Published online Mar 28, 2011. doi: 10.3748/wjg.v17.i12.1649
Revised: January 11, 2011
Accepted: January 18, 2011
Published online: March 28, 2011
AIM: To assess the application of multiple planar volume reconstruction (MPVR) and three-dimensional (3D) transparency lung volume rendering (TL-VR) with 64-row multidetector-row computed tomography (MDCT) in neonates with congenital esophageal atresia (EA) and distal tracheoesophageal fistula (TEF).
METHODS: Twenty neonates (17 boys, 3 girls) with EA and distal TEF at a mean age of 4.6 d (range 1-16 d) were enrolled in this study. A helical scan of 64-row MDCT was performed at the 64 mm × 0.625 mm collimation. EA and TEF were reconstructed with MPVR and TL-VR, respectively. Initial diagnosis of EA was made by chest radiography showing the inserted catheter in the proximal blind-ended esophageal pouch. Manifestations of MDCT images were compared with the findings at surgery.
RESULTS: MDCT showed the proximal and distal esophageal pouches in 20 cases. No significant difference was observed in gaps between the proximal and distal esophageal pouches detected by MPVR and TL-VR. The lengths of gaps between the proximal and distal esophageal pouches detected by MPVR and TL-VR correlated well with the findings at surgery (R = 0.87, P < 0.001). The images of MPVR revealed the orifice of TEF in 13 cases, while TL-VR images showed the orifice of TEF in 4 cases.
CONCLUSION: EA and distal TEF can be reconstructed using MPVR and TL-VR of 64-row MDCT, which is a noninvasive technique to demonstrate the distal esophageal pouches and inter-pouch distance in neonates with EA and distal TEF.
- Citation: Wen Y, Peng Y, Zhai RY, Li YZ. Application of MPVR and TL-VR with 64-row MDCT in neonates with congenital EA and distal TEF. World J Gastroenterol 2011; 17(12): 1649-1654
- URL: https://www.wjgnet.com/1007-9327/full/v17/i12/1649.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i12.1649
Esophageal atresia (EA), with or without tracheoesophageal fistula (TEF), is the most important and common congenital malformation of the esophagus[1,2]. The reported incidence of EA and TEF is approximately one in 3000-4500 live births[1]. The majority of patients have an associated fistula between the trachea and distal esophageal pouch. In neonates with EA and distal TEF, the anatomy of esophageal pouch, inter-pouch gap and up-growth of tracheobronchial tree, especially inter-pouch gap, not only influences the surgical approach and postoperative strategy but also predicts prognosis of such patients[1,3,4]. If a short inter-pouch gap (e.g. shorter than 2 cm) with two longer pouches is detected, esophageal reunion with a primary anastomosis is relatively easy and postoperative complications are few. On the contrary, surgical procedure may be difficult for a longer interpouch gap with hypogenetic pouches, postoperative complications such as anastomotic leak may occur and ventilatory support is possibly required.
The application of multidetector-row computed tomography (MDCT) in reconstruction of 3D volume rendering (VR) has increased due to its ability to demonstrate the anatomy of hollow viscera. MDCT can generate VR images of the trachea and esophagus quickly and non-invasively, show the anatomy and 3D relation between the two structures more clearly and accurately, and provide valuable guidance for any surgical approach[5]. Multiple planar volume reconstruction (MPVR) by MDCT is a reliable and straightforward technique to show hollow viscera in a multidirectional view and their adjacent tissues and organs clearly.
In this study, the ability of 3D TL-VR and MPVR to reconstruct EA and distal TEF in neonates was assessed by 64-row MDCT.
The study was approved by the local ethics committee.Written informed consent was obtained from the parents of patients. Twenty neonates (17 boys and 3 girls) with EA and distal TEF were included in this study. Their birth weight was 1900-4000 g (mean 2888 g). Of the 20 neonates, 18 were full-term and 2 were pre-matured at a gestational age of 35 and 36 wk, respectively. Their age at the time of CT scan was 1-16 d with a mean age of 4.6 d. All patients had different degrees of respiratory distress and were not able to swallow food and saliva.Initial diagnosis of EA was made according their clinical symptoms and chest radiography showing the inserted catheter in the proximal blind-ended esophageal pouch and intestinal gas in the upper abdomen. Nineteen cases had different degrees of aspiration pneumonia, and only one had no inflammation of the lungs. Before CT scan, the proximal pouch was suctioned with an aspirating catheter, and then air (5 mL) was hand-injected into the proximal pouch. To avoid respiratory distress, the patients were not given sedatives. Body straps were used to immobilize the patients and decrease the motion artifacts. After CT scan, all patients underwent operation, and CT manifestations were compared with the findings at surgery.
CT data were collected with a 64-row MDCT scanner (GE Lightspeed VCT64, GE Healthcare, Wis, USA). All scans were performed from the level of larynx to the diaphragm. CT scan protocol was as follows: 120 kVp, z-axis automatic tube current modulation (ATCM) (AutomA; GE Healthcare) for all children with the noise index (NI) of 10 with a mean calculated CTDI dose of 1.57 mGy, a 64 mm × 0.625 collimation, 1.375:1 pitch, 0.8 s rotation time, and 512 × 512 matrix. The scanning time was 2-3.5 s. Images were reconstructed in the axial plane at a 0.625 mm interval with a standard reconstruction algorithm. CT images were transferred to an independent workstation (Advantage Workstation 4.3; GE Medical Systems) for further image reconstruction. Chest MPVR images were collected at the axial and sagittal and coronal view with a minimum intensity projection (MinIP), and 3D transparency lung VR models of the tracheobronchial system and the esophagi with a lower threshold of -704 HU and an upper threshold of -280 HU. Gaps between the proximal and distal esophageal pouches were measured at the workstation by two consultant radiologists with experiences in pediatric radiology and pediatric computed tomography. The average findings were recorded. The total image processing time was 20-30 min in each patient. Because the proximal and distal esophageal pouches were not frequently observed in the same plane of sagittal or in coronal view of multiple plane reconstruction (MPR), pulmonary air leaks were not discovered in all cases, chest MPVR with minimum intensity projection (MinIP) was used instead of MPR. However, it should be emphasized that MPR could provide more information about esophageal walls and the surrounding tissues of esophageal pouches than MPVR or TL-VR. View of the original axial and MPR images was indispensable before the measurement of MPVR and TL-VR.
The 20 patients underwent operation within 30 h (average 19 h) after CT scan. The inter-pouch distance and caliber of fistulae were measured with a silk thread between two artery forceps during operation. Then different lengths (centimeters) of silk thread were measured with a ruler.
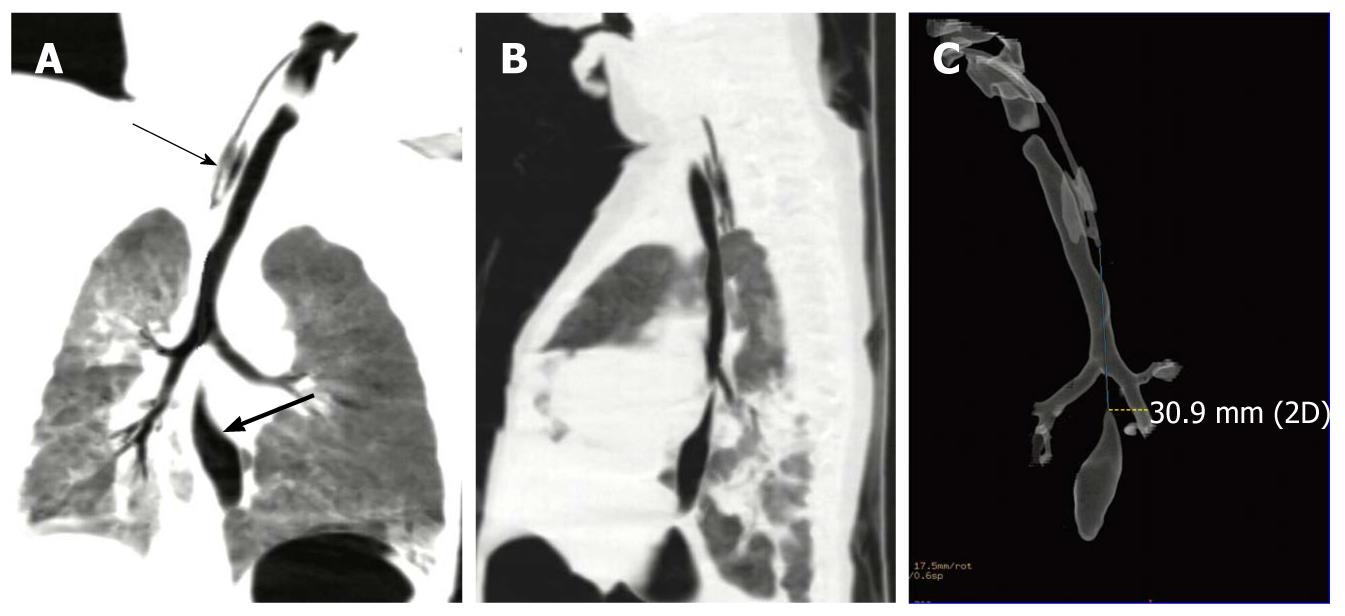
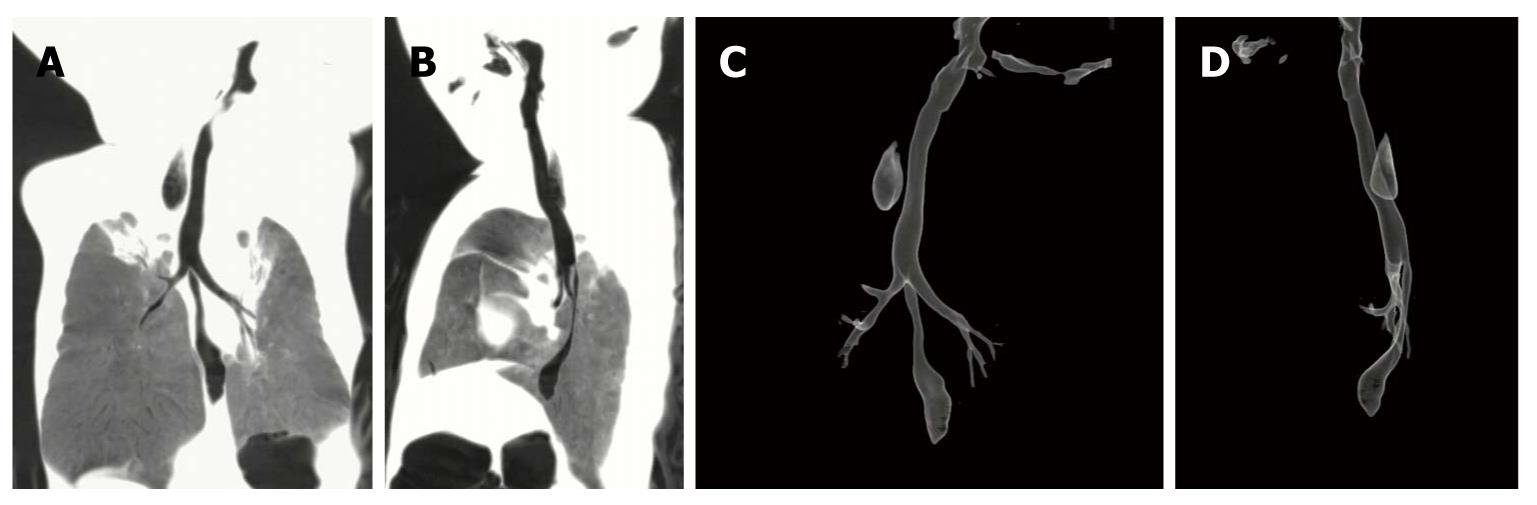
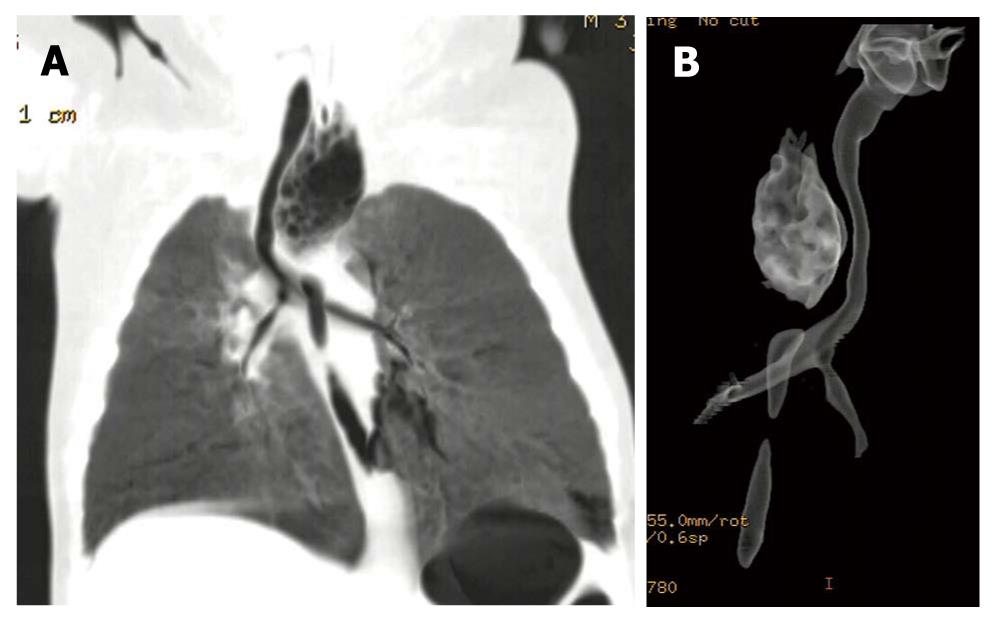
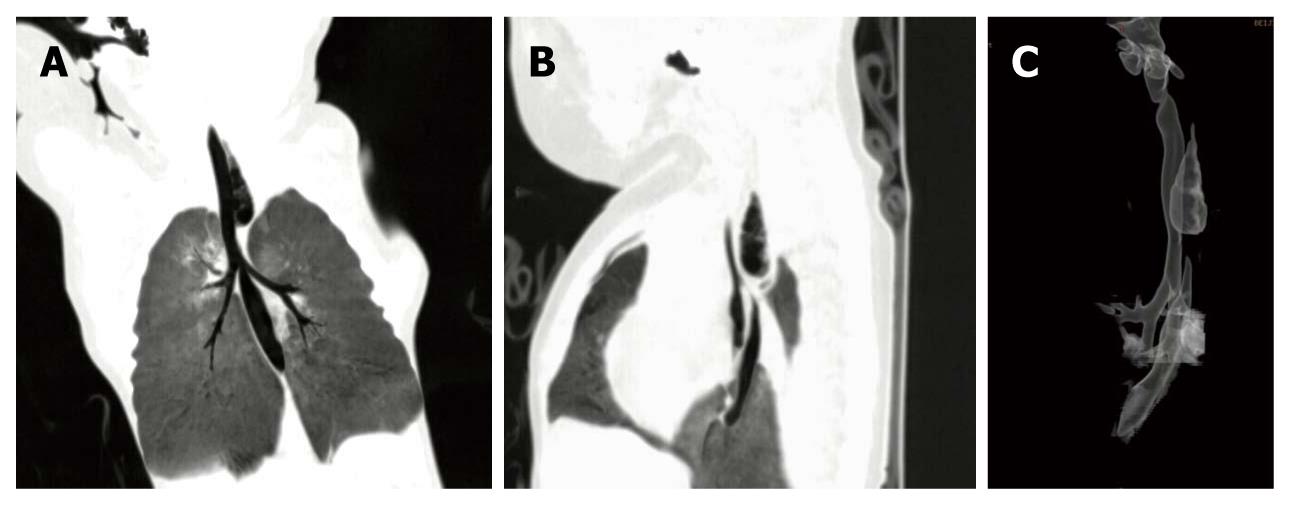
The trachea and bronchial systems including the major lobe bronchi, proximal and distal esophageal pouches, and inter-pouch gaps in 20 patients could be clearly observed on the 3D TL-VR and MPVR images. The MPVR images showed the distal fistulae including their orifice in 13 cases, while the TL-VR images revealed the distal fistulae including their orifice in only 4 cases. Zigzag artifacts were trifling in most patients because of their motion and respiration. Different lengths of the gaps and different degrees of the fistulae are shown in Figures 1, 2, 3, 4, and Figure 2 revealed that the lower fistula opened into the trachea within 1 cm of the carina.
The CT data and findings at surgery in the patients are summarized in Table 1. The inter-pouch gap was measured at surgery after the fistulae were divided in 17 cases and before the fistulae were cut in 3 cases. The diameter of fistulae was measured at surgery before the fistulae were ligated and divided in the 20 cases.
| Case | Sex | Age (d) | EI gaps (cm) | Caliber of TF (cm) | ||||
| MPVR | TL-VR | Operation | MPVR | TL-VR | Operation | |||
| 1 | M | 2 | 0.40 | 0.38 | 1.0 | IN | IN | 0.4 |
| 2 | M | 6 | 0.52 | 0.57 | 1.5 | IN | IN | 0.5 |
| 3 | M | 3 | 3.10 | 3.10 | 3.0 | 0.1 | IN | 0.5 |
| 4 | M | 3 | 0.84 | 0.74 | 1.0 | IN | IN | 0.6 |
| 5 | M | 3 | 0.54 | 0.89 | 1.2 | 0.2 | IN | 0.7 |
| 6 | F | 3 | 0.42 | 0.32 | 1.3 | 0.2 | IN | 0.7 |
| 7 | M | 2 | 1.96 | 1.80 | 2.5 | IN | IN | 1.0 |
| 8 | M | 5 | 1.72 | 1.75 | 3.0 | 0.1 | 0.2 | 0.4 |
| 91 | M | 6 | 0.58 | 0.81 | 0.6 | 0.2 | IN | 0.3 |
| 10 | F | 3 | 0.90 | 0.90 | 1.5 | 0.1 | 0.1 | 0.5 |
| 11 | M | 5 | 0.26 | 0.22 | 0.1 | 0.1 | IN | 0.6 |
| 12 | M | 11 | 0.80 | 0.83 | 1.5 | IN | IN | 0.5 |
| 131 | M | 5 | 0.70 | 0.56 | 0.7 | IN | IN | 0.4 |
| 14 | M | 1 | 0.60 | 0.51 | 1.0 | 0.1 | IN | 0.8 |
| 15 | M | 16 | 1.98 | 2.03 | 3.3 | IN | IN | 0.8 |
| 161 | M | 2 | 0.15 | 0.10 | 0.5 | 0.2 | IN | 0.5 |
| 17 | M | 1 | 0.70 | 0.94 | 1.5 | 0.2 | 0.2 | 0.4 |
| 18 | M | 6 | 1.34 | 1.60 | 2.0 | 0.1 | IN | 0.6 |
| 19 | F | 1 | 1.10 | 1.14 | 2.3 | 0.2 | 0.2 | 0.4 |
| 20 | M | 4 | 0.66 | 0.59 | 1.5 | 0.1 | IN | 0.4 |
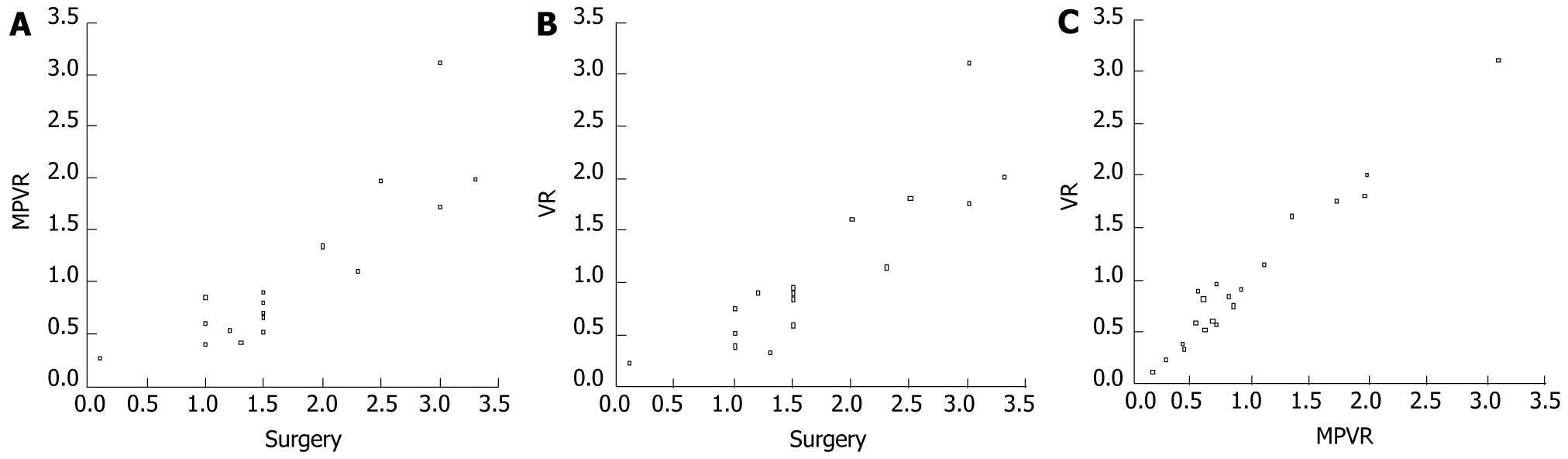
The inter-pouch distance was measured in 17 cases after the fistulae were cut. The inter-pouch gaps measured by MPVR and TL-VR correlated well with the findings at surgery (R = 0.87, P < 0.001). Scattered plots of the gaps in 17 patients after MPVR and surgery, and after TL-VR and surgery are shown in Figure 5A and B. Nevertheless, the inter-pouch gaps measured by MPVR and TL-VR were shorter than those measured at surgery except for cases 3 and 11. Paired t-test demonstrated that the preoperative CT data detected by MPVR and TL-VR were significantly different from those observed at surgery (P < 0.001) in 17 patients. On the other hand, the inter-pouch distance in 3 cases 9, 13 and 16 was measured after initial dissection of the fistulae to define the esophageal pouches, but not measured before ligation and division of the fistulae. In cases 9 and 13, the inter-pouch distance on CT images was very coincident with that observed at surgery. The findings of CT in case 16 were slightly less than those at surgery.
The inter-pouch gaps in 20 patients measured by MPVR correlated well with those measured by TL-VR (R = 0.98, P < 0.001). Statistical analysis demonstrated no significant difference in inter-pouch gaps measured by MPVR and TL-VR. A scattered plot of the gaps was determined by MPVR and TL-VR in the patients (Figure 5C).
The 13 fistulae detected by MPVR were thinner than those observed at surgery. The 13 fistulae on CT images did not correlate with those observed at surgery.
EA with or without TEF is one of the most challenging congenital anomalies in pediatric surgery because of its high morbidity and mortality[1,4]. The distance between esophageal segments and anatomy of the esophageal pouch as well as the tracheobronchial tree is highly valuable before operation. Frontal and lateral chest radiography can make unambiguous diagnosis of EA by inserting a catheter into the upper blind-ended esophageal pouch, and may give an estimate of the proximal pouch length. Nevertheless, they cannot show distal esophagus and fistulae in most patients. Although combined endoscopy and radiography can show distal esophagus and esophagus pouches[6], it is highly invasive with a low resolution[1]. Although the small size of airway in neonates naturally renders images poor in resolution, and cardiac and respiratory motion artifacts are relatively bigger in neonates than in adults, application of CT in reconstruction of EA with or without TEF has achieved encouraging results[1,2,5,7].
Our study concentrated on the application of MPVR and TL-VR with 64-row MDCT in neonates with congenital EA and distal TEF. TL-VR is a novel technique using insufflated air as a negative contrast medium and can produce high quality, more intuitive and reproducible 3D VR images[5]. MPVR technique is simple and efficient and has been widely used in demonstration of the anatomy of multi-organs. In this study, MPVR and TL-VR with MDCT clearly demonstrated the gaps between the proximal and distal esophageal pouches, tracheobronchial tree, as well as shape, size and position of the pouches. Furthermore, the fistulae and their orifice were observed on the images of some cases.
The inter-pouch gaps measured by MPVR and TL-VR were shorter than those observed at surgery in most cases (Table 1), which is not consistent with the reported findings[1,2,7]. Ratan et al[3] found that the shortest inter-pouch distance is increased to 5 mm after the fistula is divided. In our study, the inter-pouch distance was measured only once at surgery after and, before the fistula was cut. The CT data and findings at surgery were normally distributed in the 17 cases. The mean difference in MPVR and findings at surgery, and in TL-VR and findings at surgery was 0.67 ± 0.43 cm and 0.64 ± 0.42 cm, respectively, which is closely coincided with the reported findings[3]. Moreover, the lengths of gaps measured by MPVR and TL-VR correlated well with the findings at surgery (R = 0.87, P < 0.001) in 17 cases (Figure 5A and B). In addition, the inter-pouch distance in 3 cases was measured at surgery before the fistula was cut. The CT data about cases 6 and 13 were almost identical with the findings at surgery, only the CT manifestations of case 16 were slightly less than the findings at surgery.
Besides division of the fistula, there were some possible causes for the less CT data than the findings at surgery. First, about 5 mL air was injected into the proximal esophageal pouch through the catheter in order to demonstrate the pouch, which may extend the proximal pouch and shorten the inter-pouch distance. For example, the proximal pouch was distended due to filling of air and pressed the trachea rightwards in case 6 (Figure 3). It was reported that CT data correspond well with the findings at surgery although no gas is injected into the proximal esophageal pouch[1,3,7], indicating that less or no air should be injected into the proximal pouch. As an apparent exception, distention of the proximal esophageal pouch was not seen in case 3 (Figure 1) because it was short, thin and immovable as confirmed at surgery. Second, selection of opacity threshold level for TL-VR techniques can affect the measurement of diameters and lengths of lumens, and visualization of thin tracts, so does selection of window width and location of MPVR. We selected the parameters and settings according to our experience. However, the selection was not doubtlessly optimal. Considering this drawback, we correlated the inter-pouch distance, diameters and lengths of proximal and distal esophageal pouches might measured by TL-VR and MPVR with those observed on MPR images at multidirectional view to avoid erroneous results.
On the other hand, the inter-pouch gaps measured by MPVR correlated well with those measured by TL-VR (R = 0.98, P < 0.001) in the 20 cases. Statistical analysis demonstrated no significant difference in them, indicating that the two different reconstruction techniques with MDCT do not differ in evaluation of the inter-pouch distance in neonates with EA and distal TEF.
Certainly, demonstration of the fistulae is also very significant. MPVR showed the distal fistulae and their orifice in 13 cases, whereas TL-VR revealed the fistulae and their orifice in only 4 cases. The 13 fistulae shown by MPVR were thinner than those observed at surgery. The findings in 13 fistulae on CT images did not correlate with those at surgery. MDCT demonstrated the invisible and thinner fistulae because of no interior gas, as well as peristalsis and shrinkage of esophagi, mucous plugs or secretions of respiratory tracts in the fistulae, and narrower lumen of fistulae than their diameters and walls seen at surgery. In addition, selection of threshold level for TL-VR and display of MPVR in lung window may interfere with visualization of the fistula orifice. Fitoz et al[1] reported that shaded surface display (SSD) and virtual bronchoscopy reconstruction techniques can satisfactorily show the distal fistulae, which is consistent with the report of Lam et al[2]. We used TL-VR instead of SSD, and virtual bronchoscopy was not performed in our study because it is inconvenient to measure inter-pouch gaps.
The results of this study demonstrate that 3D TL-VR and MPVR with MDCT can provide valuable preoperative information about neonates with EA and distal TEF. Nevertheless, due to expenses and concept of parents, gastrostomy and multiply-staged esophageal elongation are rarely performed in our institution. Therefore, the information acquired before operation in our study mainly helped the Department of Surgery to decide whether anastomosis should be performed by an experienced surgeon or an ordinary surgeon with/without supervision, anticipate the need for ventilatory assistance after operation, decrease the anesthesia and surgery time and its attendant morbidity, and predict outcome of a case. Because we focused on visualization of distal esophageal pouches and fistulae, possible consistency of measurements between MDCT reconstruction techniques and surgery, further operative procedures and follow-up were not discussed.
TL-VR and MPVR techniques are useful for neonates with EA and distal TEF. However, in those types of esophageal atresia without distal TEF in which the distal lumen is lack of air, it seemed difficult to visualize distal pouch with MPVR and TL-VR. The distal pouch might be observed to some extent on the original axial scan images or the MPR images.
The primary pitfalls of this study are lack of measurements of inter-pouch gaps at surgery before and after ligation and division of the fistulae, the number of neonatal cases was insufficient, and selection of technical parameters and preparations before scan might not be optimal.
With increased detector rows of MDCT and development in scan and reconstruction techniques, MDCT has been increasingly applied in demonstration of the anatomy of hollow viscera. However, its application in neonates is not common. Although the number of patients was limited, our findings suggest that MDCT plays a complementary role in diagnosis of congenital EA and distal TEF. MPVR and 3D TL-VR reconstruction of MDCT are useful and noninvasive for demonstrating the distal esophageal pouches, can evaluate the distance between two esophageal pouches in rough and show distal fistulae. 3D TL-VR images can show the complex anatomic features of EA, thus enabling a better orientation before operation.
Esophageal atresia (EA) with distal tracheoesophageal fistula (TEF) is the most important and common congenital malformation of the esophagus. The anatomy of esophageal blind pouches and inter-pouch gaps not only influence the surgical approach and postoperative strategy, but also predict prognosis of EA patients. Conventional radiography cannot show distal pouches and inter-pouch gaps, for which reconstruction techniques of multidetector-row computed tomography (MDCT) are highly valuable.
The reconstruction techniques of MDCT can visualize esophageal pouch, inter-pouch gap and tracheoesophageal fistula in neonates with EA and distal TEF. Whether inter-pouch gaps determined with CT correlate well with operating measurements remains unclear.
Air was used as a negative contrast medium for visualizing esophageal pouches and fistulae by multiple planar volume reconstruction (MPVR) and 3D TL-VR reconstruction techniques of MDCT. To our knowledge, the number of cases in this study is the largest.
The reliability and accuracy of reconstruction techniques of MDCT for evaluation of EA and distal TEF still need to be confirmed by further researches. However, they are really useful and play a complementary role in diagnosis of EA and distal TEF.
EA and distal TEF: The most common congenital malformation of esophagus, in which esophagus only has the proximal and distal blind-ended pouch and distal pouch communicates with trachea through a fistula; VR: Volume rendering, a 3D reconstruction technique of MDCT; TL-VR: Transparency lung VR model, a type of VR, which utilizes air as a negative contrast medium for visualizing hollow viscera; MPVR: Multiple planar volume reconstruction, a reconstruction technique of MDCT; MinIP: Minimum intensity projection, a reconstruction pattern of MDCT.
The authors of this paper assessed the application of multiple planar volume reconstruction (MPVR) and three-dimensional (3D) transparency lung volume rendering (TL-VR) with 64-row multidetector-row MDCT in congenital EA and distal TEF in neonates, showing that MDCT plays a complementary role in diagnosis of congenital EA and distal TEF. MPVR and 3D TL-VR reconstruction of MDCT are useful and noninvasive for demonstrating the distal esophageal pouches, can evaluate the distance between two esophageal pouches in rough and show distal fistulae, thus enabling a better orientation before operation.
Peer reviewer: Kenji Miki, MD, Department of Surgery, Showa General Hospital, 2-450 Tenjin-cho, Kodaira, Tokyo 187-8510, Japan
S- Editor Tian L L- Editor Wang XL E- Editor Ma WH
| 1. | Fitoz S, Atasoy C, Yagmurlu A, Akyar S, Erden A, Dindar H. Three-dimensional CT of congenital esophageal atresia and distal tracheoesophageal fistula in neonates: preliminary results. AJR Am J Roentgenol. 2000;175:1403-1407. |
| 2. | Lam WW, Tam PK, Chan FL, Chan KL, Cheng W. Esophageal atresia and tracheal stenosis: use of three-dimensional CT and virtual bronchoscopy in neonates, infants, and children. AJR Am J Roentgenol. 2000;174:1009-1012. |
| 3. | Ratan SK, Varshney A, Mullick S, Saxena NC, Kakkar S, Sodhi PK. Evaluation of neonates with esophageal atresia using chest CT scan. Pediatr Surg Int. 2004;20:757-761. |
| 4. | Upadhyaya VD, Gangopadhyaya AN, Gupta DK, Sharma SP, Kumar V, Pandey A, Upadhyaya AD. Prognosis of congenital tracheoesophageal fistula with esophageal atresia on the basis of gap length. Pediatr Surg Int. 2007;23:767-771. |
| 5. | Soye JA, Yarr J, Dick AC, Paterson A. Multidetector-row computed tomography three-dimensional volume reformatted ‘transparency’ images to define an upper pouch fistula in oesophageal atresia. Pediatr Radiol. 2005;35:624-626. |
| 6. | Caffarena PE, Mattioli G, Bisio G, Martucciello G, Ivani G, Jasonni V. Long-gap oesophageal atresia: a combined endoscopic and radiologic evaluation. Eur J Pediatr Surg. 1994;4:67-69. |
| 7. | Luo CC, Lin JN, Wang CR. Evaluation of oesophageal atresia without fistula by three-dimensional computed tomography. Eur J Pediatr. 2002;161:578-580. |