Published online Feb 21, 2010. doi: 10.3748/wjg.v16.i7.904
Revised: December 20, 2009
Accepted: December 27, 2009
Published online: February 21, 2010
AIM: To investigate the relation between RECK methylation and clinicopathological characteristics of gastric cancer patients and evaluate the role of RECK methylation in peritoneal metastasis of gastric cancer.
METHODS: Methylation of RECK gene in 40 paired samples of gastric cancer and its corresponding adjacent normal mucosa, lymph nodes and peritoneal irrigation fluid was detected by methylation-specific polymerase chain reaction.
RESULTS: Aberrant methylation of RECK gene was detected in 27.5% (11/40) of the adjacent normal mucosa samples, in 47.5% (19/40) of gastric cancer samples, in 57.1% (12/21) of the lymph node samples, and in 35% (14/40) of peritoneal irrigation fluid samples, respectively, with a significant difference between the adjacent normal mucosa and lymph node samples (P = 0.023). Presence of RECK methylation in the primary tumor samples was significantly correlated with tumor invasion (P = 0.023). The accuracy of RECK methylation in peritoneal lavage fluid samples for the diagnosis of peritoneal metastasis of gastric cancer was 72.5% (26/40), with a sensitivity of 66.7% (6/9) and a specificity of 74.2% (23/31).
CONCLUSION: Aberrant methylation of RECK gene may provide useful information for the early diagnosis and treatment of peritoneal metastasis of gastric cancer.
-
Citation: Du YY, Dai DQ, Yang Z. Role of
RECK methylation in gastric cancer and its clinical significance. World J Gastroenterol 2010; 16(7): 904-908 - URL: https://www.wjgnet.com/1007-9327/full/v16/i7/904.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i7.904
Gastric cancer seriously threatens the human health worldwide. There is increasing evidence that almost all gastric cancers have epigenetic abnormalities that drive cancer development and progression in collaboration with genetic changes. Aberrant methylation in the promoter CpG island of tumor suppressor genes (TSG) where DNA is transcribed into RNA causes its silence. Transcription of DNA is the first major step in decoding DNA into a protein. DNA methylation can inactivate tumor suppressor genes[1]. It has been shown that aberrant methylation and diminished expression of DNA in the promoter CpG island occur in a number of tumor-related genes in gastric cancer[2]. For example, RASSF1A, a candidate tumor suppressor gene, is hypermethylated in gastric cancer[3,4], TIMP-3, a silenced tumor suppressor gene, encodes a protease inhibitor that may inhibit tissue invasion[5], and RECK, a newly discovered metastasis suppressor gene, is silenced with aberrant CpG island hypermethylation in some common tumors[6-8]. However, the relation between methylation of RECK gene and gastric cancer has not been fully studied.
In this study, RECK gene methylation was detected in samples of primary tumor tissue and its adjacent normal mucosa, metastatic lymph nodes and peritoneal irrigation fluid by methylation-specific PCR in order to find the relation between RECK methylation and clinicopathological characteristics of gastric cancer and the role of RECK methylation in diagnosis of peritoneal metastasis of gastric cancer.
Forty patients including 28 males and 12 females at the age of 34-78 years underwent resection of their gastric cancer at the First Affiliated Hospital of China Medical University from July 2008 to January 2009. All patients did not receive chemotherapy or radiotherapy before operation.
Physiological saline (50 mL) was injected into the Douglas cavity at the beginning of operation and aspirated after gentle stirring, and then peritoneal lavage fluid was collected from the cavity before operation. Half of the peritoneal lavage fluid was examined using conventional cytological methods with Papanicolaou’s staining and intact cells were harvested from the other half centrifuged at 2000 r/min for 20 min as previously described[9,10] and stored in liquid nitrogen. Samples of primary tumor tissue and its paired adjacent normal mucosa and metastatic lymph nodes were taken immediately after resection of gastric cancer and stored in liquid nitrogen until use. The diagnosis of gastric cancer was made with hematoxylin and eosin (HE) staining. Paired adjacent normal mucosa samples were obtained at least 3 cm from the distal negative surgical margin to confirm the absence of malignancy. Lymph node samples were also stained with HE to confirm the occurrence of metastasis. Differentiation of tumor cells was detected and the tumor was staged following the guidelines of International Union against Cancer (UICC).
DNA was extracted from the genome with the hydroxybenzene-chloroform extraction method, stored at -70°C, and treated with bisulfite to convert the unmethylated cytosine to uracil.
DNA was purified using a Wizard DNA clean-up system (Promega) according to its manufacturer’s instructions. A 20 μL reaction volume was consisted of 3 μL DNA, 2 μL 10 × PCR buffer, 0.8 μL dNTP, 0.4 μL primers, 0.15 μL Tap enzyme, and 13.25 μL double-distilled water. PCR conditions were as follows: pre-denaturation at 94°C for 10 min, followed by 40 cycles at 94°C for 30 s, at 54°C for 20 s, at 72°C for 30 s, and a final extension at 72°C for 5 min. Methyltransferase SssI-treated DNA in peripheral blood cells from healthy people was used as a methylation positive control, untreated DNA served as an unmethylation positive control, and double-distilled water served as a negative control[4]. The sequences of primers are as follows: unmethylation primer: UF_RECK (5'-GGTTAGTTTTTTTTTTTATTTTAGTGGTTTGA-3') and UR_RECK (5'-ATTTCCAAAACCTCCCAAAAACAAAAACA-3'), methylation primer: MF_RECK (5'-GTTAGTTTTTTTTTTTATTTTAGTGGTTCGA-3') and MR_RECK (5'-TCCAAAACCTCCCGAAAACGAAAACG-3')[8]. The PCR products (205 bp and 201 bp) were subjected to 2.5% agarose gel electrophoresis at 120 V for 40 min and quantified with the Fluor Chen 2.0 system.
Statistical analysis was performed using the SPSS13.0 software package. χ2 test and Fisher’s exact test were adopted to verify the difference. P < 0.05 was considered statistically significant.
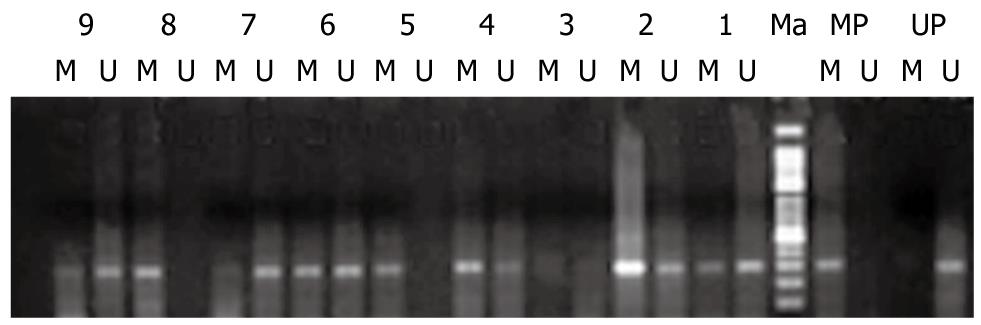
The methylation of RECK in samples of primary tumor tissue and its paired adjacent normal mucosa and metastatic lymphnode was detected by methylation special-PCR (Figure 1).
Methylation of RECK was found in 47.5% (19/40) of primary tumor tissue samples, in 27.5% (11/40) of paired adjacent normal mucosa samples, in 57.1% (12/21) of metastatic lymphnode samples, respectively. A significant relation was found between adjacent normal mucosa and metastatic lymphnode samples. RECK methylation was related with tumor invasion (P = 0.023) but not with other clinicopathological characteristics of gastric cancer patients such as age, tumor size, tumor differentiation, and Borrmann classification (Table 1).
| Variable | Patients (n) | RECK methylation | P value |
| Age (yr) | 0.689 | ||
| ≤ 65 | 32 | 16 | |
| > 65 | 8 | 3 | |
| Tumor size (cm) | 0.121 | ||
| ≤ 5 | 24 | 9 | |
| > 5 | 16 | 10 | |
| Borrmann classification | 0.199 | ||
| 1+2 | 19 | 7 | |
| 3+4 | 21 | 12 | |
| Tumor differentiation | 0.935 | ||
| Well | 25 | 12 | |
| Moderate/poor | 15 | 7 | |
| Tumor invasion | 0.023 | ||
| T1+T2 | 28 | 10 | |
| T3+T4 | 12 | 9 | |
| Nodal status | 0.199 | ||
| N- | 19 | 7 | |
| N+ | 21 | 12 |
In this study, the promoter of RECK gene was hypermethylated in 35% (14/40) of the samples. Among the 14 samples, peritoneal metastasis of gastric cancer was observed in 9. The diagnostic accuracy of RECK methylation in peritoneal lavage fluid for peritoneal metastasis of gastric cancer was 72.5%, with a sensitivity of 66.7%, a specificity of 74.2%, a PPV of 47.1%, and a NPV of 95.7% (Table 2).
| PLM | Peritoneal metastasis | |
| + | - | |
| + | 6 | 8 |
| - | 3 | 23 |
RECK methylation in peritoneal lavage fluid was found in tumors with lymph node metastasis (42.6%) and without lymph node metastasis (26.3%), although the difference between them was not statistically significant (Table 3).
| Variable | Patients (n) | RECK methylation in peritoneal lavage | P value |
| Tumor invasion | 0.193 | ||
| T1+T2 | 28 | 8 | |
| T3+T4 | 12 | 6 | |
| Nodal status | 0.273 | ||
| N- | 19 | 5 | |
| N+ | 21 | 9 |
RECK gene was discovered on chromosome region 9p13-p12 by Takahashi et al[11] in 1998. It encodes a membrane-anchored glucose protein with a relative molecular mass of 110 000. RECK protein is an important mediator of tissue remodeling to inhibit MMP-2, MMP-9 and MT1-MMP after transcription[12,13]. RECK protein limits tumor invasion and metastasis and angiogenesis through negatively regulated MMPs. It has been shown that several common tumors, such as colorectal, breast, and lung carcinomas, are linked to down-regulation of RECK[14-16]. In these tumors, RECK is down-regulated most likely as a result of inhibition at the Sp1 promoter site[17]. It was reported that down-regulation of the RECK gene is mediated by promoter methylation which causes its silence, just as other tumor suppressor genes[7,8,18]. Epigenetic alteration induced by DNA methyltransferases (DNMT) catalyzing methylation at 5 positions of cytosine ring using S-adenosylmethionine as the donor molecule for the methyl group plays an important role in tumorigenesis and progression[1]. The mechanism underlying RECK down-regulation appears to be multifactorial, and more studies are required to define its reasons.
In this study, RECK methylation was observed in samples of primary tumor tissue and its paired adjacent normal mucosa and metastatic lymph nodes from gastric cancer patients, indicating that RECK methylation in primary tumor tissue samples (47.5%) and in metastatic lymph node samples (57.1%) is much higher than that in paired adjacent normal mucosa samples (27.5%) (P = 0.023) and that RECK methylation is correlated with tumor invasion (P = 0.023). No significant difference was found in other factors, including age, tumor size, tumor differentiation, nodal status, Borrmann classification. However, Song et al[19] found that RECK expression is negatively related with lymph node metastasis and tumor stage in gastric cancer patients, which may be due to the small sample size, contamination of normal tissues, technical limitations[7], and down-regulation of RECK. Cho et al[7] showed that RECK promoter is methylated in 44% of tumor tissue samples and down-regulation of RECK is significantly correlated with promoter methylation (P < 0.05), suggesting that RECK methylation plays a significant role in inhibiting tumorigenesis and metastasis.
Methylation alteration occurs not only in solid cancer tissues but also in various remote samples from cancer patients. It has been recently reported that DNA methylation can act as a promising biomarker in early diagnosis and prognosis of gastric cancer[20]. In our study, RECK methylation in peritoneal lavage fluid was related with peritoneal metastasis of gastric cancer. Peritoneal metastasis of gastric cancer with cytologically positive peritoneal lavage was found in 9 of 14 patients with promoter hypermethylation. RECK promoter hypermethylation in peritoneal lavage showed a higher sensitivity (66.7%) for the diagnosis of peritoneal dissemination of gastric cancer than cytology. The reasons why methylation alteration acts as a biomarker are as follows. First, the methylation signal can act as a marker at a low concentration. Second, the methylation pattern and underlying DNA are more stable than RNA level and molecules[10]. However, methylation alteration in peritoneal lavage has a lower specificity for the diagnosis of peritoneal dissemination of gastric cancer, which can explained as follows. First, most cells in peritoneal lavage are mesothelial cells leading to false positive RECK methylation. Second, the discrepancy of methylation profile exists sometimes in peritoneal lavage and cancer tissue. In order to solve these problems, serial test, RECK methylation and other examinations, such as carcino-embryonic antigen in peritoneal lavage, can be used in the diagnosis of peritoneal dissemination of gastric cancer. In our study, RECK methylation in peritoneal lavage fluid was more frequently found in tumors with lymph node metastasis than in tumors without lymph node metastasis, suggesting that RECK methylation in peritoneal lavage can be considered a biomarker for predicting peritoneal metastasis of gastric cancer.
In summary, hypermethylation of RECK promoter is a common event in gastric cancer patients. RECK methylation in peritoneal lavage fluid acts as a biomarker of peritoneal metastasis of gastric cancer. Promoter hypermethylation of RECK gene provides a new tool for the prevention and treatment of gastric cancer. Further study is needed on the mechanism underlying RECK hypermethylation in gastric cancer patients.
Gastric cancer is a common tumor which seriously threatens the human health worldwide. DNA methylation in the promoter CpG island of tumor suppressor genes is one of the reasons for tumorigenesis and progression. It has been shown that DNA methylation, especially in body fluid, can act as a biomarker for predicting tumor metastasis.
RECK hypermethylation plays an important role in the epigenetic regulation of gene transcription. There is evidence that DNA promoter hypermethylation can cause transcription repression, contributing to tumorigenesis and progression. It has been recently shown that DNA methylation, especially in body fluid, can act as a biomarker for predicting tumorigenesis and prognosis. However, further study is needed on the mechanism underlying RECK hypermethylation.
RECK methylation in gastric cancer and peritoneal lavage fluid was detected, showing that RECK methylation plays an important role in diagnosing peritoneal metastasis.
Promoter hypermethylation of RECK gene provides a new tool for the prevention and treatment of gastric cancer. In addition, RECK methylation, especially in peritoneal lavage fluid, can act as a biomarker for diagnosing peritoneal metastasis.
It is a very interested topic for the readers of WJG. The results of this study show that promoter hypermethylation of RECK gene provides a new tool for the prevention and treatment of gastric cancer and RECK methylation, especially in peritoneal lavage fluid, can act as a biomarker for diagnosing peritoneal metastasis, which are of great value for the diagnosis of gastric cancer.
Peer reviewer: Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba
S- Editor Wang JL L- Editor Wang XL E- Editor Ma WH
| 1. | Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042-2054. |
| 2. | Wang JF, Dai DQ. Metastatic suppressor genes inactivated by aberrant methylation in gastric cancer. World J Gastroenterol. 2007;13:5692-5698. |
| 3. | Oliveira C, Velho S, Domingo E, Preto A, Hofstra RM, Hamelin R, Yamamoto H, Seruca R, Schwartz S Jr. Concomitant RASSF1A hypermethylation and KRAS/BRAF mutations occur preferentially in MSI sporadic colorectal cancer. Oncogene. 2005;24:7630-7634. |
| 4. | Shen WJ, Dai DQ, Teng Y, Liu HB. Regulation of demethylation and re-expression of RASSF1A gene in gastric cancer cell lines by combined treatment of 5-Aza-CdR and NaB. World J Gastroenterol. 2008;14:595-600. |
| 5. | Kang GH, Shim YH, Jung HY, Kim WH, Ro JY, Rhyu MG. CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res. 2001;61:2847-2851. |
| 6. | Chang HC, Cho CY, Hung WC. Downregulation of RECK by promoter methylation correlates with lymph node metastasis in non-small cell lung cancer. Cancer Sci. 2007;98:169-173. |
| 7. | Cho CY, Wang JH, Chang HC, Chang CK, Hung WC. Epigenetic inactivation of the metastasis suppressor RECK enhances invasion of human colon cancer cells. J Cell Physiol. 2007;213:65-69. |
| 8. | Long NK, Kato K, Yamashita T, Makita H, Toida M, Hatakeyama D, Hara A, Mori H, Shibata T. Hypermethylation of the RECK gene predicts poor prognosis in oral squamous cell carcinomas. Oral Oncol. 2008;44:1052-1058. |
| 9. | Katsuragi K, Yashiro M, Sawada T, Osaka H, Ohira M, Hirakawa K. Prognostic impact of PCR-based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. Br J Cancer. 2007;97:550-556. |
| 10. | Kamiyama H, Noda H, Takata O, Suzuki K, Kawamura Y, Konishi F. Promoter hypermethylation of tumor-related genes in peritoneal lavage and the prognosis of patients with colorectal cancer. J Surg Oncol. 2009;100:69-74. |
| 11. | Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi K, Kitaura Y, Takai S, Sasahara RM, Horimoto A. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci USA. 1998;95:13221-13226. |
| 12. | Noda M, Oh J, Takahashi R, Kondo S, Kitayama H, Takahashi C. RECK: a novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis Rev. 2003;22:167-175. |
| 13. | Clark JC, Thomas DM, Choong PF, Dass CR. RECK--a newly discovered inhibitor of metastasis with prognostic significance in multiple forms of cancer. Cancer Metastasis Rev. 2007;26:675-683. |
| 14. | Takemoto N, Tada M, Hida Y, Asano T, Cheng S, Kuramae T, Hamada J, Miyamoto M, Kondo S, Moriuchi T. Low expression of reversion-inducing cysteine-rich protein with Kazal motifs (RECK) indicates a shorter survival after resection in patients with adenocarcinoma of the lung. Lung Cancer. 2007;58:376-383. |
| 15. | Figueira RC, Gomes LR, Neto JS, Silva FC, Silva ID, Sogayar MC. Correlation between MMPs and their inhibitors in breast cancer tumor tissue specimens and in cell lines with different metastatic potential. BMC Cancer. 2009;9:20. |
| 16. | Oshima T, Kunisaki C, Yoshihara K, Yamada R, Yamamoto N, Sato T, Makino H, Yamagishi S, Nagano Y, Fujii S. Clinicopathological significance of the gene expression of matrix metalloproteinases and reversion-inducing cysteine-rich protein with Kazal motifs in patients with colorectal cancer: MMP-2 gene expression is a useful predictor of liver metastasis from colorectal cancer. Oncol Rep. 2008;19:1285-1291. |
| 17. | Sasahara RM, Takahashi C, Noda M. Involvement of the Sp1 site in ras-mediated downregulation of the RECK metastasis suppressor gene. Biochem Biophys Res Commun. 1999;264:668-675. |
| 18. | Chang HC, Cho CY, Hung WC. Silencing of the metastasis suppressor RECK by RAS oncogene is mediated by DNA methyltransferase 3b-induced promoter methylation. Cancer Res. 2006;66:8413-8420. |
| 19. | Song SY, Son HJ, Nam E, Rhee JC, Park C. Expression of reversion-inducing-cysteine-rich protein with Kazal motifs (RECK) as a prognostic indicator in gastric cancer. Eur J Cancer. 2006;42:101-108. |
| 20. | Tost J. DNA methylation: an introduction to the biology and the disease-associated changes of a promising biomarker. Methods Mol Biol. 2009;507:3-20. |