Published online Mar 7, 2010. doi: 10.3748/wjg.v16.i9.1097
Revised: November 26, 2009
Accepted: December 3, 2009
Published online: March 7, 2010
AIM: To investigate omeprazole-induced transepithelial gastric leak and its effects on the permeability of the peptides bradykinin and oxytocin.
METHODS: Rat gastric corpus tissue was isolated and mounted in an Ussing chamber apparatus to evaluate the permeability of 3H-bradykinin, 3H-oxytocin, and 14C-EDTA in the presence or absence of omeprazole. Thin-layer chromatography was performed to identify any metabolic breakdown products of the peptides resulting from permeation through the gastric tissue, and thereby calculate the true flux of the peptide.
RESULTS: The flux rate of intact 3H-bradykinin increased substantially after omeprazole addition (109.5%) compared to the DMSO vehicle control (14%). No corresponding change in flux of intact 3H-oxytocin was observed under the same conditions (11.9% and 6.4% in the DMSO- and omeprazole-treated conditions, respectively). After exposure to omeprazole, the flux rate of 14C-EDTA also increased dramatically (122.3%) compared to the DMSO condition (36.3%).
CONCLUSION: The omeprazole-induced gastric leak allows for transmucosal permeability to charged molecules as well as non-electrolytes. This induced leak will allow certain peptides to permeate.
- Citation: Gabello M, Valenzano MC, Zurbach EP, Mullin JM. Omeprazole induces gastric transmucosal permeability to the peptide bradykinin. World J Gastroenterol 2010; 16(9): 1097-1103
- URL: https://www.wjgnet.com/1007-9327/full/v16/i9/1097.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i9.1097
Our group has previously reported in patient-based (in vivo) and animal (ex vivo) studies that proton pump inhibitors (PPIs) induce a paracellular transepithelial leak in the gastric corpus[1,2]. The leak allows uncharged, non-electrolyte probes (mannitol, sucrose, polyethyleneglycol) as large as 4000 Da to cross, and is bidirectional in nature. We have also investigated the medical significance of the PPI-induced leak and have shown that the small molecule drug, digoxin, used to treat congestive heart failure and supraventricular arrhythmias, can cross the gastric mucosa in the presence of omeprazole[3]. Hopkins et al[4] earlier speculated that PPIs may allow small molecule drugs to permeate across the gastric mucosa. This finding suggests that omeprazole has the potential to alter blood kinetics of certain orally-delivered small molecule drugs whose blood levels must be carefully titrated, and co-administration may therefore pose a significant clinical risk. Kiley et al[5] have very recently shown the clinical ramifications of such PPI-induced leak coinciding with PPI-mediated inhibition of liver cytochrome degradation of certain small molecule drugs such as digoxin. It is currently unknown, however, whether the possession of charge would prevent molecules from permeating through this induced leak. The aforementioned 4000 Da molecular weight “ceiling” on the omeprazole-induced leak was based on study of uncharged molecules only. This led us to investigate a small, charged (non-metabolizable) probe, EDTA (MW 380). The exact charges on the EDTA molecule vary depending upon pH[6].
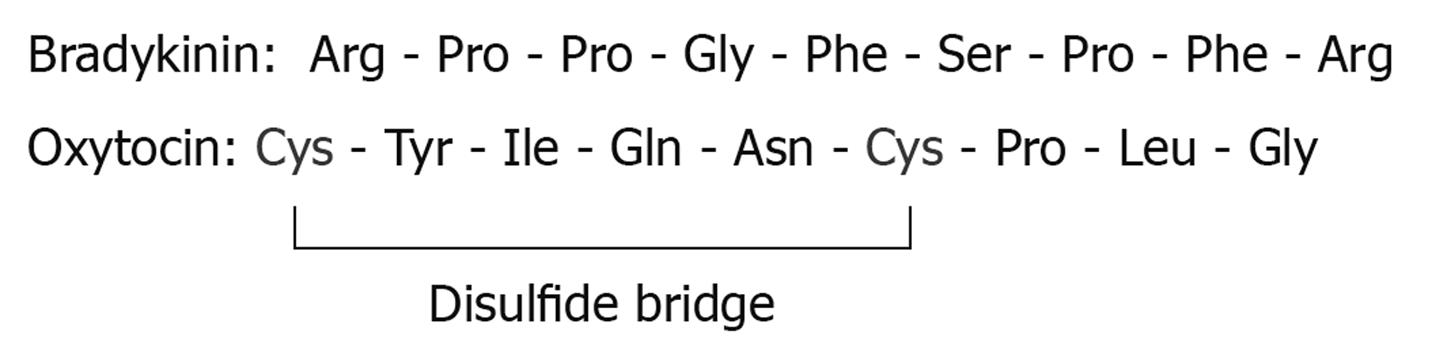
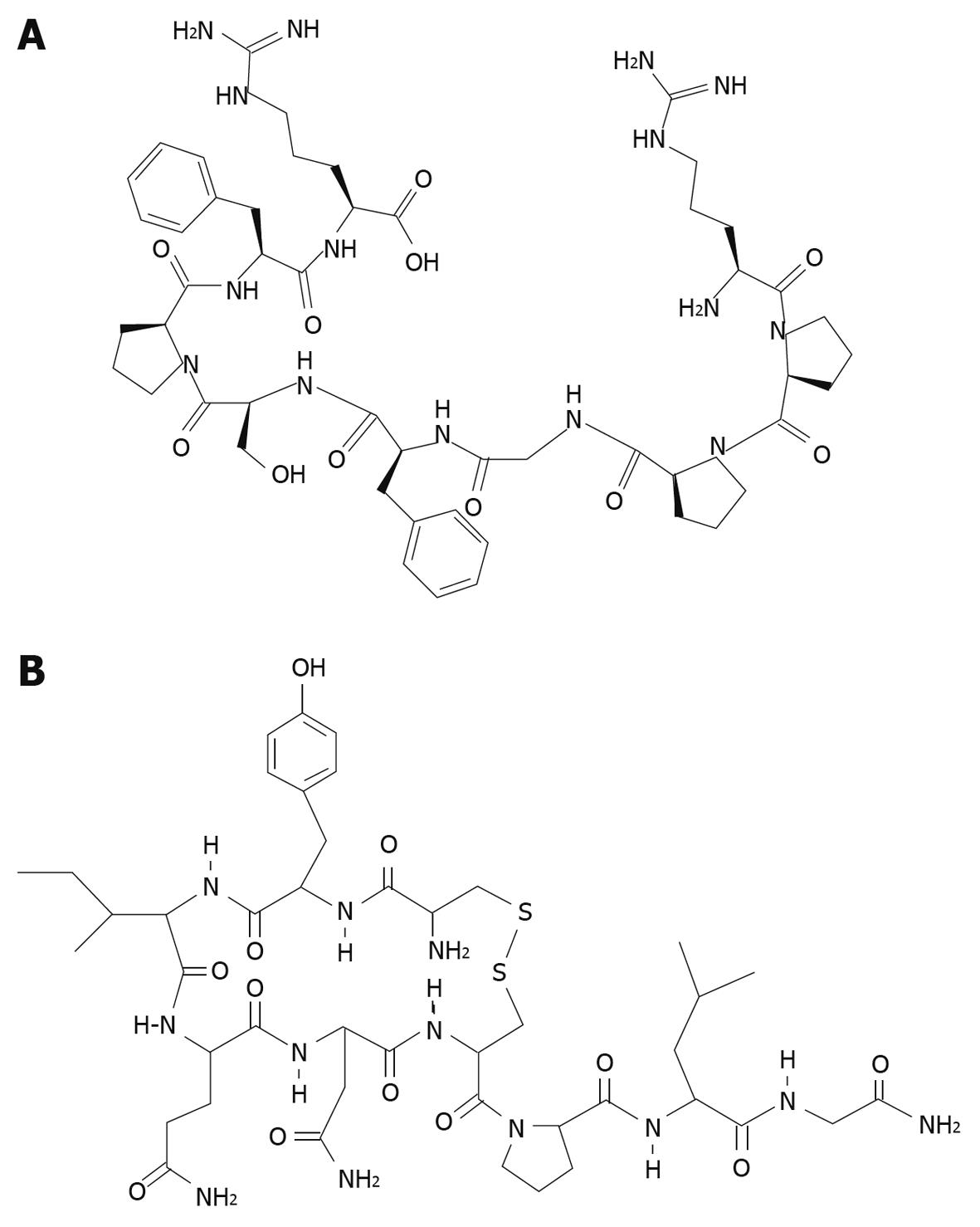
The leak induced by PPIs may carry drug delivery potential, due largely to the fairly safe profile of PPIs[7]. Although the specific mechanism of the gastric leak is unknown, the leak seems to be benign to the tissue, carrying with it no morphological damage to gastric mucosa, a major benefit for a drug delivery vehicle. This current study also examines the gastric permeability of two characteristic small peptides, bradykinin (MW 1060) and oxytocin (MW 1007) (Figure 1) in the presence and absence of omeprazole. If omeprazole were to allow these peptides to leak across the gastric mucosa into the bloodstream, they would thereby avoid proteolytic digestion by trypsin and other digestive enzymes present in the duodenum.
Sprague Dawley male rats weighing approximately 400 g were sacrificed by decapitation and the stomach was quickly removed, cut along the greater curvature, and flushed of luminal contents. The serosal membrane was then stripped from the corpus region and two equal sized pieces of corpus mucosa were mounted in two separate Ussing chambers with exposed tissue areas of 1.13 cm2[2]. Chambers were connected to 15 mL gas-lift reservoirs filled with un-buffered saline aerated with 100% O2 and bicarbonate-buffered saline (pH 7.3) aerated with 95% O2/5% CO2 on the mucosal and serosal sides, respectively. Un-buffered saline was used on the mucosal side to allow for luminal acidification. Saline temperature was held constant at 37°C by a jacketed water bath surrounding the reservoirs. Thirty minutes at 37°C was allowed for tissue equilibration. In paired experiments, one tissue served as (vehicle) “control”, and the other as omeprazole-treated “experimental”.
Electrical parameters were monitored throughout the duration of the experiment. Tissues were maintained under open circuit conditions. Ag/AgCl electrodes were bridged to saline in chambers with 4% agarose bridges stored in 1 mol/L NaCl. Tissue voltage (potential difference), short circuit current, and transepithelial resistance were recorded approximately every five minutes for the duration of the experiment, by passing a one-second current pulse equal to biological current to bring the potential difference to zero using a current/voltage clamp (McGrath Research & Technology). Transepithelial resistance was calculated by dividing open circuit potential difference by short circuit current (Ohm’s law).
Mucosal and serosal saline pH measurements were taken at approximately 5 min intervals using a manual pH electrode (Denver Instruments) to ensure that the tissue was secreting acid. Mucosal pH readings were also taken before and after the addition of omeprazole to document the action of omeprazole on inhibiting acid secretion. Tissue viability was confirmed at the end of each experiment by tissue exposure to 10 mmol/L amiloride in the mucosal fluid compartment in order to inhibit short circuit current.
After 30 min of tissue equilibration time in Ussing chambers, dibutyryl cyclic AMP (dBcAMP) (Sigma) was added at a final concentration of 1 mmol/L to the serosal fluid chambers to stimulate acid secretion. Forty minutes following dBcAMP addition, protease inhibitors, pepstatin (1.8 μmol/L) and leupeptin (5 μmol/L), were added to the mucosal and serosal sides to prevent extracellular degradation of the peptide molecules of interest. Radiolabeled peptides under study [3H-bradykinin, 3H-oxytocin (PerkinElmer)] along with their unlabeled forms were added five minutes after the addition of protease inhibitors to the mucosal fluid of both chambers to achieve final concentrations of 0.3 mmol/L bradykinin or 5 μmol/L oxytocin in the mucosal fluid. The addition of the isotope marked the beginning of the flux period. Five hundred microliters of samples were taken from the serosal fluid compartment (opposite from which the isotope was added) for liquid scintillation counting (LSC). Samples were only taken from the mucosal fluid compartment at the end of the experiment to determine the specific activity of radioactivity initially added. The amount of radioactivity present in the mucosal compartment does not significantly change throughout the duration of the experiment. Serosal samples were initially taken at 45 min intervals for 90 min for the 3H-bradykinin and 3H-oxytocin fluxes to establish the basal permeability rate (ρmoles/min per cm2) across the tissue. About 200 μmol/L omeprazole (Sigma) (in DMSO) was then added to the mucosal and serosal sides of one chamber and an equivalent amount of DMSO (Sigma) was also added to both sides of the control chamber. Another series of serosal fluid samples were taken for LSC at 45 min intervals for the remaining 90 min of 3H-bradykinin and 3H-oxytocin flux experiments to determine flux rate of the radiolabeled drugs after omeprazole addition. In all cases of fluid sampling for liquid scintillation counting, an equal amount of fresh buffered saline was added back to the serosal side to prevent hydrostatic gradients from developing across the tissue. The flux of each isotope (ρmole/min per cm2) was determined by calculating the linear regression slope of the graph of cpm vs time, and converting cpm to picomoles after determining the specific activity of the drug in the mucosal fluid.
A similar flux protocol was followed for 14C-EDTA experiments, with the following differences: 14C-EDTA (ARC, Inc.) was added to the serosal fluid compartment 45 min after the addition of dBcAMP (no protease inhibitors were added) along with its unlabeled form to achieve a final concentration of 10 μmol/L. Five hundred and fifty microliters of samples were taken from the mucosal fluid compartment (opposite from which the isotope was added) for LSC every 15 min for 75 min. About 200 μmol/L omeprazole (or DMSO) was then added and another series of mucosal fluid samples were taken every 15 min for the remaining 45 min of the flux period. Fresh unbuffered saline was added back to the mucosal fluid after each sample was taken.
Additional saline samples were taken from the mucosal and serosal fluid compartments one minute before the addition of omeprazole/DMSO and at the end of the entire flux period to allow for thin-layer chromatography (TLC) analysis of radioactivity that crossed the tissue. TLC was performed to account for the potential contribution of radiolabeled metabolites to total radioactivity crossing the epithelial barrier, in the event of drugs entering gastric epithelial cells and metabolites being effluxed. Saline samples were taken from both surfaces of the tissue to determine possible degradation of the molecule upon exposure to the mucosal surface of the tissue as well as to analyze the chemical nature of the radioactivity that crossed the tissue. Saline samples from bradykinin and oxytocin flux experiments were concentrated by evaporation (Savant Speed Vac) to dryness and then resuspended in small volumes of double deionized water. TLC was performed on silica gel 60 plates with a 254 fluorescent indicator (Kodak) by spotting very small amounts of the concentrated sample. Plates were then placed in a sandwich tank and a variety of mobile phases were employed, including butanol/acetic acid/water (120:30:50), isopropanol/water/NH4OH (120:30:1), isopropanol/water (120:30), and ethyl acetate/methanol/water (81:11:8). After completion of the mobile phase, plates were sprayed with ninhydrin reagent to visually detect the location of proline or tyrosine, likely amino acid degradation products of bradykinin or oxytocin, respectively, and examined under a 254 UV lamp for detection of bradykinin or oxytocin, indicated by blocked fluorescence.
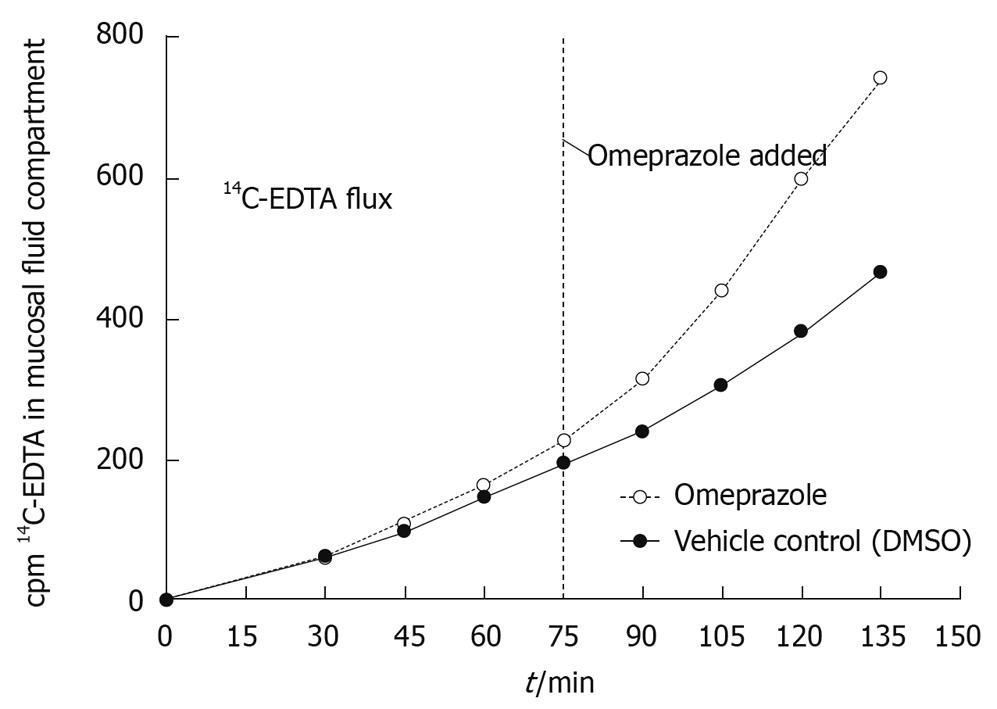
Ex vivo analyses indicate that omeprazole increases the rate at which the non-metabolizable, paracellular probe, 14C-EDTA, diffuses across rat gastric mucosa compared to the vehicle control (DMSO). The amount of radiolabeled EDTA in the mucosal fluid compartment increased linearly over time in both conditions, but upon the addition of omeprazole to one chamber, there was a dramatic increase in the rate of diffusion of radioactive EDTA seen entering the mucosal fluid compartment (Figure 2). The observed increase in flux after treatment with omeprazole is continuous and nearly immediate in each of three experiments. When comparing the post-omeprazole flux values to the post-DMSO flux values, the flux rate of EDTA after the addition of omeprazole increased by 122.3% ± 8.5% vs 36.3% ± 11.5% for DMSO (Table 1). This evidences that the paracellular leak induced by omeprazole does allow a charged particle, 14C-EDTA, to diffuse across the gastric mucosa. In Table 1 the values listed show the picomoles/min per cm2 of 14C-EDTA crossing the gastric mucosa in the DMSO and omeprazole conditions, with corresponding percent increases after the addition of each compound. Data from three experiments (and three animals) are shown. There is a consistent dramatic percent increase from the pre- to post-omeprazole addition compared to the pre- to post-DMSO (control) condition. The mean ± SE of the mean is also listed from the three experiments.
| Flux value (ρmole/min per cm2) | % increase in flux | |||||
| Pre-DMSO | Post-DMSO | Pre-omeprazole | Post-omeprazole | After DMSO | After omeprazole | |
| Experiment 1 | 1.78 | 2.03 | 1.52 | 3.18 | 14.0 | 109.0 |
| Experiment 2 | 1.65 | 2.36 | 1.58 | 3.48 | 43.0 | 120.0 |
| Experiment 3 | 1.50 | 2.28 | 1.69 | 4.03 | 52.0 | 138.0 |
| mean ± SE | 1.64 ± 0.08 | 2.22 ± 0.10 | 1.60 ± 0.05 | 3.56 ± 0.25 | 36.3 ± 11.5 | 122.3 ± 8.5 |
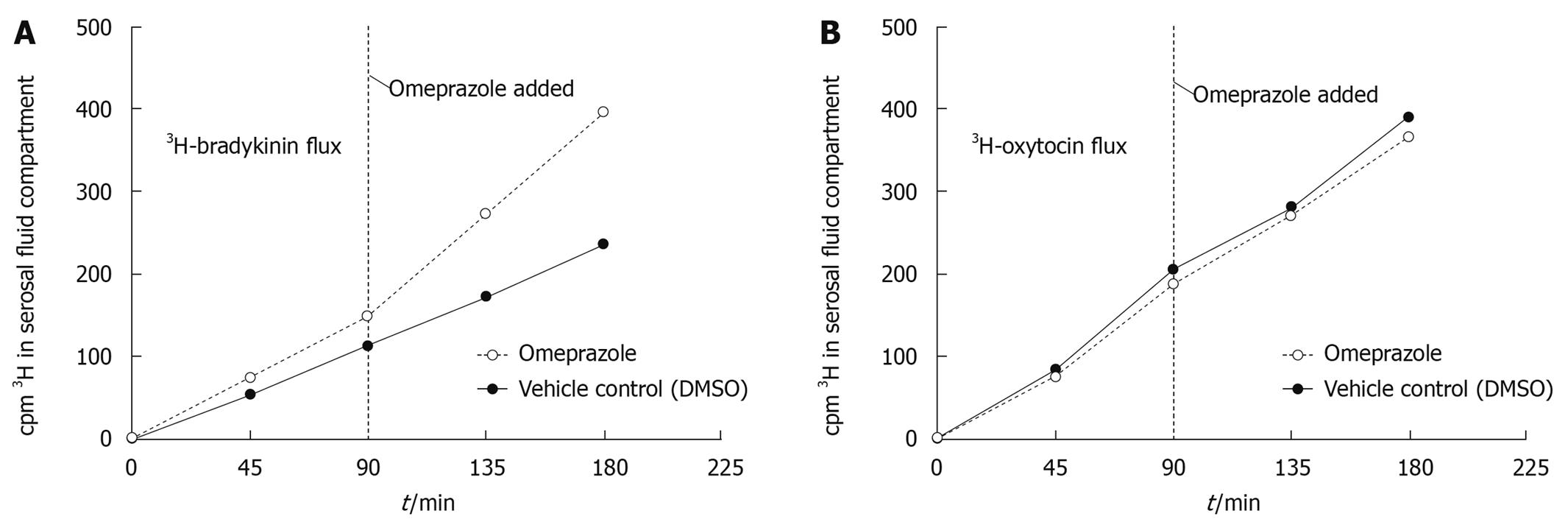
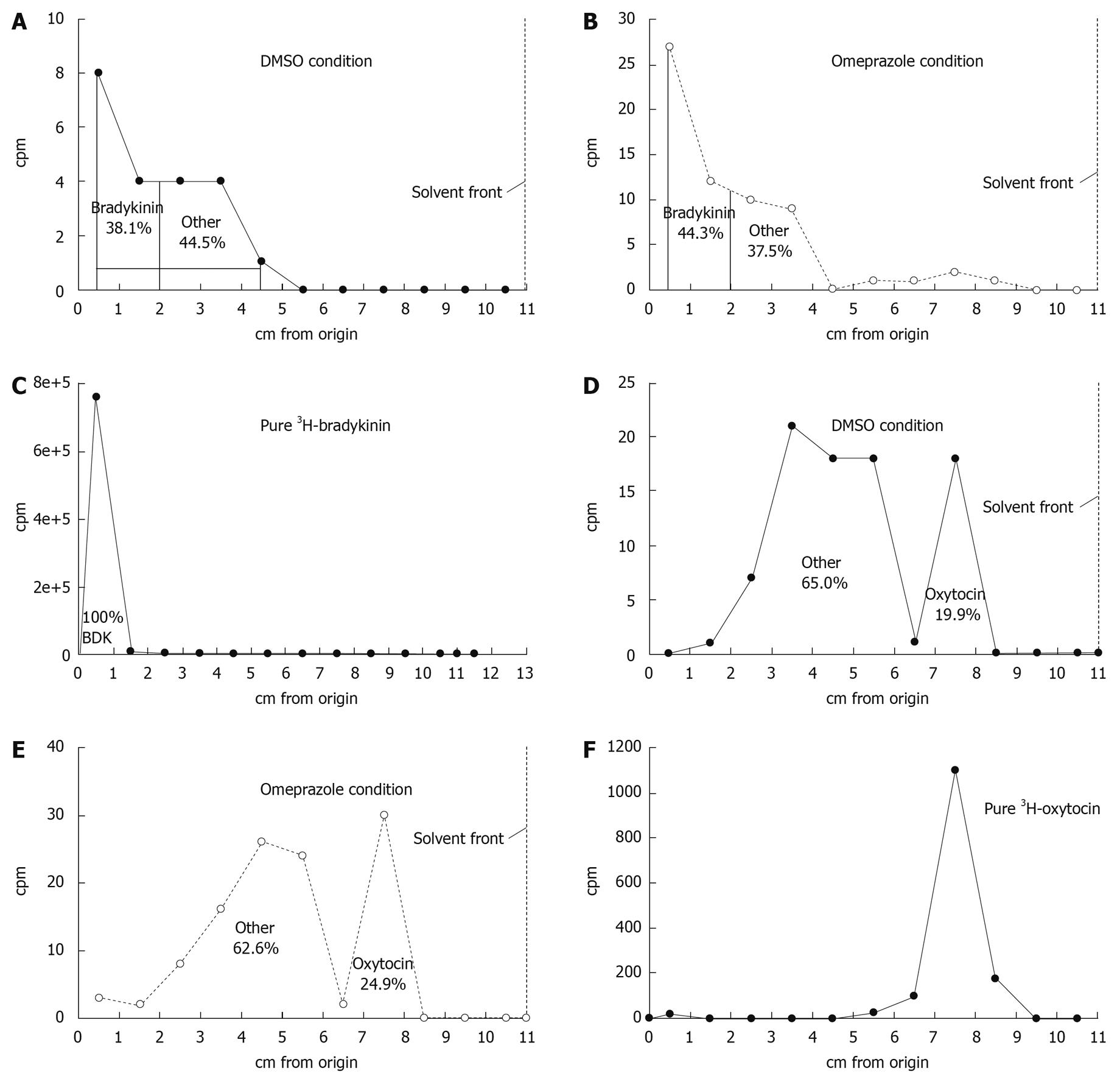
The total amount of tritium diffusing across the gastric mucosa (in a mucosal to serosal direction) also increased in the presence of omeprazole compared to DMSO in a 3H-bradykinin flux experiment (Figure 3A), but did not increase under the same conditions in a 3H-oxytocin flux (Figure 3B). Since, unlike EDTA, peptides are subject to metabolism by the gastric tissue, TLC was performed to analyze the total radioactivity from each flux experiment and differentiate intact 3H-bradykinin or 3H-oxytocin (data not shown) from 3H-metabolites. TLC results revealed the presence of 3H-metabolites in the serosal fluid after a 180 min 3H-bradykinin flux experiment; however no substantial difference in relative proportions of 3H-bradykinin and 3H-metabolites between the omeprazole and DMSO conditions was seen (Figure 4A and B). Intact 3H-bradykinin migrated with a known pure 3H-bradykinin and unlabeled bradykinin standard (Figure 4C). After TLC analysis, flux values were adjusted to reflect the total amount of radiolabeled intact bradykinin or oxytocin crossing the gastric mucosa over the 180 min flux period. After the addition of omeprazole, there was a 109.5% ± 38.5% (range) increase in the rate of 3H -bradykinin flux compared to the DMSO vehicle control increase of only 14.0% ± 12.0% (range) (Table 2). Both bradykinin experiments performed yielded similar results, with omeprazole increasing 3H-bradykinin flux dramatically more than DMSO did. There was, however, no dramatic difference in flux rate of 3H-oxytocin after the addition of omeprazole or DMSO, with percent increases in flux being 11.9% ± 42.6% (range) and 6.4% ± 9.9% (range) in the DMSO- and omeprazole-treated conditions, respectively (Table 3). These figures reflect correction of the data for oxytocin metabolites in the 3H-cpm coming into the serosal fluids compartment (Figure 4D-F), as was done for the bradykinin flux measurements. It can thus be concluded that permeability to bradykinin is increased by omeprazole, but permeability to oxytocin is not.
| Flux value (ρmole/min per cm2) | % increase in flux | |||||
| Pre-DMSO | Post-DMSO | Pre-omeprazole | Post-omeprazole | After DMSO | After omeprazole | |
| Experiment 1 | 10.2 | 10.4 | 12.4 | 21.2 | 2.0 | 71.0 |
| Experiment 2 | 5.0 | 6.3 | 5.0 | 12.4 | 26.0 | 148.0 |
| mean ± range | 7.6 ± 2.6 | 8.35 ± 2.1 | 8.7 ± 3.7 | 16.8 ± 4.4 | 14.0 ± 12.0 | 109.5 ± 38.5 |
| Flux value (fmole/min per cm2) | % increase in flux | |||||
| Pre-DMSO | Post-DMSO | Pre-omeprazole | Post-omeprazole | After DMSO | After omeprazole | |
| Experiment 1 | 20.9 | 32.3 | 38.7 | 45.0 | 54.5 | 16.3 |
| Experiment 2 | 51.5 | 35.7 | 31.3 | 30.2 | -30.7 | -3.5 |
| mean ± range | 36.2 ± 15.3 | 34.0 ± 1.7 | 35.0 ± 3.7 | 37.6 ± 7.4 | 11.9 ± 42.6 | 6.4 ± 9.9 |
Tables 2 and 3 show the actual flux values in mole/min per cm2 and fmole/min per cm2 pre- and post- omeprazole (or DMSO) addition. All values are corrected after TLC analysis of radioactivity. Two experiments were performed for each peptide (3H-bradykinin and 3H-oxytocin).
In previous patient-based[1] and animal model studies[2,3] our group has shown that proton pump inhibitors induce a transmucosal, paracellular “leak” in the gastric corpus. Using nonelectrolyte probes, it was shown that molecules as large as 4 kDa can permeate through this induced leak. Although there are many biomedical considerations to such leak, one possibility is that the leak could be used for oral administration of molecules (e.g. peptides, proteins) that normally could not cross the GI barrier into the bloodstream and/or are metabolized in the lumen (e.g. duodenum) before uptake is possible. The field of drug delivery has long pursued agents capable of inducing transmucosal permeation without damaging the mucosal tissue.
In this study we have shown that a relatively small, charged molecule (EDTA) can pass through the omeprazole-induced gastric leak, suggesting that the possession of charge by a molecule will not prevent it from crossing through the leak. It made it reasonable to assume that other charged molecules would likely also diffuse through the “pore”. We can only speculate, however, that the same would be true for a larger, charged molecule. If a charged molecule with a larger radius than EDTA (one closer to the size of the opening) were to come in contact with the omeprazole-induced pore, it may be more likely repelled because of its close proximity to the lining of the pore. In other words, if a charged molecule is small enough compared to the pore, it does not come close enough to be repelled by fixed charges lining the pore.
In the EDTA studies reported here the radioactivity coming across the gastric mucosa after each flux experiment would be all true EDTA because EDTA does not permeate cell membranes, is metabolized only negligibly and is known to only travel paracellularly[8,9]. We saw only one peak of radioactivity (data not shown), but because of the many forms molecular EDTA takes on at different pHs, consistent co-migration results were difficult to attain. Given the fact that EDTA will alter its charge as a function of pH it is worth highlighting that EDTA fluxes in this study were conducted in the serosal to mucosal direction. Serosal pH in these studies was maintained at pH 7.3. However in this direction, EDTA must traverse the intercellular space before presenting to the tight junction barrier. We do not know the exact pH of the intercellular fluid, but note that it is in communication with the pH 7.3 serosal fluid compartment.
Because charge was thus found to not be exclusionary for passage through the leak induced by omeprazole, the permeability of two small, similar size peptides (bradykinin and oxytocin) in the presence and absence of omeprazole was also investigated. Similar to EDTA, based on their amino acid moieties, peptides can have dynamically changing charges at different pHs. We observed that bradykinin (MW 1060) was found to diffuse through the omeprazole-induced leak while oxytocin (MW 1007) did not show an increase in flux in the presence of omeprazole. There are obvious differences in the molecular structures of bradykinin and oxytocin (Figure 5) albeit their sizes are very similar. Bradykinin is an open chain of amino acids, while oxytocin is more globular and rigid, containing a disulfide bridge. Also noteworthy is that bradykinin, but not oxytocin, has basic amino acids at its C and N termini. These structural points may be pivotal to permeation through a PPI-induced “pore”.
Because the gastric leak induced by omeprazole appears to be benign to the tissue, with no histological pathology or increased occurrence of apoptosis, it is possible it may have value as a drug delivery vehicle for certain biological drugs. Melittin has been used in epithelial cell culture as a barrier manipulator, but was found to cause hemolysis and cytotoxicity in the process of enhancing permeability[10]. The local toxicity caused by sodium caprate, another drug delivery agent, is a major downfall in its use in absorption enhancement[11]. Yamamoto et al[12], in fact, reported a linear relationship between efficacy of various absorption enhancers and their toxicity. Contrary to these other barrier manipulators that are shown to enhance the barrier with negative local and systemic repercussions, omeprazole is a well-tolerated, widely-used, FDA-approved medication that opens the gastric paracellular barrier (tight junction) upstream from the duodenum with seemingly no physiological downside. The leak exhibits a size limit, is highly specific to what types of molecules permeate, and is reversible upon discontinuation of PPI therapy. We postulate that these specificity characteristics are precisely why this leak occurs with little or no tissue morbidity.
We suspect that this PPI-induced transepithelial gastric leak to bradykinin is paracellular and not transcellular because we already demonstrated that the PPI-induced leak is bidirectional[2]. In addition, the gastric epithelium is not specialized to transport molecules transcellularly like the duodenum. The gastric mucosa is not absorptive, as the majority of gastrointestinal absorption occurs downstream in the small intestine. Finally, however, note that there is evidence that as a result of PPIs raising gastric luminal pH, the protonation of certain molecules may be changed causing an alteration of their diffusion through the gastric epithelium[13]. This remains another possible mechanism of how bradykinin may have crossed the gastric mucosa in the presence of omeprazole elevating mucosal fluid pH.
The ability of bradykinin to permeate suggests that this leak may be useful as a drug delivery vehicle for synthetic peptides. The inability of oxytocin to pass suggests that the structure, charge, and hydrophobicity of a peptide will matter considerably to its paracellular permeation, and the exact criteria for permeation of future candidate peptides need to be determined. Future studies will look into establishing both the inclusionary and exclusionary structural criteria of peptide passage through the leak, as well as examine if the leak admits oligonucleotide permeation.
The very recently discovered transmucosal gastric leak caused by proton pump inhibitor (PPI) drugs may have use in allowing oral delivery of certain peptides and oligonucleotides that might otherwise have to be injected. PPIs have little if any morbid effects on the gastric mucosa, and thus the induced leak appears to not be harming the tissue.
PPIs are very widely utilized drugs for treatment of reflux disease and gastritis. The ability of PPIs to induce a gastric leak is just beginning to be explored. In this manuscript a positive aspect of such leak, namely a delivery route for other oral medications that normally have no way into the bloodstream, is explored.
In previous work, this leak was shown to be bidirectionally symmetric and allow only molecules less than 10 kDa to pass. In this current study the leak is shown to allow charged molecules in general to pass, and specifically allow the peptide, bradykinin, to pass. The inability of the similarly-sized peptide, oxytocin, to pass through the leak suggests that the leak possesses considerable specificity in the types of molecules it will admit.
The field of drug delivery has long sought agents that can “open up” epithelial barriers but not cause morbidity for the tissues in the process. It is a significant problem given the physiological importance of these barriers and the noxious nature of many constituents of luminal fluid compartments.
These studies utilize the Ussing chamber technique wherein gastric mucosal epithelial tissue is placed between two fluid-filled chambers. The passage across the tissue of radiolabeled probes, in this case, peptides, is then observed and measured, as a function of PPI exposure. These molecules are in all probability moving paracellularly across the tissue, and likely diffusing through altered epithelial tight junctions.
This is a very interesting paper in which the investigators examined the gastric permeability of two characteristic small peptides, bradykinin and oxytocin in the presence or absence of omeprazole. This is a carefully conducted study by a well qualified group of investigators.
Peer reviewer: Parimal Chowdhury, Professor, Department of Physiology and Biophysics, College of Medicine, University of Arkansas for Medical Sciences, 4301 W Markham Street, Little Rock, AR 72205, United States
S- Editor Tian L L- Editor O'Neill M E- Editor Ma WH
| 1. | Mullin JM, Valenzano MC, Whitby M, Lurie D, Schmidt JD, Jain V, Tully O, Kearney K, Lazowick D, Mercogliano G. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther. 2008;28:1317-1325. |
| 2. | Murray LJ, Gabello M, Rudolph DS, Farrell CP, Morgan M, Martin AP, Underwood JC, Valenzano MC, Mullin JM. Transmucosal gastric leak induced by proton pump inhibitors. Dig Dis Sci. 2009;54:1408-1417. |
| 3. | Gabello M, Valenzano MC, Barr M, Zurbach P, Mullin JM. Omeprazole Induces Gastric Permeability to Digoxin. Dig Dis Sci. 2009;Epub ahead of print. |
| 4. | Hopkins AM, McDonnell C, Breslin NP, O’Morain CA, Baird AW. Omeprazole increases permeability across isolated rat gastric mucosa pre-treated with an acid secretagogue. J Pharm Pharmacol. 2002;54:341-347. |
| 5. | Kiley CA, Cragin DJ, Roth BJ. Omeprazole-associated digoxin toxicity. South Med J. 2007;100:400-402. |
| 6. | Harris D, Freeman WH. Quantitative chemical analysis. 7th ed. New York: Freeman and Company 2007; . |
| 7. | Mullin JM, Gabello M, Murray LJ, Farrell CP, Bellows J, Wolov KR, Kearney KR, Rudolph D, Thornton JJ. Proton pump inhibitors: actions and reactions. Drug Discov Today. 2009;14:647-660. |
| 8. | Darwish NM, kratzer FH. Metabolism of ethylenediaminetetraacetic acid (edta) by chickens. J Nutr. 1965;86:187-192. |
| 9. | Bjarnason I, Smethurst P, Levi AJ, Peters TJ. Intestinal permeability to 51Cr-EDTA in rats with experimentally induced enteropathy. Gut. 1985;26:579-585. |
| 10. | Maher S, Feighery L, Brayden DJ, McClean S. Melittin as an epithelial permeability enhancer I: investigation of its mechanism of action in Caco-2 monolayers. Pharm Res. 2007;24:1336-1345. |
| 11. | Motlekar NA, Srivenugopal KS, Wachtel MS, Youan BB. Oral delivery of low-molecular-weight heparin using sodium caprate as absorption enhancer reaches therapeutic levels. J Drug Target. 2005;13:573-583. |
| 12. | Yamamoto A, Uchiyama T, Nishikawa R, Fujita T, Muranishi S. Effectiveness and toxicity screening of various absorption enhancers in the rat small intestine: effects of absorption enhancers on the intestinal absorption of phenol red and the release of protein and phospholipids from the intestinal membrane. J Pharm Pharmacol. 1996;48:1285-1289. |
| 13. | Endo H, Yoshida H, Ohmi N, Higuchi S. Effects of lansoprazole, clarithromycin and pH gradient on uptake of[14C]amoxycillin into rat gastric tissue. J Antimicrob Chemother. 2001;47:405-410. |