Published online Mar 21, 2011. doi: 10.3748/wjg.v17.i11.1501
Revised: December 13, 2010
Accepted: December 20, 2010
Published online: March 21, 2011
AIM: To study the HER-2/neu protein expression and gene amplification in gastric carcinoma and their relation.
METHODS: One hundred and forty-five formalin-fixed and paraffin- embedded tumor tissue samples from Chinese gastric carcinoma patients were studied with immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) methods. Clinicopathologic data about all patients were collected.
RESULTS: The levels of HER-2 3+, HER-2 2+ and HER2 1+ were measurable in 6.9%, 8.3% and 17.2% of the samples, respectively. No HER-2 was stained in 67.6% of the samples. FISH showed that HER-2 gene was amplified in 18 samples, 10 HER-2 3+ samples, 5 HER-2 2+ samples, and 3 HER-2 1+ samples with IHC staining. HER-2 status was not correlated with the sex and age of patients, and tumor size, location or differentiation, but with the depth of invasion, TNM stage, lymph node and distant metastasis as well as histopathological classification of gastric cancer (P < 0.05).
CONCLUSION: All samples with IHC as HER-2 expression should be analyzed with FISH. Detection of HER-2 gene amplification can assess the malignant biological behaviors and prognosis of gastric cancer.
- Citation: Yan SY, Hu Y, Fan JG, Tao GQ, Lu YM, Cai X, Yu BH, Du YQ. Clinicopathologic significance of HER-2/neu protein expression and gene amplification in gastric carcinoma. World J Gastroenterol 2011; 17(11): 1501-1506
- URL: https://www.wjgnet.com/1007-9327/full/v17/i11/1501.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i11.1501
Gastric cancer is the second most common cause of cancer-related death worldwide. Surgery is the only curative procedure for localized gastric cancer, which is at the advanced stage when diagnosed in most cases. Currently available agents are not very effective, resulting in a high recurrence rate, a low survival rate, and a poor prognosis of advanced gastric cancer patients. Thus, treatment of gastric cancer remains a challenge for physicians. New targeted therapies for advanced gastric carcinoma are needed, which may open up a new avenue for cancer treatment. Current targeted therapy for advanced gastric carcinoma depends on the evaluation of target gene status[1-3].
HER-2/neu or CerbB-2 is a member of growing factors (EGFR, erbB-2, erbB-3 and erbB-4) in the HER family with intrinsic protein tyrosine kinase activity, and its increased activity is the assumed mechanism underlying cell transformation[4]. It is an oncogene that regulates the biological functions such as cell proliferation, differentiation, motility, and apoptosis. It was reported that HER-2 is over-expressed in cancer of breast, lungs, salivary gland, ovary, colon, prostate and pancreas[5,6]. HER-2 plays an important role in activation of HER-2 protein, and is over-expressed in 10%-38% of gastric cancer patients[7]. However, the correlation between the expression of HER-2 protein and the prognosis of gastric cancer is still controversial. Different studies showed different HER-2 expression levels in gastric carcinoma[8-10].
Detecting the HER-2 status in gastric carcinoma is a prerequisite of monoclonal antibody therapy for gastric cancer. In this study, immunohistochemistry (IHC) was used in detecting HER-2 oncoprotein and fluorescent in situ hybridization (FISH) was used as a follow-up test for ambiguous results.
Paraffin-embedded tumor tissue blocks were provided by Cancer Hospital of Fudan University and Institute of Traditional Chinese Medicine of Jiangsu Province. One hundred and forty-five (95 males and 50 females) out of the 476 Chinese patients with gastric adenocarcinoma, admitted to Department of Surgery, Cancer Hospital of Fudan University, and Institute of Traditional Chinese Medicine of Jiangsu Province in 2006-2009 for surgery, were enrolled in this study. Tumor tissue blocks from patients with full clinical data (including diagnosis, age, sex, address, disease history, etc.) were cut into 20 slides. The average age of the patients was 60 years. Cancer was classified as stage I in 32 cases, stage II in 35 cases, stage III in 37 cases, and stage IV in 41 cases, respectively, according to the TNM Cancer Staging System of the American Joint Committee of Cancer. The study was approved by The Review Board of Fudan University Cancer Hospital and Institute of Traditional Chinese Medicine of Jiangsu Province. Informed consent was obtained from all patients.
Tumor tissue was cut into 4-μm thick sections which were stained with hematoxylin and eosin for histopathological types, differentiation stage, IHC and FISH evaluation.
Slides were deparaffinized, rehydrated, and heated in a microwave oven containing 0.01 mol/L citrate buffer (pH 6.0) for 5 min at 100°C for antigen retrieval, cooled for 20 min and washed with water and a buffer solution. Peroxidase was applied for 5 min and washed with a buffer solution for 2 × 5 min. Primary antibody diluted at 1:200 was applied for 30 min, link and streptavidin were applied for 10 min, respectively, and then washed with a buffer solution for 2 × 5 min. The bound antibody was visualized using a DAB-chromogen substrate. The sections were then counterstained with hematoxylin, covered with a cover-slip. Negative control was stained by omission of the primary antibody. Over-expression of HER-2 protein in paraffin-embedded invasive breast carcinoma tissue slides was used as a positive control.
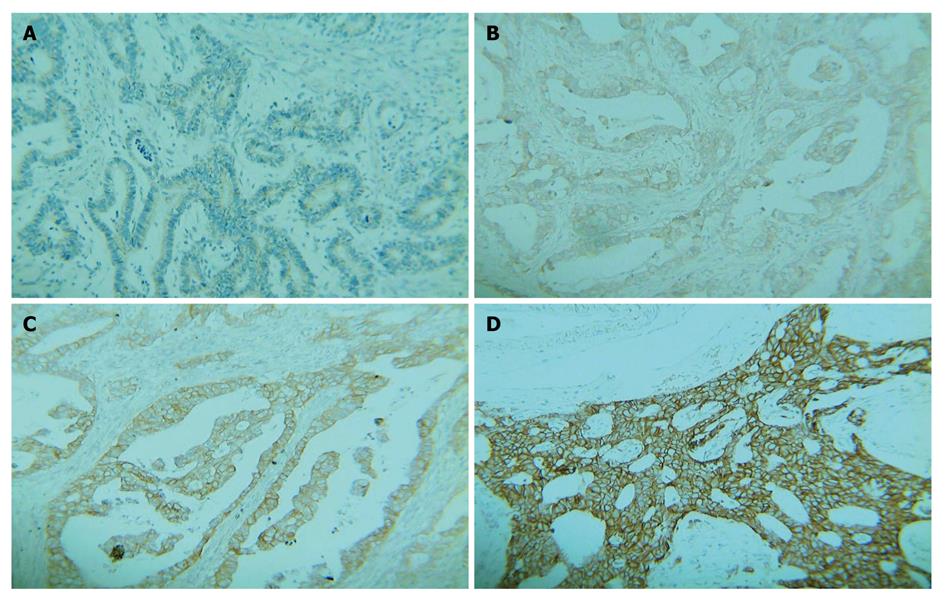
A strong brown staining was located in cell membrane of malignant cells using this staining method. The DAKO Hercep Test™ Protocol System[11] was used to grade the membrane staining. The staining was scored as negative (0) when no membrane was stained or when membrane was stained in less than 10% of tumor cells, weakly positive (1+) if focal membrane was stained in more than 10% of tumor cells, intermediately positive (2+) if complete membrane was weakly- moderately stained in more than 10% of tumor cells, and strongly positive (3+) if complete membrane was intensely stained in more than 10% of tumor cells. Scores 0 and 1 were considered negative, while scores 2 and 3 were considered positive.
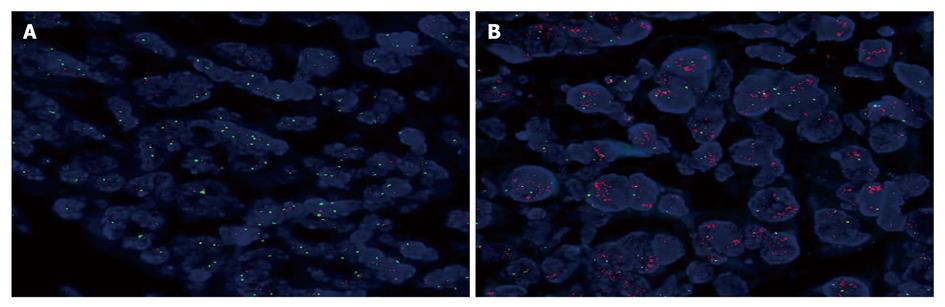
HER-2 gene was amplified with dual-color FISH using a Passvision HER-2 DNA probe kit (Vysis Inc. Downers Grove, IL, USA) according to its manufacturer’s instructions. Briefly, hybridization buffer, DNA probe, and purified water were centrifuged, and heated to 73°C for 5 min in a water bath. Slides were immersed in a denaturing bath (70% formamide 2 × SSC) for 5 min at 73°C, followed by dehydration in increasing ethanol concentrations, and then dried. The probe mixture was applied to each slide. The slides were placed in a 42°C incubator for 30 min, washed with 0.4 × SSC/0.3% NP-40 for 2 min, air-dried in darkness, counterstained with 4’,6-diamidino-2-phenylindole (DAPI), and covered with a cover-slip. HER-2/neu-spectrum orange probe contains a DNA sequence specific for the HER-2 human gene locus and hybridized to the region 17q11.2-q12 of human chromosomes. CEP17 (chromosome enumeration probe 17)/spectrum green probe containing alpha-satellite DNA that hybridizes to the D17Z1 locus (centromere region of chromosome 17) was used as a control. Nuclei were counterstained with DAPI. The slides were observed under a B × 60 fluorescence microscope equipped with a digital camera (DP50; Olympus, Tokyo, Japan) and the images were captured on a Windows PC with the Viewfinder Lite software. A cell was considered to be amplified when a definite cluster or more than 10 signals for HER-2 were found. Known positive and negative cells were used as controls for each FISH. Gene amplification was scored when a minimum of 20 cancer cell nuclei exhibited a HER-2/CEP17 ratio ≥ 2, or when a HER-2 signal cluster was observed.
Statistical analysis of data was performed by Student’s t test. P < 0.05 was considered statistically significant.
HER-2 protein status in 145 gastric carcinoma tissue samples was determined with immunohistochemical staining (Figure 1). Of the 145 gastric carcinoma tissue samples, 98 (67.6%) were scored as 0, 25 (17.2%) as 1, 12 (8.3%) as 2, and 10 (6.9%) as 3. The positive rate was approximately 15.2% (22/145).
The HER-2/CEP17 ratio was determined using the rate of HER-2/neu signals and CEP17 signals in 20 nuclei. The total number of HER-2/neu signals was divided by the total number of CEP17 signals, with a ratio was ≥ 2 according to the indications given by the Abbott-Vysis Company. IHC showed complete membrane immunostaining (2+ and 3+). In addition, 123 samples negative for HER-2 over-expression with IHC staining were also analyzed by FISH. HER-2 gene was amplified in 18 out of the 145 gastric carcinoma tissue samples, including HER-2 3+ in 10 samples, HER2 2+ in 5 samples, and HER2 1+ in 3 samples with IHC staining (Table 1). The 10 tumors with strong complete membrane immunostaining (3+) exhibited HER-2 gene amplification, accounting for 55.6% (10/18) of all HER-2 amplifications. The positive amplification rate of HER-2 gene was about 12.4% (18/145) in all gastric carcinoma tissue samples (Figure 2).
| HER-2 FISH | Immunohistochemistry for HER-2 | Total | |||
| 0 (n = 98) | 1 (n = 25) | 2 (n = 12) | 3 (n = 10) | ||
| Negative | 98 | 22 | 7 | 0 | 127 |
| Positive | 0 | 3 | 5 | 10 | 18 |
The clinicopathological differences were observed in gastric carcinoma tissue samples with or without HER-2 protein expression or HER-2 gene amplification. The HER-2 protein expression and HER-2 gene amplification rates were 86.4 % (19/22) and 94.4 % (17/18) in intestinal type gastric carcinomas, respectively (P <0.05). The HER-2 status was correlated with the depth of invasion, TNM stage, lymph node and distant metastasis (P <0.05). However, no significant relation was found between clinicopathologic variables (sex and age of the patients, and tumor diameter, differentiation, location) (Table 2).
| Clinicopathologic data | n | HER-2 (IHC) | P value | HER-2 (FISH) | P value | ||
| Pos | % | Amp | % | ||||
| Sex | |||||||
| Male | 95 | 14 | 14.7 | NS | 10 | 10.5 | NS |
| Female | 50 | 8 | 16.0 | 8 | 16.0 | ||
| Age (yr) | |||||||
| > 60 | 68 | 10 | 14.7 | NS | 7 | 10.3 | NS |
| ≤ 60 | 77 | 12 | 15.6 | 11 | 14.3 | ||
| Diameter of the tumor (cm) | |||||||
| > 5 | 85 | 15 | 17.6 | NS | 12 | 14.1 | NS |
| ≤ 5 | 60 | 7 | 11.7 | 6 | 10.0 | ||
| Location | |||||||
| Cardia or fundus of stomach | 55 | 8 | 14.5 | 7 | 12.7 | ||
| Body of stomach | 39 | 5 | 12.8 | NS | 4 | 10.3 | NS |
| Sinus ventriculi/ostium pyloricum | 51 | 9 | 17.6 | 7 | 13.7 | ||
| Differentiation | |||||||
| Well | 36 | 6 | 16.7 | 5 | 13.9 | ||
| Moderately | 44 | 5 | 11.4 | NS | 4 | 9.1 | NS |
| Pooly/undifferentiated | 65 | 11 | 16.9 | 9 | 13.8 | ||
| Serosa invasion | |||||||
| Positive | 107 | 20 | 18.7 | < 0.05 | 17 | 15.9 | < 0.05 |
| Negative | 38 | 2 | 5.3 | 1 | 2.7 | ||
| Lymph node metastasis | |||||||
| Positive | 91 | 20 | 22.0 | < 0.05 | 18 | 19.8 | < 0.05 |
| Negative | 54 | 2 | 3.7 | 0 | 0.0 | ||
| Distant metastasis | |||||||
| Positive | 11 | 10 | 90.9 | < 0.05 | 9 | 81.8 | < 0.05 |
| Negative | 134 | 12 | 9.0 | 8 | 6.0 | ||
| TNM stage | |||||||
| I + II | 67 | 3 | 4.5 | < 0.05 | 2 | 2.99 | < 0.05 |
| III + IV | 78 | 19 | 24.4 | 16 | 20.5 | ||
| Histopathological classification(Lauren) | |||||||
| Intestinal | 86 | 19 | 22.1 | 17 | 19.8 | ||
| Diffuse | 37 | 2 | 5.4 | < 0.05 | 0 | 0.0 | < 0.05 |
| Non classified | 22 | 1 | 4.5 | 1 | 4.5 | ||
Gastric cancer is the second most frequent cause of cancer-related death worldwide and the most common cancer in Asian countries[12]. Although various therapies for gastric carcinoma are available, such as gastrectomy with extensive lymphadenectomy and surgery combined with chemotherapy, the control of advanced stage gastric cancer is still a challenge for physicians. In recent years, molecular target therapy is a new treatment modality for gastric cancer and HER-2 has been identified as a potential therapeutic target. However, molecular target therapy for gastric cancer depends on the evaluation of the target gene status.
HER-2 amplification and over-expression play a key role in initiation, progression, and metastasis of some common cancers, including breast and gastric cancer. HER-2 status has been recognized as an important prognostic factor for cancer. The survival time of patients with breast cancer and positive HER-2 disease is significantly shorter than that of those with HER-2 negative tumors[13-15]. Thus, detecting HER-2 status is of important significance in diagnosis of gastric cancer.
It was reported that the HER-2 over-expression rate in gastric carcinoma is 8.2%-34.0%[2,16-18], whereas the concordance of FISH and IHC is 93.5% in diagnosis of gastric cancer[9]. In the present study, the HER-2 gene amplification was evaluated using the FISH method and the HER-2 protein expression levels were compared. The positive rate of HER-2 gene amplification was 12.4% (18/145). Recent studies showed that the HER-2 gene amplification rate in gastric carcinoma is 8.2%-5%[19,20], which is consistent with our findings (12.4%). HER-2 gene amplification is a golden criterion for target therapy with trastuzumab. In this study, the HER-2 gene was amplified in all samples with oncoprotein over-expression at 3+ level, but HER-2 gene was not amplified at 0 level in all samples, which is consistent with the reported data[21], suggesting that target therapy is not necessary for gastric cancer patients with HER-2 protein expression but necessary for those with HER-2 protein over-expression at 3+ level. Although FISH shows a high sensitivity and specificity and remains a criterion for determining gene amplification status, IHC for HER-2 protein expression may be a good alternative when FISH cannot be performed.
In this study, the HER-2 protein expression and the HER-2 gene amplification rates were 86.4% (19/22) and 94.4% (17/18), respectively, in most intestinal types of gastric carcinoma (P < 0.05), and the HER-2 status was correlated with the depth of invasion, TNM stage, lymph node and distant metastasis of gastric cancer (P < 0.05), with no significant relation found between clinicopathologic variables (sex and age of patients, and tumor diameter, differentiation, and location), which is consistent with the reported findings[22]. Furthermore, it has been shown that HER-2 status is only correlated with the histopathological classification of gastric cancer[23]. It was also reported that HER-2 over-expression is correlated with well or moderately differentiated gastric cancer but not with pathologic stage of gastric cancer or the age and sex of gastric cancer patients[24].
In conclusion, HER-2 status is correlated with the depth of invasion, TNM stage, lymph node and distant metastasis of gastric cancer. Detecting HER-2 status may contribute to the target therapy for gastric carcinoma using trastuzumab.
HER-2 protein over-expression and gene amplification in gastric carcinoma are the prerequisite for monoclonal antibody therapy. Detecting HER-2 status in gastric carcinoma is very important in clinical practice. HER-2 protein expression and gene amplification were detected using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) in this study.
HER-2 is over-expressed in 10%-38% of gastric cancer patients. However, few studies are available on HER-2 status in gastric carcinoma in China.
In this study, HER-2 over-expression was detected with IHC and HER-2 gene amplification was found with FISH. FISH testing is unnecessary for patients with HER-2 protein expression at 0 or 3+ level and necessary for patients with HER-2 protein expression at 1+ or 2+ levels. HER-2 status was correlated with the depth of invasion, TNM stage, lymph node and distant metastasis of gastric cancer.
IHC may be used to screen the HER-2 status in gastric carcinoma patients. FISH testing may be necessary for patients with HER-2 protein expression at 1+ or 2+ levels. The screening method may determine whether target therapy is necessary for gastric carcinoma using trastuzumab.
IHC and FISH are the abbreviation forms of IHC and FISH, respectively. HER-2 status contains negative or positive HER-2 protein expression, HER-2 gene amplification or no HER-2 gene amplification.
The authors showed the expression of HER-2 in approximately one third of patients. Interestingly, they found that HER-2 was amplified not only in IHC +3 cases but also in some of IHC +2 and even +1 cases, suggesting that FISH should be performed in all cases with positive IHC and HER-2 expression is correlated with the key indicators for cancer progression, such as TNM stage and metastasis.
Peer reviewer: Jean François Beaulieu, Professor, Department of Anatomy and Cell Biology, Faculty of Medicine and Heath Sciences, Université de Sherbrooke, Sherbrooke, Qué, J1H 5N4, Canada
S- Editor Sun H L- Editor Wang XL E- Editor Zheng XM
| 1. | Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30:1415-1425. |
| 2. | Takehana T, Kunitomo K, Kono K, Kitahara F, Iizuka H, Matsumoto Y, Fujino MA, Ooi A. Status of c-erbB-2 in gastric adenocarcinoma: a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int J Cancer. 2002;98:833-837. |
| 3. | Yu GZ, Chen Y, Wang JJ. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol. 2009;135:1331-1339. |
| 4. | Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, Yarden Y. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci USA. 1999;96:4995-5000. |
| 5. | Yu D, Hung MC. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19:6115-6121. |
| 6. | Høgdall EV, Christensen L, Kjaer SK, Blaakaer J, Bock JE, Glud E, Nørgaard-Pedersen B, Høgdall CK. Distribution of HER-2 overexpression in ovarian carcinoma tissue and its prognostic value in patients with ovarian carcinoma: from the Danish MALOVA Ovarian Cancer Study. Cancer. 2003;98:66-73. |
| 7. | Wang L, Habuchi T, Takahashi T, Kamoto T, Zuo T, Mitsumori K, Tsuchiya N, Sato K, Ogawa O, Kato T. No association between HER-2 gene polymorphism at codon 655 and a risk of bladder cancer. Int J Cancer. 2002;97:787-790. |
| 8. | Rüschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, Chenard MP, Penault-Llorca F, Nagelmeier I, Schlake W. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299-307. |
| 9. | Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805. |
| 10. | Gürel S, Dolar E, Yerci O, Samli B, Oztürk H, Nak SG, Gülten M, Memik F. The relationship between c-erbB-2 oncogene expression and clinicopathological factors in gastric cancer. J Int Med Res. 1999;27:74-78. |
| 11. | Selvarajan S, Bay BH, Chng MJ, Tan PH. The HercepTest and routine C-erbB2 immunohistochemistry in breast cancer: any difference? Ann Acad Med Singapore. 2004;33:473-476. |
| 12. | Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ. Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer. 2002;2:37. |
| 13. | Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353:1652-1654. |
| 14. | Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361-370. |
| 15. | Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463-475. |
| 16. | Lee KE, Lee HJ, Kim YH, Yu HJ, Yang HK, Kim WH, Lee KU, Choe KJ, Kim JP. Prognostic significance of p53, nm23, PCNA and c-erbB-2 in gastric cancer. Jpn J Clin Oncol. 2003;33:173-179. |
| 17. | Aoyagi K, Kohfuji K, Yano S, Murakami N, Miyagi M, Takeda J, Shirouzu K. Evaluation of the epidermal growth factor receptor (EGFR) and c-erbB-2 in superspreading-type and penetrating-type gastric carcinoma. Kurume Med J. 2001;48:197-200. |
| 18. | Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW, Heiss MM. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol. 2000;18:2201-2209. |
| 19. | Kimura M, Tsuda H, Morita D, Ichikura T, Ogata S, Aida S, Yoshizumi Y, Maehara T, Mochizuki H, Matsubara O. A proposal for diagnostically meaningful criteria to classify increased epidermal growth factor receptor and c-erbB-2 gene copy numbers in gastric carcinoma, based on correlation of fluorescence in situ hybridization and immunohistochemical measurements. Virchows Arch. 2004;445:255-262. |
| 20. | Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273-278. |
| 21. | Bizari L, Borim AA, Leite KR, Gonçalves Fde T, Cury PM, Tajara EH, Silva AE. Alterations of the CCND1 and HER-2/neu (ERBB2) proteins in esophageal and gastric cancers. Cancer Genet Cytogenet. 2006;165:41-50. |
| 22. | Chen B, Luo RC, Cui F, Qian XY. [Association of HER-2/neu expression with prognosis of gastric cancer]. Nanfang Yike Daxue Xuebao. 2006;26:344-347. |
| 23. | Barros-Silva JD, Leitão D, Afonso L, Vieira J, Dinis-Ribeiro M, Fragoso M, Bento MJ, Santos L, Ferreira P, Rêgo S. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer. 2009;100:487-493. |
| 24. | Kim MA, Jung EJ, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. Evaluation of HER-2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real-time quantitative polymerase chain reaction. Hum Pathol. 2007;38:1386-1393. |