Published online Mar 14, 2011. doi: 10.3748/wjg.v17.i10.1267
Revised: November 11, 2010
Accepted: November 18, 2010
Published online: March 14, 2011
AIM: To evaluate the effect of portal vein thrombosis and arterioportal shunts on local tumor response in advanced cases of unresectable hepatocellular carcinoma treated by transarterial chemoembolization.
METHODS: A retrospective study included 39 patients (mean age: 66.4 years, range: 45-79 years, SD: 7) with unresectable hepatocellular carcinoma (HCC) who were treated with repetitive transarterial chemoembolization (TACE) in the period between March 2006 and October 2009. The effect of portal vein thrombosis (PVT) (in 19 out of 39 patients), the presence of arterioportal shunt (APS) (in 7 out of 39), the underlying liver pathology, Child-Pugh score, initial tumor volume, number of tumors and tumor margin definition on imaging were correlated with the local tumor response after TACE. The initial and end therapy local tumor responses were evaluated according to the response evaluation criteria in solid tumors (RECIST) and magnetic resonance imaging volumetric measurements.
RESULTS: The treatment protocols were well tolerated by all patients with no major complications. Local tumor response for all patients according to RECIST criteria were partial response in one patient (2.6%), stable disease in 34 patients (87.1%), and progressive disease in 4 patients (10.2%). The MR volumetric measurements showed that the PVT, APS, underlying liver pathology and tumor margin definition were statistically significant prognostic factors for the local tumor response (P = 0.018, P = 0.008, P = 0.034 and P = 0.001, respectively). The overall 6-, 12- and 18-mo survival rates from the initial TACE were 79.5%, 37.5% and 21%, respectively.
CONCLUSION: TACE may be exploited safely for palliative tumor control in patients with advanced unresectable HCC; however, tumor response is significantly affected by the presence or absence of PVT and APS.
- Citation: Vogl TJ, Nour-Eldin NE, Emad-Eldin S, Naguib NN, Trojan J, Ackermann H, Abdelaziz O. Portal vein thrombosis and arterioportal shunts: Effects on tumor response after chemoembolization of hepatocellular carcinoma. World J Gastroenterol 2011; 17(10): 1267-1275
- URL: https://www.wjgnet.com/1007-9327/full/v17/i10/1267.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i10.1267
Hepatocellular carcinoma (HCC) is one of the most common neoplasms in the world and its incidence is increasing worldwide. It is associated with liver cirrhosis in 80% of cases and it is the leading cause of death among cirrhotic patients[1]. Liver resection and liver transplantation are curative therapeutic options[2]. Likewise, percutaneous ablative treatments such as percutaneous ethanol injection (PEI)[3], radiofrequency ablation[4], microwave ablation[5] or MR-guided laser-induced thermotherapy (LITT)[6] can also be applied as therapeutic options with curative potential. However, curative treatments are applicable in only 30%-40% of cases due to the presence of multifocal tumors or limited hepatic reserve at the time of diagnosis[7,8]. Transarterial chemoembolization (TACE) was introduced as a palliative local therapeutic option for the treatment of unresectable HCC. The goal of palliation is to control symptoms, improve life quality and prolong survival[8,9]. Different studies have investigated the predictors of survival after TACE and concluded that the prognosis is multifactorial, including the extent of the tumor at diagnosis and also the extent of liver injury[8,10,11].
The purpose of this study was to evaluate retrospectively the role of TACE in local tumor control of unresectable HCC and the effect of portal vein thrombosis (PVT) and arterioportal shunts (APS), as well as other associated pathological factors, on the local tumor response in advanced cases.
Approval of this study was obtained from the institutional review board, and informed consent was obtained from all patients including approval of the protocol of treatment and the anonymous use of the data for research purposes.
The medical records of all patients who had unresectable hepatocellular carcinoma and who were treated with TACE between March 2006 and October 2009 were retrospectively evaluated. A total of 39 patients (33 males and 6 females) ranging in age from 45-79 years (mean: 66.4 years, SD: 7) with unresectable HCC were treated with repetitive TACE. The total number of sessions was 219, range: 1-17, mean: 5.6 sessions, SD: 3.7. Patient demographics, lesion pathology, treatment, and outcome data, including all histopathology reports and imaging studies, were collected from the electronic medical record archiving system and were subsequently analyzed. Special emphasis was placed on the stage of disease at the time of first embolization. The number, location and size of liver tumors were obtained by reevaluating the original CT and magnetic resonance imaging (MRI) scans. The patient and tumor characteristics are summarized in Table 1.
| Total number | 39 |
| Age (yr) | Range: 45-79, mean: 66.4, SD: 7 |
| Gender (M:F) | 33:6 |
| Underlying liver pathology | |
| Hepatitis C virus | 17 |
| Hepatitis B virus | 5 |
| Alcoholic cirrhosis | 7 |
| Toxic cirrhosis | 2 |
| Cryptogenic cirrhosis | 8 |
| Child-Pugh Score | |
| Child A | 27 |
| Child B | 12 |
| Tumor number | |
| Solitary | 20 |
| Multiple | 19 |
| 2 lesions | 6 |
| 3 lesions | 3 |
| 4 lesions | 1 |
| > 5 lesions | 9 |
| Tumor margin definition | |
| Well defined margin | 20 |
| Ill defined margin | 19 |
| Portal vein thrombosis | 19 |
| Main (partial thrombosis) | 3 |
| Main lobar branch | 10 |
| Segmental branch | 6 |
| A-P shunt | 7 |
All patients involved in the study were not eligible for surgical or local ablative therapies. The inclusion criteria of the study were: (1) Tumors of any size associated with portal vein thrombosis, either partial thrombosis of the main portal vein or segmental portal vein branch thrombosis; (2) Tumors associated with APS with or without PVT; (3) Large solitary tumors of more than 5 cm in maximal diameter, multinodular or bilobar tumors; and (4) Patients with Child-Pugh Scores between A and B.
The exclusion criteria were: (1) Patients with poor performance status (Karnofsky index < 50%); (2) Nutritional impairment; (3) Presence of ascites; (4) Encephalopathy (5) High serum total bilirubin level (> 3 mg/dL); (6) Serum albumin level < 2.0 mg/dL; (7) Renal failure (serum creatinine level > 2 mg/dL); (8) Cardiovascular or respiratory failure; (9) Florid infection; (10) Main PV complete thrombosis; and (11) Extrahepatic tumor manifestation.
The protocol for management was decided by a multidisciplinary team composed of hepat ic surgeons, interventional radiologists, hepatologists and medical oncologists. The end point of TACE therapy was defined as stable disease for two successive sessions or disease progression.
TACE was performed with a treatment interval of 4-6 wk. For patients with bilobar disease, the treatment was performed to control disease in the lobe with higher tumor burden as seen on MRI performed immediately before the procedure; the second lobe was treated in another session. All angiographies were performed on an Axiom Multistar system (Siemens; Erlangen). After the introduction of a 5 French sheath into a femoral artery, an angiographic survey of the abdominal vessels was performed using a 5 F pigtail catheter in the first TACE course. After exclusion of the presence of a right hepatic artery by selective catheterization of the mesenteric artery, indirect portography followed, outlining the portal circulation in the venous phase. Afterwards, a 5 French Cobra catheter was placed in the coeliac trunk and advanced beyond the gastroduodenal artery. If possible, the tip of the catheter was advanced further into segmental arteries adjacent to the tumor.
The embolization suspension, containing 5-10 mg/m2 mitomycin C as a chemotherapeutic agent and 1-10 mL Lipiodol, an iodized oil, was administered, followed by injection of 60-180 mg degradable starch microspheres (EmboCept, PharmaCept) for vascular occlusion. The embolization material was injected slowly with fluoroscopic control until stasis of blood flow was observed. Devascularization after embolization was confirmed by an additional angiographic study of the hepatic artery.
TACE procedure was performed on an outpatient basis. After the procedure, patients were observed for 10-12 h to ensure adequate hydration and symptomatic treatment of pain and vomiting. After the observation time, patients who had remained symptom-free were discharged to the care of the referring oncologist. If complications developed, patients were to be readmitted immediately. Complications were evaluated adopting the Society of Interventional Radiology (SIR) criteria[12]. Major complications were defined as any event that resulted in additional treatment including an increased level of care, hospital stay beyond observation status (including readmission after initial discharge, substantial morbidity and disability and death. All other complications were classified as minor.
The local tumor response to the treatment protocol was evaluated using the response evaluation criteria in solid tumors (RECIST) and MRI volumetric assessment.
According to RECIST criteria, complete response was defined as the complete disappearance of all recognizable tumor in the liver confirmed at 4 wk after the procedure. Partial response was defined as a reduction of at least 30% in the sum of the longest diameter of the lesions, taking as reference the baseline study, and was confirmed at 4 wk. Stable disease was defined when neither partial response nor progressive disease criteria were met, taking as reference the smallest sum of the longest diameter recorded since treatment started. Progressive disease was defined as the appearance of new lesions or as an increase of at least 20% in the sum of the longest diameter of the lesions, taking as reference the smallest-sum longest diameter recorded since treatment started[13].
To evaluate the local tumor response using volumetric MRI measurements, tumor volumes were calculated before treatment, one month after the first TACE session and after the last TACE session. Tumor volumes were calculated with the ellipsoidal volume formula: Volume = (length × width × height × 0.523). For assessment of multicentric tumors; in patients with up to 3 focal lesions, the sum of all tumor volumes was calculated. For patients with more than 3 tumors, the sum of the largest 3 tumors was calculated.
The percentage of volume changes before and after treatment was then calculated. The local tumor response was considered as progressive if there was increase in the post-treatment volume (after the last TACE session) compared to the pretreatment volume, and as regressive if there was reduction in the post-treatment volume compared to the initial volume.
The morphologic tumor response (number, size and volume) was evaluated on MRI in consensus by two senior radiologists. For initial treatment planning, unenhanced and contrast-enhanced [application of 0.1 mmol of gadopentate dimeglumine (Magnevist, Schering, Berlin, Germany) per kilogram body weight] T1-weighted gradient-echo sequences (FLASH-2D) with transversal and sagittal slice orientation (TR/TE:135/6 ms; FA 80°; FOV 350 mm; matrix 134 × 256; slice thickness 8 mm) MRI studies were carried out for all patients with a conventional 1.5-T system (Magnetom Symphony; Siemens, Erlangen, Germany). Additional non-enhanced T2-weighted turbo-spin-echo (TSE) sequences (TR/TE: 3 800/92 ms; FA 150°; FOV 350 mm; matrix 115 × 256; slice thickness 8 mm) and contrast-enhanced dynamic VIBE sequences (TR/TE: 4.5/1.8 ms; FA 15°; FOV 350 mm; matrix 128 × 256; slice thickness 8 mm) were used for the differentiation of the lesions.
Lipiodol retention in the tumor and the liver parenchyma was verified with non-enhanced CT examinations. In addition, CT allows optimal comparison between results on follow-up images in the subsequent sessions and can efficiently exclude major post-procedure complications such as pancreatitis, hepatic infarction, mesenteric ischemia, and ascites or ectopic embolization. CT was performed 24 h after TACE using the spiral technique (slice thickness, 8 mm) on fourth-generation scanners (Somatom plus or Somatom plus 4, Siemens, Erlangen, Germany).
For assessment of different risk factors, patients were divided into two groups according to the following: (1) Presence or absence of PVT; (2) Presence or absence of APS; (3) Child-Pugh scores (A or B); (4) Number of focal lesions (solitary or multiple); and (5) Definition of the focal lesions (well defined or ill defined margin). Patients were further divided into groups according to the underlying liver pathology (HCV, HBV, alcoholic, toxic and cryptogenic cirrhosis). Data were statistically described in terms of range, mean ± SD, frequencies (number of cases) and relative frequencies (percentages) when appropriate. Comparison of quantitative variables between different groups was done using Wilcoxon Mann Whitney U test for independent samples. Comparison of quantitative variables over the study period was done using Wilcoxon matched-pairs test comparisons. A probability value (P value) less than 0.05 was considered statistically significant. The influence of variables on prognosis was evaluated by univariate analysis. When the result was statistically significant, multivariate regression analysis was used to analyze the factors influencing the prognosis to avoid any confounding interaction between them.
Survival times were calculated beginning with the dates of the first TACE treatment using the Kaplan-Meier method. The log-rank test was used to determine the significance of the difference between patient survival rates in different patient groups.
The treatment protocols were well tolerated by all patients, with only minor side effects and no major complications. Post-embolization syndrome in the form of nausea and vomiting occurred in 9 patients (23%) and a dull aching upper abdominal pain persisting for 24 h in 11 patients (28.2%). There was no mortality within 30 d from the time of TACE.
Local tumor morphologic evaluations according to RECIST criteria were as follows: no patient achieved complete response, partial response in one patient (2.6%), stable disease in 34 patients (87.1%) and progressive disease in 4 patients (10.3%). The comparisons of the local tumor response according to RECIST criteria in different patient groups are summarized in Table 2.
| Total | B | APS | Child A | Child B | Solitary | Multiple | Well defined | Ill defined | |||
| Yes | No | Yes | No | ||||||||
| No. of patients | 39 | 19 | 20 | 7 | 32 | 27 | 12 | 20 | 19 | 20 | 19 |
| Partial response | 1 (2.6) | 0 | 1 (5) | 0 | 1 (3.1) | 0 | 1 (8.3) | 0 | 1 (5.2) | 1 (5) | 0 |
| Stable disease | 34 (87.1) | 18 (94.7) | 16 (80) | 6 (85.7) | 28 (87.5) | 24 (88.9) | 10 (83.3) | 18 (90) | 16 (84.2) | 17 (85) | 17 (89.4) |
| Progressive disease | 4 (10.3) | 1 (5.3) | 3 (15) | 1 (14.2) | 3 (9.30) | 3 (11.1) | 1 (8.3) | 2 (10) | 2 (10.5) | 2 (10) | 2 (10.5) |
The local tumor response using MR volumetric measurements revealed the following results: tumor volume reduction in 17 patients (43.6%) and tumor volume progression in 22 patients (56.4%). The changes of the tumor volumes after TACE and the local tumor response are summarized in Tables 3 and 4.
| Total | PVT | APS | Child A | Child B | Solitary | Multiple | Well defined | Ill defined | |||
| Yes | No | Yes | No | ||||||||
| No. of patients | 39 | 19 | 20 | 7 | 32 | 27 | 12 | 20 | 19 | 20 | 19 |
| Initial tumor volume (mL) | |||||||||||
| Range | 1.2-1358 | 1.2-1358 | 28.8-301.9 | 28.9-237 | 1.2-1358 | 1.1-1355 | 56.7-1358 | 1.2-1355 | 23.4-1358 | 1.2-301.9 | 28.8-1358 |
| Mean | 227.6 | 354.5 | 107 | 109.2 | 253.5 | 162.3 | 374.5 | 246 | 208.2 | 97.0 | 365 |
| SD | 318.76 | 418.2 | 78.5 | 75.2 | 345.8 | 275.8 | 370.5 | 317 | 327.2 | 60.0 | 411 |
| Tumor volume 1 mo after the 1st TACE | |||||||||||
| Range | 1.3-1637.5 | 1.3-1637.5 | 15.6-334.8 | 39-239 | 1.3-637.5 | 1.3-1500 | 23.7-1637.5 | 1.3-1499.7 | 15.6-1637.5 | 1.3-334.9 | 39-1637.5 |
| Mean | 244.5 | 402.6 | 94.3 | 126.5 | 270.3 | 159.6 | 435.6 | 242.6 | 246.6 | 65.3 | 412 |
| SD | 369 | 480.1 | 72.8 | 85.0 | 402.2 | 284.7 | 470.0 | 334.3 | 411.9 | 75.3 | 473 |
| Tumor volume at the end of treatment | |||||||||||
| Range | 1.6- 2053.4 | 1.6-2053.4 | 9.4-403.1 | 94.5-308 | 1.6-2053.4 | 1.6-2053.4 | 22.5-1637.5 | 1.6-2053.4 | 9.4-1637.4 | 1.6-403.1 | 83-2053.4 |
| Mean | 286.1 | 486 | 96.2 | 188.6 | 307.5 | 197.4 | 485.6 | 280.8 | 291.7 | 74.3 | 509.1 |
| SD | 453.2 | 583.7 | 100 | 72.5 | 490 | 391 | 534.2 | 452.9 | 465.8 | 69.5 | 568 |
| Total | PVT | APS | Child A | Child B | Solitary | Multiple | Well defined | Ill defined | |||
| Yes | No | Yes | No | ||||||||
| No. of patients | 39 | 19 | 20 | 7 | 32 | 27 | 12 | 20 | 19 | 20 | 19 |
| Tumor volume reduction after last TACE session | 17 (43.6) | 5 (26.3) | 12 (60) | 0 (0) | 17 (53.1) | 13 (48.1) | 4 (33.3) | 9 (45) | 8 (42.1) | 13 (65) | 4 (21.05) |
| Tumor volume progression after last TACE session | 22 (56.4) | 14 (73.7) | 8 (40) | 7 (100) | 15 (46.9) | 14 (51.9) | 8 (66.7) | 11 (55) | 11 (57.9) | 7 (35) | 15 (78.9) |
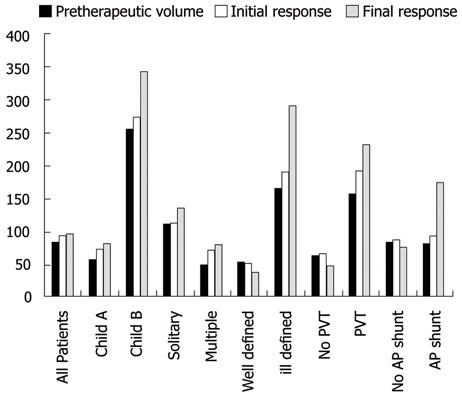
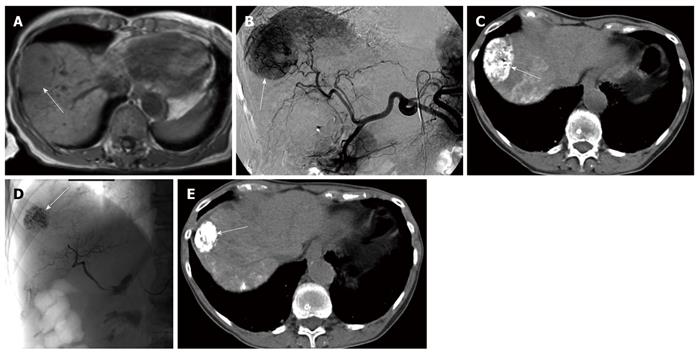
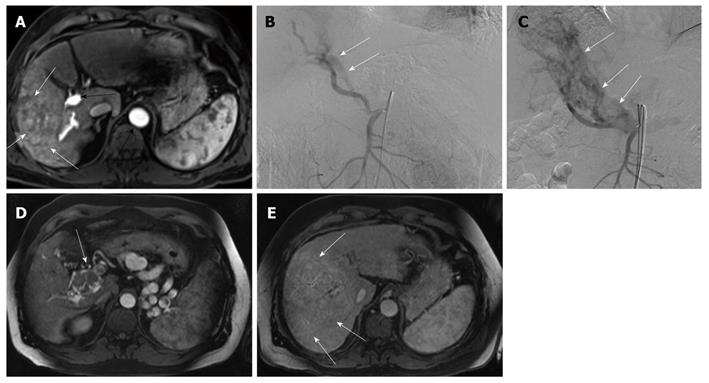
The local tumor response evaluated using the percentage of volume changes between the pre- and post-treatment volumes was statistically significant and influenced by the following factors: the presence or absence of PVT, presence or absence of APS and tumor margin definition (P = 0.018, 0.008 and 0.001, respectively) (using Mann-Whitney-U-test), as tumor volume regression was detected more in the absence of PVT and APS and in tumors with well defined margins (Figure 1). Multivariate analysis (using multiple regression test) showed that the tumor margin definition seems a more significant factor compared to the presence of PVT or AP shunts (P = 0.002).
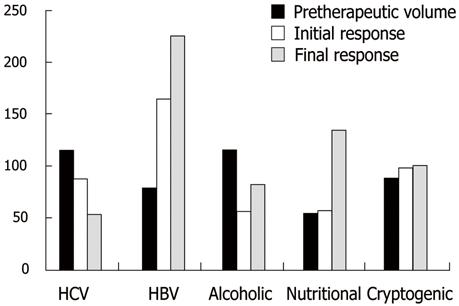
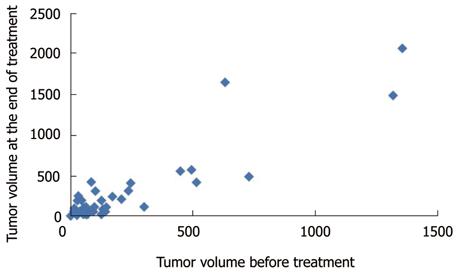
The local tumor response was insignificantly affected by the number of focal lesions and Child-Pugh score of the patient (P = 0.478 and 0.893, respectively). Correlation between the underlying liver pathology and the percentage of volume changes between the pre- and post-treatment volumes was statistically significant (P = 0.034) (using Kruskal Wallis-test). However, correlation between the different patient groups failed to prove statistical significance (using Conover-Iman-Test). The changes of the median tumor volumes are illustrated in Figure 2. The correlation between the pre- and post-treatment tumor volumes for all patients is illustrated in a scatter gram (Figure 3).
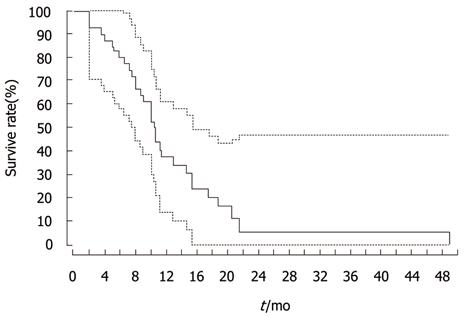
The overall survival rates calculated from the time of first TACE sessions for all patients at 6, 12 and 18 mo were 79.5%, 37.5% and 21%, respectively (Figure 4). The median survival time was 10 mo. There was an insignificant difference in the survival rates between different patient groups on analysis by log-rank test (PVT: P = 0.653, APS: P = 0.822, number of lesions: P = 0.26, margin definition of lesion: P = 0.155 and Child-Pugh score: P = 0.09) (Figure 4).
Despite the remarkable advancement and availability of novel curative options, a great proportion of HCCs are still not eligible for curative treatment due to advanced tumor stage or poor hepatic functional reserve[7]. There is no evidenced-based knowledge regarding a standard systemic chemotherapy protocol that improves the overall survival in advanced HCC patients[14]. Combination chemotherapy using different mixtures of doxorubicin, 5-FU, mitomycin C, bleomycin, cisplatin, interferon (PIAF), gemcitabine and mitoxantrone has been employed in the treatment of advanced HCC. Although some of the combination regimes have shown improved tumor control in phase II studies, most of them fail to demonstrate any survival advantage in randomized phase III studies[15].
New research trials utilizing sorafenib have demonstrated survival extension in two phase III trials in North America, Europe and in the Asia-Pacific area, which respectively reported a median survival after treatment of 10.7 and 6.5 mo[16]. However, drug-related adverse effects including diarrhea, hand-foot skin reaction, anorexia, alopecia, weight loss, dry skin, abdominal pain and voice changes have been reported[17].
Attention has been focused on locoregional approaches for the palliative treatment of HCC in recent years. Although TACE is widely used in the palliative treatment of unresectable HCC, its role remains controversial[18-20]. It has been shown to provide reasonable survival advantages in two randomized control trials and a meta-analysis[21].
Different reports have investigated the predictors of survival after TACE for unresectable HCC. These predictors may be related to the tumor burden (number of focal lesions, size of the tumor, tumor/liver volume ratio and PVT), the liver function (Child-Pugh score), health status (constitutional syndrome and Karnofsky index) or may be related to the treatment protocol[8,10,20,22,23]. In a large scale study of 8510 patients who underwent TACE, multivariate analyses revealed the following variables to be independent predictors of patient prognosis: degree of liver damage, maximum tumor size, number of lesion(s) and portal vein invasion[8]. Portal vein invasion showed much higher risks than the other variables.
The endpoint in cancer research is overall survival. Nonetheless, other potential endpoints, such as response rate and time to progression, are currently used. In this study, the predictors of local tumor response after TACE were evaluated, as well as the role of TACE in local tumor control for unresectable advanced HCC. Since volumetric quantification can lead to a different assessment result compared with uni- and bi-dimensional measurement techniques[24], the local tumor response to the TACE treatment was evaluated using both RECIST criteria and MRI volumetric measurements. According to RECIST criteria, tumor stability was achieved in 34/39 patients (87.1%), and partial response in 1 patient (2.6 %), while progression occurred in 4 patients (10.3%) and, by volumetric measurements, reduction of the tumor volume occurred in 17 patients (43.6%) and progression in (56.4%), taking into consideration the inclusion of advanced pretreatment tumor stage regarding the size, number and portal vein invasion. This may emphasize the role of TACE in local tumor control even in the advanced stages of HCC. Univariate analysis revealed the following 4 variables as significant prognostic factors: PVT, APS, tumor margin definition and underlying liver pathology (Figures 5 and 6). The clinical significance of the presence of PVT, APS and tumor margin ill definition is clearly demonstrated by the progressive increase in the corresponding median tumor volumes at the end of TACE treatment (Figure 1). Multivariate analysis showed that the tumor margin ill definition affects the local tumor response more significantly compared to the presence of PVT or AP shunts.
TACE has been limited in palliative treatment of cases associated with major portal vein (PV) invasion due to the possibility of liver failure following embolization and that it may predispose to hepatic infarction. Transcatheter arterial chemo infusion (TACI) has been an option in such cases[25]. However, recent studies have shown that TACE using less aggressive embolization can be performed safely in patients with major PV thrombosis with no increase in morbidity or mortality[26-28]. The current study included patients with partial thrombosis of the main PV or thrombosis of one of its lobar branches or segmental branches. The procedure was tolerated by all patients and none of the patients developed major complications that required prolonged hospitalization. Chung et al[29] explained the prognostic significance of PVT by suggesting that the presence of tumor thrombi in the PV results in extensive tumor spread throughout the liver.
Arterioportal shunts associated with HCC have been reported in about 28.8% to 63.2% of HCCs. Ngan et al[30] explained the poor prognostic clinical significance by suggesting that the shunt flow to the portal vein aggravates the portal venous pressure, which induces life-threatening conditions such as esophageal varix, ascites and hepatic encephalopathy. In this present study 7/39 patients (17.9%) had associated APS, six of these cases were associated with ill defined tumor margin and four cases were associated with PVT. The MR volumetric measurements revealed progression of the tumor in the 7 patients (100%) and the percentage of volume changes between both groups (APS and no APS) were statistically significant. Huang et al[31] explained poor response to TACE by the fact that during TACE, oil emulsion may be diverted into the portal vein branches and delivered to non-tumor hepatic tissue instead of being deposited intratumorally. Although the univariate analysis revealed a statistical significance between the underlying liver pathology and the local tumor response, the multivariate analysis failed to prove the clinical significance, probably because of the sample size and the registered data which are located out of the overlapping area statistically.
Limitations of this study are the retrospective design and the heterogeneous population. However, this study at least illustrates the role of TACE in local tumor control of unresectable HCC, even in advanced cases, and the prognostic factors affecting the tumor response.
In conclusion, TACE may be applied in the palliative treatment of patients with unresectable advanced HCC for local tumor control. The local tumor response to TACE is significantly affected by the presence or absence of portal vein thrombosis, arterioportal shunt, tumor margin definition and the underlying liver pathology.
To date, there is no evidence-based knowledge regarding a standard systemic chemotherapy protocol that improves the overall survival in advanced hepatic cancer (HCC). Attention has been focused on regional liver approaches for the palliative treatment of HCC in recent years, including transarterial hepatic chemoembolization (TACE). This therapy been shown to provide reasonable survival advantages in two randomized control trials and a meta-analysis. However, the factors that determine tumor response under the effect of local chemotherapy are still under research and evaluation.
This research focused on the vascular factors that may affect the tumor response and patient survival in patients undergoing TACE therapy in advanced HCC. Both portal vein thrombosis (PVT) and arterioportal shunts (APS) were found to be statistically significant factors influencing reduced tumor responsiveness to TACE therapy. Other pathological factors which may determine the tumor end response are the underlying liver pathology, Child-Pugh status and tumor margin definition.
The study concludes that TACE may be exploited safely for palliative tumor control in patients with advanced unresectable HCC; however tumor response is significantly affected by the presence or absence of PVT and APS.
Transarterial Chemoembolization is a minimally invasive therapeutic procedure which consists of: catheterization of the hepatic artery to inject the chemotherapeutic medications selectively in the feeding vessels of the liver tumor. The therapeutic medications consist of chemotherapeutic agent (which inhibits tumor cell proliferation) in a mixture with occlusive material (to block the feeding vessels of the tumor). Both agents work together to suppress tumor activity and kill tumor cells. Portal vein thrombosis: thrombosis of the major blood vessel of the liver that carries the nutritive materials from the gastrointestinal tract to be metabolized in the liver. The hepatic portal vein is responsible for 75% of blood supply to the liver. Arterioportal shunt: This is an abnormal communication between the hepatic artery and portal vein tributaries.
I think this article is well written.
Peer reviewer: Satoshi Mamori, MD, PhD, Department of Gastroenterology and Hepatology, Shinko Hospital, 1-4-47 Wakihama-cho, Chuo-ku, Kobe, Hyogo 651-0072, Japan
S- Editor Sun H L- Editor Logan S E- Editor Ma WH
| 1. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. |
| 2. | Llovet JM, Sala M. Non-surgical therapies of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2005;17:505-513. |
| 3. | Ishii H, Okada S, Nose H, Okusaka T, Yoshimori M, Takayama T, Kosuge T, Yamasaki S, Sakamoto M, Hirohashi S. Local recurrence of hepatocellular carcinoma after percutaneous ethanolinjection. Cancer. 1996;77:1792-1796. |
| 4. | Sartori S, Tombesi P, Macario F, Nielsen I, Tassinari D, Catellani M, Abbasciano V. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology. 2008;248:670-679. |
| 5. | Ishida T, Murakami T, Shibata T, Inoue Y, Takamura M, Niinobu T, Sato T, Nakamura H. Percutaneous microwave tumor coagulation for hepatocellular carcinomas with interruption of segmental hepatic blood flow. J Vasc Interv Radiol. 2002;13:185-191. |
| 6. | Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions). Radiology. 2002;225:367-377. |
| 7. | Molinari M, Kachura JR, Dixon E, Rajan DK, Hayeems EB, Asch MR, Benjamin MS, Sherman M, Gallinger S, Burnett B. Transarterial chemoembolisation for advanced hepatocellular carcinoma: results from a North American cancer centre. Clin Oncol (R Coll Radiol). 2006;18:684-692. |
| 8. | Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461-469. |
| 9. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. |
| 10. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. |
| 11. | Zhang JW, Feng XY, Liu HQ, Yao ZW, Yang YM, Liu B, Yu YQ. CT volume measurement for prognostic evaluation of unresectable hepatocellular carcinoma after TACE. World J Gastroenterol. 2010;16:2038-2045. |
| 12. | Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guide lines. J Vasc Interv Radiol. 2003;227:407-413. |
| 13. | Padhani AR, Ollivier L. The RECIST (Response Evaluation Criteria in Solid Tumors) criteria: implications for diagnostic radiologists. Br J Radiol. 2001;74:983-986. |
| 14. | Palmer DH, Hussain SA, Johnson PJ. Systemic therapies for hepatocellular carcinoma. Expert Opin Investig Drugs. 2004;13:1555-1568. |
| 15. | Yau T, Chan P, Epstein R, Poon RT. Evolution of systemic therapy of advanced hepatocellular carcinoma. World J Gastroenterol. 2008;14:6437-6441. |
| 16. | Di Lorenzo G, Imbimbo M, Leopardo D, Marciano R, Federico P, Buonerba C, Salvatore B, Marinelli A, Palmieri G. A long-lasting response to sorafenib treatment in an advanced hepatocellular carcinoma patient. Int J Immunopathol Pharmacol. 2010;23:951-954. |
| 17. | Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223-240. |
| 18. | Llovet JM, Sala M, Castells L, Suarez Y, Vilana R, Bianchi L, Ayuso C, Vargas V, Rodés J, Bruix J. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology. 2000;31:54-58. |
| 19. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. |
| 20. | O’Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325-331. |
| 21. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. |
| 22. | Martins A, Cortez-Pinto H, Marques-Vidal P, Mendes N, Silva S, Fatela N, Glória H, Marinho R, Távora I, Ramalho F. Treatment and prognostic factors in patients with hepatocellular carcinoma. Liver Int. 2006;26:680-687. |
| 23. | Ikeda M, Okada S, Yamamoto S, Sato T, Ueno H, Okusaka T, Kuriyama H, Takayasu K, Furukawa H, Iwata R. Prognostic factors in patients with hepatocellular carcinoma treated by transcatheter arterial embolization. Jpn J Clin Oncol. 2002;32:455-460. |
| 24. | Sohaib SA, Turner B, Hanson JA, Farquharson M, Oliver RT, Reznek RH. CT assessment of tumour response to treatment: comparison of linear, cross-sectional and volumetric measures of tumour size. Br J Radiol. 2000;73:1178-1184. |
| 25. | Kim JH, Yoon HK, Kim SY, Kim KM, Ko GY, Gwon DI, Sung KB. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther. 2009;29:1291-1298. |
| 26. | Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol. 2007;18:1517-1526; quiz 1527. |
| 27. | Kiely JM, Rilling WS, Touzios JG, Hieb RA, Franco J, Saeian K, Quebbeman EJ, Pitt HA. Chemoembolization in patients at high risk: results and complications. J Vasc Interv Radiol. 2006;17:47-53. |
| 28. | Georgiades CS, Hong K, D’Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653-1659. |
| 29. | Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165:315-321. |
| 30. | Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. 1997;52:36-40. |
| 31. | Huang MS, Lin Q, Jiang ZB, Zhu KS, Guan SH, Li ZR, Shan H. Comparison of long-term effects between intra-arterially delivered ethanol and Gelfoam for the treatment of severe arterioportal shunt in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:825-829. |