such as Bax and Bcl-2, may be associated with gastric injury and repair induced by GI-R. Although Bcl-2 and Bax are expressed in gastric mucosa, their presence in normal gastric mucosa is controversial. Liu et al[13] have reported that Bcl-1 mRNA and protein are expressed in the gastric gland zone at a middle level and Bax protein is expressed in the epithelial cells of normal gastric mucosa. Xia et al[14] have found that, in intact gastric tissue, Bcl-2 and Bax are localized predominantly in the glandular base region in chief cells in normal rat gastric mucosa. However, a conflicting study has found that no expression of Bcl-2 protein is detected in the glandular epithelium of normal gastric mucosa[15]. On the other hand, Bcl-2 and Bax show significant changes in many conditions including gastric cancer, gastritis, and GI-R[15-18]. El Eter et al[15] have reported that cytoplasmic expression of Bcl-2 protein is observed in the superficial portion of gastric mucosa sections obtained from rats subjected to GI-R injury.
The available data on expression of Bcl-2 and Bax proteins in the stomach, and their relation to apoptosis of gastric mucosal cells, seem equivocal, thus, a further study of Bcl-2 and Bax expression in the stomach is clearly important. In the present study, we used an immunohistochemical assay and western blotting to determine the changed courses of Bcl-2 and Bax at different reperfusion durations after GI-R, and whether ERK1/2 activation was involved in this process.
Groups of six adult Sprague-Dawley rats, regardless of sex, weighing 220-270 g, were provided by the Experimental Animal Centre of Xuzhou Medical College. All experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Rats were housed under controlled temperature (22-24°C) and photoperiod (12 h light/12 h dark), and allowed food and water ad libitum. Rats were fasted for 24 h before the experiment, but were allowed free access to tap water. Animals were randomly assigned to groups: GI-R (different reperfusion time points after 30 min of ischemia); PD98059 + R3h (PD98059 + reperfusion for 3 h after 30 min of ischemia); and vehicle control (PD98059 replaced with vehicle but otherwise the same as PD98059 + R3h). PD98059 was given 20 min before operation [150 μg/kg, administered intraperitoneally (i.p.), dissolved in dimethyl sulfoxide]. A sham group in which only the same surgical procedure without clamping the celiac artery was performed served as a control.
PowerVisionTM two-step immunohistochemistry detection kit were purchased from Zhongshan Biotech Co. (Beijing, China), anti-Bcl-2 and anti-Bax polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), alkaline-phosphorylase-tagged goat anti-rat IgG antibody, PD98059 was from Promega (Madison, WI, USA), and sodium pentobarbital was purchased from Sigma (St. Louis, MO, USA).
GI-R models were induced according to the method of Qiao et al [7]. The randomly grouped rats were all anesthetized with sodium pentobarbital (40 mg/kg, i.p.). Their abdomens were incised along the midline, and the celiac artery and its adjacent tissues were carefully isolated. The celiac artery was clamped with a small non-traumatic vascular clamp for 30 min to induce gastric ischemia and then released for 0, 0.5, 1, 3, 6, 24, 48 and 72 h to allow reperfusion. Following reperfusion, the rats were sacrificed and the stomachs were removed immediately. The stomachs were incised along the greater curvature and flushed with ice-cold PBS (0.1 mol/L). One half of the gastric mucosa was frozen at -80°C for western blotting, and the other was fixed in Bouin’s fixative for immunohistochemical staining.
The fixed stomach was embedded in paraffin, sliced into 4-μm-thick sections, and mounted on glass slides. The immunohistochemistry was performed with a PowerVision two-step immunohistochemistry detection kit. The sections were stained with 3,3’-diaminobenzidine (DAB), then counterstained using hematoxylin. The sections were examined with a microscope (Model IX71; Olympus, Tokyo, Japan). Gastric mucosal cells with brown granules visible in the cytoplasm or nucleus were considered positive. The number of positive cells per section was counted in 10 random lower-power (× 10) fields, and the percentage of positive cells (positive cells/total cells × 100%) was calculated. Three non-consecutive sections were selected from each specimen and those indexes were averaged.
The frozen gastric mucosa was homogenized with a Teflon glass homogenizer in 1:10 (w/v) ice-cold homogenization buffer consisting of 50 mmol/L 3-(N-morpholino) propanesulfonic acid (MOPS, pH 7.4), 50 mmol/L NaF, 20 mmol/L sodium pyrophosphate (NaPPi), 20 mmol/L b-glycerophosphate, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L phenylmethylsulphonyl fluoride, 10 mg/mL leupeptin, 10 mg/mL aprotinin and 10 mg/mL pepstatin A. The homogenate was centrifuged at 800 g for 15 min at 4°C, and the supernatant was retained as cytoplasmic parts. Protein concentrations were determined by Coomassie brilliant blue protein assay. The proteins was heated at 100°C for 5 min with loading buffer containing 0.125 mol/L Tris-HCl (pH 6.8), 20% glycerol, 4% SDS, 10% mercaptoethanol and 0.002% bromophenol blue, then separated by 10% SDS-PAGE. The proteins were isolated by 12.5% SDS-PAGE and transferred to a nitrocellulose membrane. The blots were incubated with 4% bovine serum albumin in TBST (10 mmol/L Tris, pH 7.5; 150 mmol/L NaCl, 0.05% Tween-20) at 4°C for 6 h and probed with primary antibodies (anti-Bcl-2 polyclonal antibody 1:500, anti-Bax polyclonal antibody 1:400) at 4°C overnight. Membranes were rinsed and incubated with secondary antibody for 2 h and were detected with an NBT/BCIP assay kit. After immunoblotting, the bands were scanned and analyzed by Image J software. The optical density (OD) of the band in each lane was expressed as the fold change versus the OD of the sham control.
All results are presented as mean ± SD. Comparisons between two groups were made with Student’s t test; multiple-group analyses were made by one-way ANOVA. Statistical analyses were performed with SPSS for Windows version 11.5. P < 0.05 was considered statistically significant.
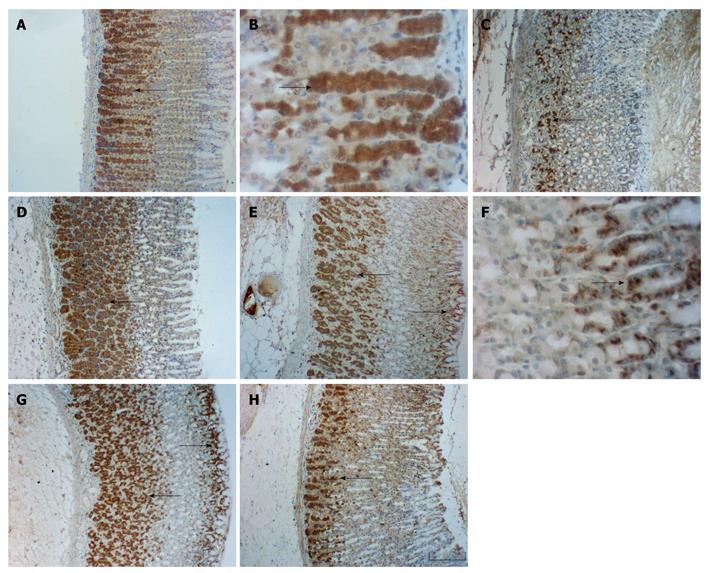
Immunohistochemical staining clearly showed that Bcl-2 and Bax were expressed and limited to the cytosol of the cells in the gastric mucosa (Figure 1). Bcl-2 expressing cells were found predominantly in the lower part of the gastric gland, and were present only in gland cells of the stomach fundus (Figure 1A-D). Bax positive cellular distribution was similar to that of Bcl-2, with prominent expression in the base, but staining for Bax was also noticeable in the cells of the pit. Bax appeared to be absent from the middle part of the gastric gland, and weak expression of Bax was detected in most cells of the gastric mucosa (Figure 1E-H). The control sample (sham-operated) obtained prior to the ischemic period showed a normal appearance of Bcl-2 (Figure 1A and B) and Bax (Figure 1E and F), and there were significant differences between the GI-R (Figure 1C, D, G and H) and control groups (Figure 1A and E) in the quantities of Bcl-2 and Bax immunoreactive cells.
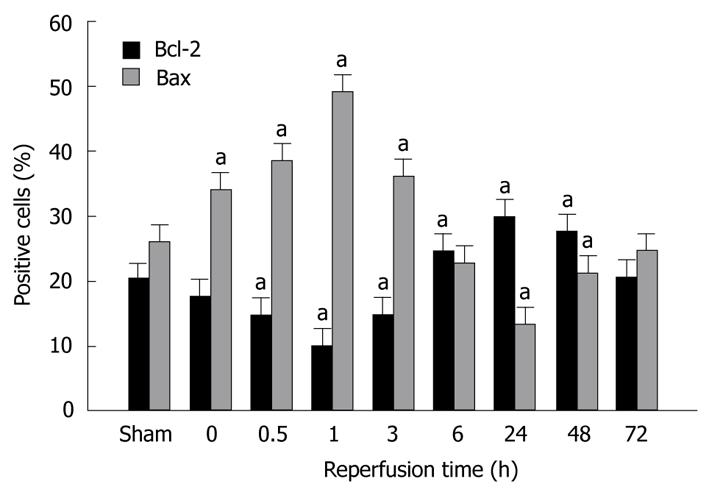
The percentage of Bcl-2 and Bax positive cells in various groups is shown in Figure 2. Bcl-2 and Bax positive cells decreased in the early phase of reperfusion, with a nadir (10.02% ± 1.21%) at 1 h of reperfusion, then increased significantly after reperfusion for 3 h, with a peak (29.76% ± 3.32%) at 24 h of reperfusion, and returned to near the base level (20.47% ± 2.97%) at 72 h of reperfusion. The opposite pattern was observed for Bax positive cells. The percentage of Bax positive cells increased in the initial stages of reperfusion, reached the highest Bax positive cell count (49.34% ± 3.83%) at 1 h of reperfusion, then decreased gradually, with its nadir (13.36% ± 3.05%) at 24 h of reperfusion, and recovered to base level (24.94% ± 2.83%) at 72 h of reperfusion.
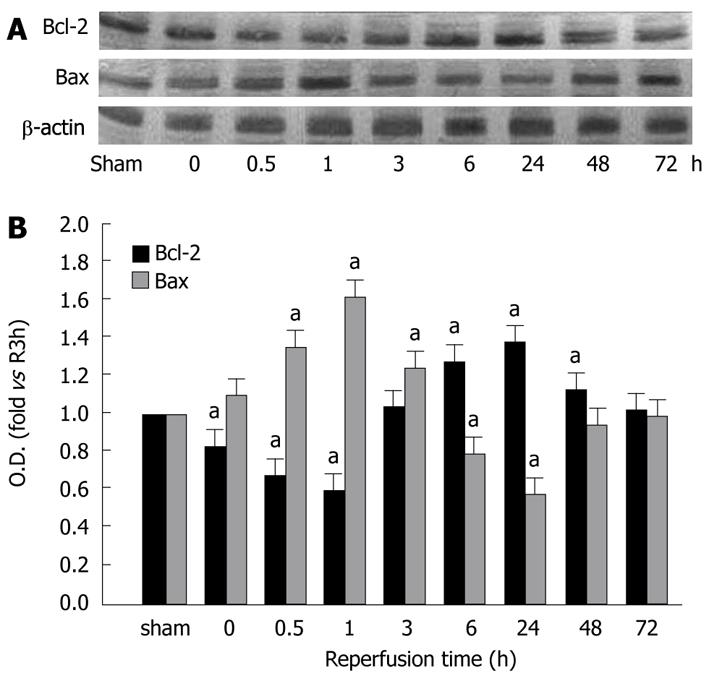
Figure 3 show the Bcl-2 and Bax protein levels in the gastric mucosa in different groups of the study. After reperfusion, expression of Bcl-2 protein was significantly lower than that of the controls, and the lowest level was observed at 1 h of reperfusion (0.59% of sham group, P < 0.05). A peak of Bcl-2 protein expression was displayed in the 24 h reperfusion group (1.36 fold vs sham group, P < 0.05). The Bax protein level increased in the early stage of reperfusion, reached its peak (1.62-fold vs sham group, P < 0.05) at 1 h of reperfusion, and then decreased gradually to its lowest levels (0.57% of sham group, P < 0.05) at 24 h of reperfusion. At 72 h of reperfusion, Bcl-2 and Bax protein levels were the same as that of the control group.
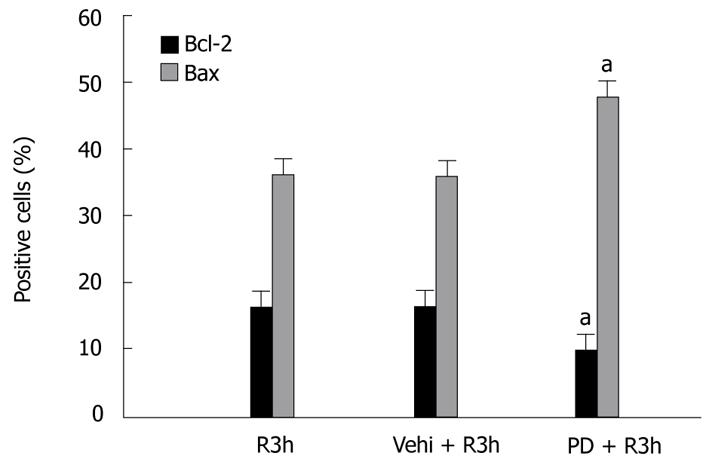
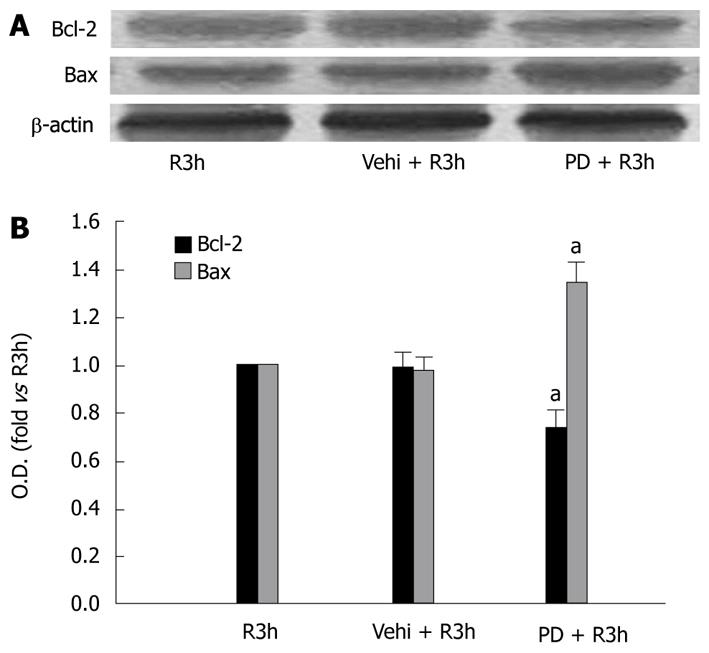
PD98059 is a specific upstream inhibitor of ERK1/2. By immunohistochemical assay, we found PD98059 had significant effects on Bcl-2 and Bax expression in GI-R. Compared with the control group (R3h group), the PD98059 + R3h group showed a fall in the number of Bcl-2 positive cells (0.58% of R3h group, P < 0.05) but an increase in Bax positive cells (1.31-fold vs R3h group, P < 0.05) (Figure 4). To ascertain the effect of PD98059 on expression levels of Bcl-2 and Bax protein, western blotting was performed. In the PD98059 + R3h group, gastric mucosal Bcl-2 protein level was 74% of that in the R3h group (P < 0.05), whereas Bax protein level was 1.35-fold more than that in the R3h group (P < 0.05) (Figure 5).
Previous studies have shown that many stress conditions, such as hemorrhagic shock, burns, sepsis, major surgery, ischemia and trauma can lead to GI-R injury. In recent years, studies on GI-R injury have revealed that reactive oxygen species, endothelin, microvascular dysfunction, polymorphonuclear leukocyte infiltration, nitric oxide release, gastric acid secretion and decreased prostaglandin concentrations may play a role in the pathogenesis of gastric mucosal injury induced by GI-R[1,5,19-25]. Although the gastric mucosa is vulnerable to damage by various factors, it can quickly repair the damage[26]. Mucosal integrity is maintained by a balance between proliferation and apoptosis of the gastric mucosal cells. To understand better the causes of gastric lesions, it is important to study the imbalance between proliferation and apoptosis[27,28].
In previous experiments[7], we have shown the changed courses of gastric mucosal injury and repair induced by GI-R, and the role of ERK1/2 in this process. Our results indicated clearly that the gastric mucosal injury induced by GI-R was mainly the result of reperfusion. The serious gastric mucosal lesions occurred in the initial stages of reperfusion and the aggravating processes of mucosal lesions were at 1 h after reperfusion, which were maintained about 3 h after reperfusion. The gastric mucosal repairs started after 3 h of reperfusion, and the complete recovery took almost 3 d. Based on these facts, it indicated that gastric mucosa has an amazing self-repairing ability. ERK1/2 are important members of the mitogen-activated protein kinase family. The activation of ERK1/2 participates in the regulation of cellular injury and repair in many tissues. Our researches have also shown that the p-ERK1/2 protein level decreased at 0.5 h after reperfusion began, and then gradually increased, reaching its peak after 3 h of reperfusion. Inhibition of the activation of ERK1/2 aggravated the gastric mucosal injury, with apoptosis increased and proliferation reduced in the gastric mucosal cells at the same duration of reperfusion. Therefore, activated ERK1/2 inhibited apoptosis and promoted proliferation in gastric mucosal cells.
Apoptosis and proliferation are fundamental mechanisms for cell death and survival and differentiation in the gastric mucosa. The status of the Bcl-2 family proteins determines whether a cell will live or die through the regulation of cytochrome c release from the mitochondria[29,30]. Bcl-2 protein mainly inhibits apoptosis and facilitates cellular survival and differentiation, whereas overexpression of Bax protein induces apoptosis and inhibits the effect of Bcl-2[31-33]. Our data showed that Bcl-2 expression decreased significantly after the start of the reperfusion, reaching its nadir at 1 h, before increasing gradually to a peak after 24 h of reperfusion. The pattern of change in Bax expression was opposite to that of Bcl-2 expression. Bax expression increased at first, reaching its maximum after 1 h of reperfusion, and then decreased. Bcl-2 and Bax recovered gradually to base level at 72 h of reperfusion. PD98059, a specific upstream inhibitor of ERK1/2, downregulated expression of Bcl-2 and upregulated expression of Bax in GI-R. These results suggest that the course of expression of Bcl-2 and Bax were closely correlated with p-ERK1/2. Activation of ERK1/2 causes upregulation of Bcl-2 and downregulation of Bax.
In conclusion, Bcl-2 and Bax played a pivotal role in GI-R injury and repair by activation of ERK1/2. Bcl-2 was involved in recovery of GI-R-mediated gastric mucosa injury by promoting cellular proliferation, and Bax was involved in gastric mucosal injury induced by GI-R by promoting apoptosis.
It is well known that many hemorrhagic and stress conditions lead to gastric ischemia-reperfusion (GI-R) injury. Gastric ulceration is very prevalent in humans and is usually preceded by burns, sepsis, major surgery, ischemia, trauma and other heterogeneous forms of stress. Erosions in the gastric mucosa can be demonstrated in as many as 75%-100% of patients within 24 h of admission to the intensive care unit (ICU). Clinically apparent gastrointestinal bleeding can occur in as many as 25% of ICU patients.
In recent years, studies on GI-R injury have focused on its pathogenic and underlying molecular mechanism. In recent years, studies on GI-R injury have revealed that reactive oxygen species, endothelin, microvascular dysfunction, polymorphonuclear leukocyte infiltration, nitric oxide release, gastric acid secretion and decreased prostaglandin concentrations during reperfusion may play a role in the pathogenesis of gastric mucosal injury induced by GI-R. Mucosal integrity is maintained by the equilibrium between proliferation and apoptosis of the gastric mucosal cells. To understand better the pathogenesis of gastric lesions, it is of great importance to study the imbalance between proliferation and apoptosis.
This is believed to be the first study to investigate changes in expression of Bcl-2 and Bax at different times of reperfusion after gastric ischemia, and whether extracellular signal-regulated kinase 1/2 activation was involved in this process.
Not only does our study provide insights into the mechanism of gastric mucosal tissue injury and repair; it also provides information that could potentially guide development of a new therapeutic strategy.
The quality of the paper is excellent and deserves a fast publication, considering the contribution importance.
Peer reviewer: Dr. José Liberato Ferreira Caboclo, Professor, Rua Antônio de Godoy, 4120, São José do Rio Preto, Brazil
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Du DS, Ma XB, Zhang JF, Zhou XY, Li Y, Zhang YM, Qiao WL. The protective effect of capsaicin receptor-mediated genistein postconditioning on gastric ischemia-reperfusion injury in rats. Dig Dis Sci. 2010;55:3070-3077. |
| 2. | Nakamori Y, Komatsu Y, Kotani T, Kojima S, Takeuchi K. Pathogenic importance of cysteinyl leukotrienes in development of gastric lesions induced by ischemia/reperfusion in mice. J Pharmacol Exp Ther. 2010;333:91-98. |
| 3. | Suzuki S, Suzuki H, Horiguchi K, Tsugawa H, Matsuzaki J, Takagi T, Shimojima N, Hibi T. Delayed gastric emptying and disruption of the interstitial cells of Cajal network after gastric ischaemia and reperfusion. Neurogastroenterol Motil. 2010;22:585-593, e126. |
| 4. | Du D, Ma X, Zhang J, Zhang Y, Zhou X, Li Y. Cellular and molecular mechanisms of 17beta-estradiol postconditioning protection against gastric mucosal injury induced by ischemia/reperfusion in rats. Life Sci. 2010;86:30-38. |
| 5. | Peskar BM, Ehrlich K, Schuligoi R, Peskar BA. Role of lipoxygenases and lipoxin A(4)/annexin-1 receptor in gastric protection induced by 20% ethanol or sodium salicylate in rats. Pharmacology. 2009;84:310-313. |
| 6. | Li L, Zhang YM, Qiao WL, Wang L, Zhang JF. Effects of hypothalamic paraventricular nuclei on apoptosis and proliferation of gastric mucosal cells induced by ischemia/reperfusion in rats. World J Gastroenterol. 2007;13:874-881. |
| 7. | Qiao WL, Wang L, Zhang YM, Zhang JF, Wang GM. Extracellular signal-regulated kinase 1- and 2-mediated gastric mucosal injury and repair in gastric ischemia-reperfusion of rats. J Gastroenterol. 2006;41:1158-1168. |
| 8. | Zhang YM, Wei EQ, Hu X, Qiao WL, Shi Y, Xu M, Zhang JF. The role of nuclear factor-kappaB in the effect of angiotensin II in the paraventricular nucleus in protecting the gastric mucosa from ischemia-reperfusion injury in rats. J Gastroenterol. 2008;43:687-698. |
| 9. | Boucher MJ, Morisset J, Vachon PH, Reed JC, Lainé J, Rivard N. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem. 2000;79:355-369. |
| 10. | Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82:1111-1129. |
| 11. | Rezvani M, Barrans JD, Dai KS, Liew CC. Apoptosis-related genes expressed in cardiovascular development and disease: an EST approach. Cardiovasc Res. 2000;45:621-629. |
| 12. | Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol. 2001;96:16-26. |
| 13. | Liu J, Li ZS, Wan XJ, Wang W. Expression and function of apoptosis-related genes Bcl-2/Bax and Fas/Fas L in the course of stress ulcer. Zhonghua Yixue Zazhi. 2003;83:504-509. |
| 14. | Xia HH, Zhang GS, Talley NJ, Wong BC, Yang Y, Henwood C, Wyatt JM, Adams S, Cheung K, Xia B. Topographic association of gastric epithelial expression of Ki-67, Bax, and Bcl-2 with antralization in the gastric incisura, body, and fundus. Am J Gastroenterol. 2002;97:3023-3031. |
| 15. | El Eter E, Hagar HH, Al-Tuwaijiri A, Arafa M. Nuclear factor-kappaB inhibition by pyrrolidinedithiocarbamate attenuates gastric ischemia-reperfusion injury in rats. Can J Physiol Pharmacol. 2005;83:483-492. |
| 16. | Smith L, Berrieman HK, O’Kane SL, Campbell A, Maraveyas A, Cawkwell L. Immunohistochemical detection of apoptotic markers in gastric cancer. Oncol Res. 2006;15:441-444. |
| 17. | Yamasaki E, Wada A, Kumatori A, Nakagawa I, Funao J, Nakayama M, Hisatsune J, Kimura M, Moss J, Hirayama T. Helicobacter pylori vacuolating cytotoxin induces activation of the proapoptotic proteins Bax and Bak, leading to cytochrome c release and cell death, independent of vacuolation. J Biol Chem. 2006;281:11250-11259. |
| 18. | Liu HF, Liu WW, Wang GA, Teng XC. Effect of Helicobacter pylori infection on Bax protein expression in patients with gastric precancerous lesions. World J Gastroenterol. 2005;11:5899-5901. |
| 19. | Konturek SJ, Brzozowski T, Konturek PC, Schubert ML, Pawlik WW, Padol S, Bayner J. Brain-gut and appetite regulating hormones in the control of gastric secretion and mucosal protection. J Physiol Pharmacol. 2008;59 Suppl 2:7-31. |
| 20. | Brzozowski T, Konturek PC, Konturek SJ, Drozdowicz D, Kwiecieñ S, Pajdo R, Bielanski W, Hahn EG. Role of gastric acid secretion in progression of acute gastric erosions induced by ischemia-reperfusion into gastric ulcers. Eur J Pharmacol. 2000;398:147-158. |
| 21. | Ishii M, Shimizu S, Nawata S, Kiuchi Y, Yamamoto T. Involvement of reactive oxygen species and nitric oxide in gastric ischemia-reperfusion injury in rats: protective effect of tetrahydrobiopterin. Dig Dis Sci. 2000;45:93-98. |
| 22. | Szlachcic A, Brzozowski T, Majka J, Pajdo R, Konturek PC, Pawlik M, Kwiecien S, Drozdowicz D, Bielanski W, Konturek SJ. Involvement of orexigenic peptides in the mechanism of gastric mucosal integrity and healing of chronic gastric ulcers. Curr Pharm Des. 2010;16:1214-1223. |
| 23. | Cabeza J, Motilva V, Martín MJ, de la Lastra CA. Mechanisms involved in gastric protection of melatonin against oxidant stress by ischemia-reperfusion in rats. Life Sci. 2001;68:1405-1415. |
| 24. | Kim H, Hwan Kim K. Role of nitric oxide and mucus in ischemia/reperfusion-induced gastric mucosal injury in rats. Pharmacology. 2001;62:200-207. |
| 25. | Tanaka J, Yuda Y, Inouye S, Yamakawa T. The role of nitric oxide in the gastric acid secretion induced by ischemia-reperfusion in the pylorus-ligated rat. Eur J Pharmacol. 2001;424:69-74. |
| 26. | Szabó I, Tarnawski AS. Apoptosis in the gastric mucosa: molecular mechanisms, basic and clinical implications. J Physiol Pharmacol. 2000;51:3-15. |
| 27. | Wada K, Nakajima A, Takahashi H, Yoneda M, Fujisawa N, Ohsawa E, Kadowaki T, Kubota N, Terauchi Y, Matsuhashi N. Protective effect of endogenous PPARgamma against acute gastric mucosal lesions associated with ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2004;287:G452-G458. |
| 28. | Villegas I, Martín AR, Toma W, de la Lastra CA. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, protects against gastric ischemia-reperfusion damage in rats: role of oxygen free radicals generation. Eur J Pharmacol. 2004;505:195-203. |
| 29. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. |
| 30. | Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051-3060. |
| 31. | Konturek PC, Brzozowski T, Konturek SJ, Pajdo R, Konturek JE, Kwiecień S, Taut A, Hahn EG. Apoptosis in gastric mucosa with stress-induced gastric ulcers. J Physiol Pharmacol. 1999;50:211-225. |
| 32. | Lindsten T, Zong WX, Thompson CB. Defining the role of the Bcl-2 family of proteins in the nervous system. Neuroscientist. 2005;11:10-15. |