Published online Feb 21, 2010. doi: 10.3748/wjg.v16.i7.868
Revised: December 11, 2009
Accepted: December 18, 2009
Published online: February 21, 2010
AIM: To investigate feasibility, morbidity and surgical mortality of a docetaxel-based chemotherapy regimen randomly administered before or after gastrectomy in patients suffering from locally-advanced resectable gastric cancer.
METHODS: Patients suffering from locally-advanced (T3-4 any N M0 or any T N1-3 M0) gastric carcinoma, staged with endoscopic ultrasound, bone scan, computed tomography, and laparoscopy, were assigned to receive four 21 d/cycles of TCF (docetaxel 75 mg/m2 day 1, cisplatin 75 mg/m2 day 1, and fluorouracil 300 mg/m2 per day for days 1-14), either before (Arm A) or after (Arm B) gastrectomy. Operative morbidity, overall mortality, and severe adverse events were compared by intention-to-treat analysis.
RESULTS: From November 1999 to November 2005, 70 patients were treated. After preoperative TCF (Arm A), thirty-two (94%) resections were performed, 85% of which were R0. Pathological response was complete in 4 patients (11.7%), and partial in 18 (55%). No surgical mortality and 28.5% morbidity rate were observed, similar to those of immediate surgery arm (P = 0.86). Serious chemotherapy adverse events tended to be more frequent in arm B (23% vs 11%, P = 0.07), with a single death per arm.
CONCLUSION: Surgery following docetaxel-based chemotherapy was safe and with similar morbidity to immediate surgery in patients with locally-advanced resectable gastric carcinoma.
- Citation: Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, Roth A, Schuller JC, Fiori G, Orsi F, Bonomo G, Crosta C, Huber O. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol 2010; 16(7): 868-874
- URL: https://www.wjgnet.com/1007-9327/full/v16/i7/868.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i7.868
In spite of a declining incidence in the Western world, gastric cancer is still a major malignant disease in many populations, and the second leading cause for cancer mortality worldwide[1]. While localized disease, limited to the submucosa, can be best treated surgically, with a long-term survival of 70%-95%, the prognosis of locally-advanced tumor is poorer, due to a high unresectability rate at presentation, and a much higher relapse rate after radical surgery[2], thus demanding further studies regarding adjuvant and neoadjuvant treatment. Docetaxel (Taxotere®; Sanofi-Aventis, Paris, France) has been approved for treatment of metastatic gastric cancer, when combined with cisplatin and infused fluorouracil (TCF regimen), showing superiority in survival, time to progression, and response rate (RR) vs cisplatin/fluorouracil (CF) in a randomized phase III trial[3]. A better RR for docetaxel/cisplatin (TC) vs epirubicin/cisplatin/protracted venous infusion fluorouracil (ECF) has been documented in a randomized phase II trial[4]. These data suggested investigational use TCF in a preoperative neoadjuvant setting. This analysis aimed to test the hypothesis that preoperative chemotherapy with TCF does not influence negatively the results of subsequent surgery, when compared to immediate surgery. Primary endpoints of this study were operative morbidity and mortality rates; secondary endpoints were surgical and pathological assessments of downstaging and assessment by the surgeon as to whether the surgery was curative or not.
Patients with histologically-proven locally-advanced resectable gastric carcinoma (T3-4 any N M0 or any T N1-3 M0 as defined in the 1997 TNM classification) were screened for eligibility. Other inclusion criteria were: World Health Organization (WHO) performance status ≤ 2; age 18-75 years; adequate blood counts (white blood cell count ≥ 4000/mm3, platelets ≥ 100 000/mm3); normal renal (calculated creatinine clearance ≥ 60 mL/min) and liver function. Patients suffering from Siewert type I cardia location adenocarcinoma (extended mostly into the lower esophagus) were excluded. All patients underwent chest X-ray, gastric endoscopic ultrasound (EUS), spiral thoraco-abdominal computerized tomography (CT) scan, bone scintigraphy and staging laparoscopy to define nodal status and rule out distant deposits and/or peritoneal seeding.
The trial was approved in all centers by relevant ethics committees. All patients gave written informed consent for participation in the trial.
Patients were stratified by center, tumor size, tumor location (cardia adenocarcinoma Siewert II and III vs tumors of the rest of the stomach) and nodal status (N+ vs N-). Patients received four 21-d/cycles of TCF (docetaxel 75 mg/m2, 1-h IV infusion, day 1; cisplatin 75 mg/m2 4-h IV infusion, day 1; and 5-fluorouracil (5-FU) 300 mg/m2 per day continuous IV infusion, days 1 to 14), either preoperatively (Arm A) or postoperatively (Arm B). Just before starting chemotherapy all patients underwent placement of a totally implantable central venous port. In Arm A, a re-evaluation was performed after 2 cycles. If local progression had occurred, then the patient immediately underwent surgery. Otherwise two more TCF cycles were administered and surgery was performed within 3-5 wk after day 1 of the last cycle. In Arm B, surgery was scheduled to take place within 1 wk after randomization. Postoperative TCF was to be initiated 3 to 6 wk after surgery.
Data about postoperative course and complications were reported on hospital cards by surgical teams. In addition, an epidemiology nurse was in charge of regularly collecting microbiology data with respect to nosocomial infections (surgical site, pulmonary, urinary, and/or intravascular catheter infections). Data on hospital infections were regularly submitted to the infection central committees on a 3-mo basis.
As conclusive assessments of surgical procedures remain difficult, and there is a lack of consensus on how to define complications and to stratify them by severity, we decided to apply a single classification, proposed by Dindo et al[5], which is based on the evaluation of a cohort of 6336 patients and the results of a survey. In this classification, the therapy used to correct a specific complication is the cornerstone in ranking a complication. For example, life-threatening complications requiring an intermediate or intensive care management (IC/ICU) have to be differentiated from complications treated on the ward; as such complications are associated with a high mortality, stress for the patients, and substantial resource consumption. Therefore, registered complications in both groups were analyzed accordingly, with the exception of those classified as grade I (deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic or radiological interventions). This grade also included wound infections treated at bedside.
A careful intraoperative staging of disease was first performed, in order to rule out peritoneal seeding, ovarian involvement, “drop” metastasis in the pelvis, or periaortic gross adenopathy. Attention was directed to the liver, greater omentum and root of the mesentery below the transverse colon. The stomach was always inspected and gently palpated to assess the location and the extent of the tumor and to exclude direct invasion of adjacent structures. Frozen sections of every suspect tissue were obtained (e.g. preaortic, infracolic nodes); intraoperative, histology-proven recognition of metastatic spread was considered an exclusion criterion. The extent of gastrectomy depended on the proximal distance of the tumor from the cardia; therefore, total gastrectomy was performed in all patients with cardia locations and in those having antral and body tumors in whom a 6-cm gross proximal margin could not be obtained. Subtotal distal gastrectomy was performed in the others (almost exclusively small-size body or antral locations). Proximal gastric resection was never carried out, as total gastrectomy with 2-3 cm extent to abdominal esophagus was chosen for all cardia locations.
Lymphadenectomy included excision of all N1 and most N2 stations (stations 7, 8, 9 and station 11), according to the classification of the Japanese Research Society for the Study of Gastric Cancer (JRSGC)[6,7] (Table 1). Hepatoduodenal ligament nodes (station 12) were also dissected, limiting the lymphadenectomy to the 12a station (left side of the hepatic artery), and leaving undissected the parts b and p of the station (right side of the ligament and just posteriorly to the portal vein, respectively). Lymph nodes of the surgical specimen were routinely dissected by experienced pathologists, using standard techniques; in some cases, depending on the pathologist’s judgement, clearing fixatives were used prior to the dissection. Splenectomy was only carried out in cases of direct invasion of the spleen by the tumor, or gross appearance of metastatic nodes at station 10 (splenic hilum). Caudal pancreas was always preserved, according to the Maruyama’s technique, even when splenectomy was performed, unless tumor direct involvement was clinically evident.
| D1 | D2 | |
| Upper third | 1-2-3-4 | D1+5-6-7-8-9-10-11-110 |
| Middle third | 1-3-4-5-6 | D1+2-7-8 -9 -10 -11 |
| Lower third | 3-4-5-6 | D1+1-7-8-9 |
Surgeons were asked to document the extent of node dissection and to state whether the procedure was likely to be curative as follows: (1) Absolutely curative: absence of hepatic and/or peritoneal metastasis; serosa not involved; no infiltration within 10 mm of the proximal resection line; (2) Relatively curative: as above, but serosa involved, and/or cancer infiltrates within 10 mm of the proximal resection line, and/or nodal involvement (N stage) equals D number; and (3) Non-radical: resection line involvement; any residual disease after resection.
Reconstruction technique (Roux-en-Y, Braun or others) was entirely left to the discretion of the surgeon.
Pathological response to chemotherapy was centrally evaluated and classified as follows: (1) Complete response (pCR): No residual tumor could be found after in toto examination of the potential tumor site. Acellular mucus or acellular necrosis in the gastric wall or in lymph nodes was not considered as residual tumor and therefore was not taken into consideration for staging (neither for T, nor for N); (2) Partial microscopic response: Microscopic residual tumor (persistence of microscopic islands of tumor cells); (3) Partial macroscopic response: Macroscopic residual tumor, but overt necrosis or calcification, or downstaging of the tumor; and (4) No response: Only minor necrosis and no downstaging of the tumor.
Initially a target sample size at 240 patients was set, assuming a 3-year event-free survival rate of 20% in arm B and 35% in arm A (+ 15%). Trial was prematurely stopped at 70 randomized patients, due to insufficient accrual. Only two centers out of nine showed a good accrual rate, whereas most participating groups were not ready to be involved in such a multi-disciplinary approach. Moreover, some patients refused to participate to this kind of trial because they wanted to be operated on as soon as possible. For this reason, these study results are underpowered to detect any possibly significant differences in the experimental groups. Nevertheless, results were descriptively compared between the two arms on an intention-to-treat basis, using Mann-Whitney U-test and Kruskal-Wallis test to compare means and medians, respectively. χ2 or Fisher’s exact test were used to compare proportions. All tests were two-sided. A P value less than 0.05 was assumed significant. Intention-to-treat principle was adopted.
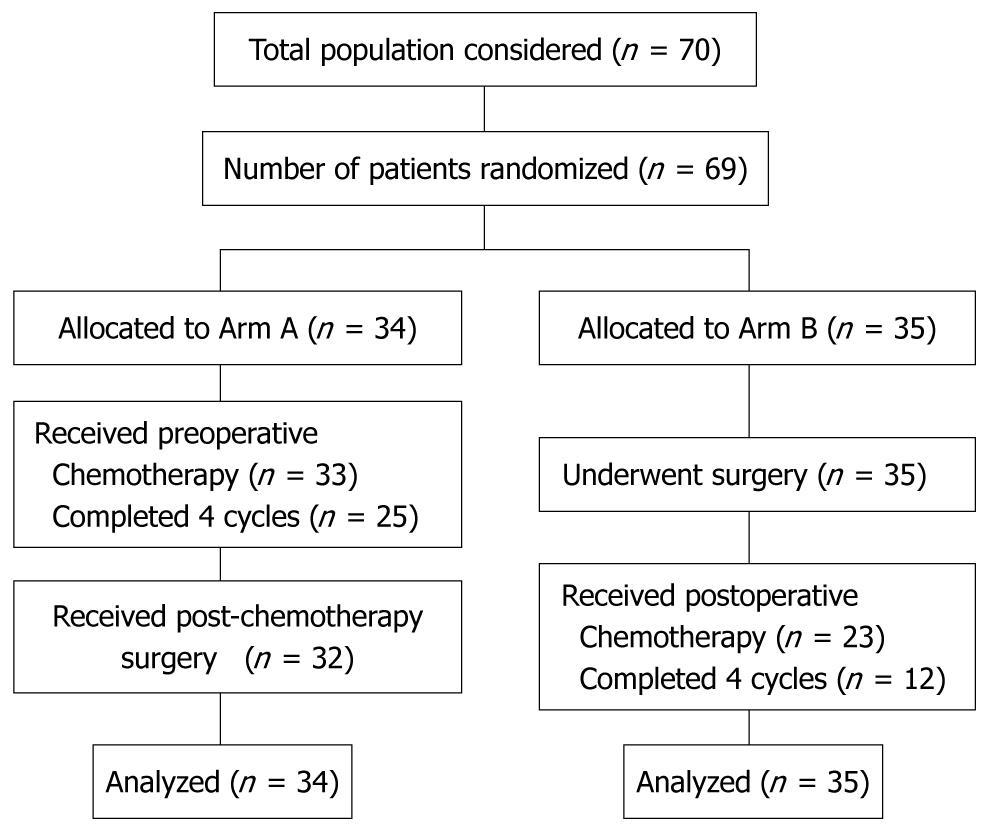
This trial was activated in November 1999 and closed in November 2005 due to insufficient accrual. From December 1999 to August 2005 a total of 70 patients were enrolled from 9 Institutions in three countries. Eighty-five percent of included patients were from two Institutions; from Milan (IEO-European Institute of Oncology) and Geneva (University Hospitals of Geneva). One patient withdrew consent, did not receive any chemotherapy, and was excluded from the analysis. Of the remaining 69 patients, 34 were randomized to Arm A (TCF followed by surgery) and 35 to Arm B (surgery followed by TCF). One patient in Arm A did not receive any chemotherapy because he died before starting. This patient was included in the analysis, in agreement with the intention-to-treat principle. A trial profile, conforming to the Consolidated Standards of Reporting Trials (CONSORT) is shown in Figure 1. The two groups of patients were similar with respect to various characteristics (Table 2).
| Arm A (n = 34) | Arm B (n = 35) | |
| Age (yr): median (range) | 57 (25-75) | 59 (39-76) |
| Male (%) | 68 | 71 |
| PS 0/1/2 (%) | 91/6/3 | 86/14/0 |
| Tumor site (%) | ||
| Cardia | 21 | 20 |
| Fundus/body | 38 | 40 |
| Antrum/pylorus | 41 | 40 |
| Stage (by EUS + CT scan) | ||
| IB | 2 | 1 |
| II | 14 | 13 |
| III | 18 | 21 |
Table 3 shows details about surgical procedures performed and pathology reports. Thirty-two patients in Arm A (94%) underwent laparotomy: 29 (85%) had an R0 resection, and two a non-radical resection; one had no resection due to unsuspected peritoneal carcinomatosis. All 35 patients in Arm B underwent laparotomy; 32 (91%) had an R0 resection, two a non-radical resection and one no resection due to peritoneal carcinomatosis. In Arm A, pathological response was complete in 4 patients (11.7%), and partial (macro- or microscopic) in 18 (55%). The respective proportions of total vs subtotal gastrectomies, D ≥ 2 vs D1 lymph node dissections, median number of excised lymph nodes and of metastatic nodes were very similar in the two arms of the study, and all differences were not statistically significant.
| Arm A (n = 32) | Arm B (n = 35) | |
| Complete resection R0 (%) | 29 (85) | 32 (91) |
| Non-radical resection (%) | 2 (5.8) | 2 (5.7) |
| No resection pM1 (peritoneum) (%) | 1 (2.9) | 1 (2.8) |
| pCR n (%) | 4 (11.7) | NA |
| pPR n (%) | 18 (55) | NA |
| Total gastrectomy | 20 | 24 |
| Subtotal gastrectomy | 11 | 10 |
| D-2 lymphadenectomy | 29 | 31 |
| D-3 lymphadenectomy | - | 2 |
| Excised lymph nodes median (range) | 20 (9-39) | 26 (13-76) |
| Metastatic lymph nodes median (range) | 1 (0-23) | 5 (0-50) |
Postoperative mortality and morbidity events are detailed in Table 4; they are stratified by severity, applying the classification proposed by Dindo et al[5]. In Arm A these included four septic intraabdominal complications (one anastomotic leak, two abdominal abscesses and one infected fluid peritoneal collection), one gastro-jejunal anastomosis bleeding, one pneumonia requiring ICU admission, one pulmonary embolism, one urinary infection, and one fever of unknown origin. Morbidity events in Arm B included three septic intraabdominal complications (one anastomotic leak, one abdominal abscess, and one infected fluid peritoneal collection), and six extra abdominal infections (one infected mediastinal collection, three pneumonias requiring ICU admission, and two central venous catheter-related blood stream infections). Three re-operations were performed, one in Arm A due to anastomotic hemorrhage, and two in Arm B, due to infected mediastinal collection and pleural empyema complicating severe pneumonia, respectively. Overall, 9 morbidity events occurred in each arm (28.5% in Arm A and 25.7% in Arm B). Two postoperative deaths occurred, in Arm B, as a consequence of multiple organ failure (MOF) complicating mediastinal infected fluid collection, in spite of re-operation. All these differences were not statistically significant (P = 0.86).
| Type of complication | Arm A (n = 32) | Arm B (n = 35) |
| Anastomotic leak | 1-IVb | 1-IVb |
| Abdominal abscess | 2-IIIa | 1-IIIa |
| Infected peritoneal collection | 1-IIIa | 1-IIIa |
| Anastomotic bleeding | 1-IIIb (re-operation) | - |
| Pneumonia requiring ICU | 1-IVa | 3-IVa-IVa, V (re-operation, death) |
| Pulmonary embolism | 1-II | - |
| Urinary infection | 1-II | - |
| Fever of unknown origin | 1-II | - |
| Mediastinal infected collection + MOF | - | 1-V (re-operation, death) |
| Central venous catheter-related blood stream infection | - | 2-II |
| Totala | 9 (28.5%), 1 re-operation | 9 (25.7%), 2 re-operations, 1 death |
A total of 189 TCF cycles were administered; 118 in Arm A and 71 in Arm B (Table 5). In Arm A, 25 patients (74%) received all 4 cycles, two patients 3 cycles, and six patients 2 cycles. In Arm B, only 12 patients received all 4 cycles (34%), five patients 3 cycles, two patients 2 cycles, four patients 1 cycle and 12 patients received no cycle. A 64-year-old female patient who had received one cycle of preoperative TCF died after severe worsening of performance status and dyspnoea. Excluding this case, serious adverse events (SAEs) occurred more frequently in Arm B. In Arm A, 13 SAEs in 10 patients were observed (13 SAEs out of 118 cycles = 11%), 7 of them infectious (3 febrile neutropenia). In Arm B, 16 SAEs occurred in 14 patients (16 SAEs out of 71 cycles = 23%). All these differences were not statistically significant (P = 0.07 and 0.15, respectively).
A 58-year-old male patient in Arm B died suddenly 38 d after gastrectomy from severe arrhythmia and pulmonary infection. Table 5 details reasons for cessation of therapy in the two arms of the study.
Prognosis of locally-advanced gastric cancer is generally poor in Western surgical and population-based series, with 5-year overall survival rates of 25% or less[8], in spite of complete excision of the gastric and nodal components of the disease[2]. This is the consequence of a high relapse rate after radical surgery, and has prompted many studies in the last decade, aimed at improving these results by means of adjuvant and neoadjuvant treatments[9-12]. Both these approaches remain controversial and are under current investigation. A large randomized trial [Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC)][13], demonstrated a survival benefit with the use of perioperative chemotherapy (i.e. pre- and postoperatively delivered) as compared with surgery alone. Similarly, the FFCD 9703 trial[14] showed an improvement in both disease free survival (DFS) and overall survival (OS) with the use of perioperative chemotherapy (FP regimen: 5-FU continuous infusion + cisplatin) as compared to surgery. To date, no trial has so far investigated the effects of the same chemotherapy regimen given either pre- or postoperatively.
Although our study is underpowered to detect any possible significant difference in short-term postoperative outcome, it gives some preliminary answers to questions not yet available in the medical literature that could be interesting for future studies. The first relevant information provided by the present study is the safety of surgery following a preoperative docetaxel-based chemotherapy regimen. In fact, we did not register any mortality and we had a 28.5% morbidity rate, without any significant difference between pre- and postoperative administration of chemotherapy. Although our patient population was slightly different from that of the MAGIC and FFCD trials, since Type I Siewert adenocarcinomas of the lower third of the esophagus were excluded, results of our study compare favorably with those of the MAGIC trial, where a 45% morbidity rate and 5% mortality rate were observed. In the FFCD trial, postoperative morbidity was 21% in the surgical arm, and 28% in the perioperative chemotherapy arm, whereas surgical mortality was 5% for both groups. A possible favorable factor was that 85% of patients in our series were operated on in two high-volume institutions where D2 gastrectomy is routinely carried out as standard treatment of gastric cancer. Our results support the conclusions that D2 gastrectomy can be considered a safe treatment of gastric cancer in Western patients, at least when performed in experienced centers[15], and that gastric cancer resection should probably be added to the growing list of procedures which are safer when performed in high-volume institutions[16,17]. This could explain why reports from single large volume institutions continue to demonstrate low operative mortality after D2 radical gastrectomy, while randomized trials show no survival benefit and severely increased surgical mortality after this procedure[18,19].
In addition, our data indicate that chemotherapy-related SAEs tended to be more frequent in Arm B (adjuvant) than in Arm A (neoadjuvant), suggesting that lower patient tolerance to treatment is a key factor in determining higher toxicity. This could be explained by an increased risk of gastrointestinal toxicity after surgery, when patients are already deeply affected in their eating capacity by the gastrectomy. For instance, it was shown that, in a population of 23 patients followed for dietary intake and nutritional status after total gastrectomy, no patient reached recommended dietary allowances by first monthly follow up[20].
Our trial confirms the difficulties in administering intensive adjuvant chemotherapy in gastric cancer. In the MAGIC trial, 34% of patients who completed preoperative chemotherapy and surgery did not start postoperative chemotherapy, mostly owing to early progression, patient refusal and/or surgical complication. In the weekly-PELF trial[21], only 14% of experimental arm patients completed the scheduled adjuvant treatment without time and/or dose modifications. Even without preoperative chemotherapy, 12 patients in this series did not receive any adjuvant chemotherapy. The most frequent reasons were patient refusal and medical decision. However, even when adjuvant chemotherapy was started, only 34% of the patients received all the four cycles. In the ACT-GC Group Trial (Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer), evaluating an oral fluoropyrimidine as adjuvant agent, enrollment was stopped after 1 year as a consequence of the higher rate of overall survival in the S-1 treated group than in controls who had only surgery[22]. Nevertheless, among the 517 patients who received S-1, 71 refused to continue treatment because of adverse events, and in 72 the decision of the investigators was to terminate treatment because of adverse events or complications (143/571, 25.04%). The dose of S-1 was reduced in 219 of the 517 treated patients (42.4%).
Laparoscopy has been reported to improve clinical staging when compared to conventional methods, identifying unexpected peritoneal or liver metastases in up to 20% of operable patients[23,24]; consequently, a significant proportion of patients can avoid unnecessary laparotomy[25]. Although in our study staging laparoscopy has been confirmed as an effective tool to demonstrate peritoneal deposits even when missed by preoperative CT scan, minimal peritoneal deposits were found and biopsied at laparotomy in 3 patients previously judged peritoneal seeding-free at laparoscopy. These false-negative results of laparoscopy occurred within the omentum and/or the lesser sac, emphasizing the limits of staging laparoscopy to demonstrate a minimal peritoneal spread in these difficult locations. Similarly, in a recent report, the sensitivity for detecting peritoneal carcinomatosis was 85% for laparoscopy[26,27]. The possible role of the preoperative PET scan in reducing the rate of false negative results of staging laparoscopy is currently under investigation, with conflicting preliminary evidence[28,29]; it seems a priori highly improbable that such small-volume disease could be detected by this imaging technique.
Finally, our data confirm the huge difficulties in performing this kind of study, which requires a high level of cooperation between different disciplines. Principal investigators analyzed the reasons for the slow accrual of patients for their neoadjuvant study with FAMTX[30] for operable gastric cancer, and observed that around half of the participating centers were not ready for such a multi-disciplinary approach, not believing in the potential efficacy of the neoadjuvant treatment. Moreover, several patients refused to participate in this kind of trial because they wanted to be operated on as soon as possible.
In conclusion, our study does not provide information on efficacy of preoperatively-delivered TCF, due to early discontinuation for slow accrual. It is also underpowered to detect any possible significant differences in short-term postoperative outcome. Nevertheless, data regarding TCF efficacy and feasibility in the preoperative setting and TCF feasibility in the adjuvant setting could be interesting for future studies. In particular, neoadjuvant TCF achieved promising results with a 12% pCR rate. This evidence prompts further studies, since patients achieving a pCR tend to have a much better outcome, as underlined in a recent phase II trial of preoperative chemo-radiation therapy for resectable gastric cancer[31]. Surgery was safe after TCF preoperative chemotherapy, while toxicity (especially gastrointestinal) made adjuvant postoperative TCF more difficult to administer fully compared to the neoadjuvant setting. These data are consistent with the results of the recent FFCD trial[14], where postoperative chemotherapy was completed in less than 50% of the patients. This should be carefully considered when an intensive adjuvant chemotherapy regimen is planned.
In spite of a declining incidence in the Western world, gastric cancer is still a major malignant disease in many populations, and the second leading cause for cancer mortality worldwide. While localized disease, limited to the submucosa, can be best treated surgically, with a long-term survival rate of 70%-95%, the prognosis of locally-advanced tumor is poorer, due to a high unresectability rate at presentation, and a much higher relapse rate after radical surgery. Docetaxel (Taxotere®; Sanofi-Aventis, Paris, France) has been approved for treatment of metastatic gastric cancer, when combined with cisplatin and infused fluorouracil (TCF regimen), showing superiority in survival, time to progression, and response rate (RR) vs cisplatin/fluorouracil (CF) in a randomized phase III trial.
The above mentioned results obtained in metastatic disease suggested the investigational use of the TCF regimen in a preoperative neoadjuvant setting. The present trial aimed to test the hypothesis that preoperative chemotherapy with TCF does not influence negatively the results of subsequent surgery, when compared to immediate surgery.
This trial proved that surgery is safe after TCF preoperative chemotherapy, while toxicity (especially gastrointestinal) makes adjuvant postoperative TCF more difficult to administer fully compared to the neoadjuvant setting. Moreover, neoadjuvant TCF achieved promising results with a 12% pCR (pathological complete response) rate.
Obtained data regarding TCF efficacy and feasibility in the preoperative setting and TCF feasibility in the adjuvant setting could be interesting for future studies. In fact, patients achieving a pCR tend to have a much better oncology outcome. Finally, data here presented are consistent with the results of the recent FFCD trial, where postoperative chemotherapy was completed in less than 50% of the patients. This should be carefully considered when an intensive adjuvant chemotherapy regimen is planned.
This is an interesting report of the efficacy of neoadjuvant chemotherapy for advanced gastric cancer.
Peer reviewers: Takayoshi Kiba, MD, PhD, Foundation for Biomedical Research and Innovation, Translational Research Informatics Center, 1-5-4 Minatojima-minamimachi, Chou-ku, Kobe, Hyogo 650-0047, Japan; Toru Ishikawa, MD, Department of Gastroenterology, Saiseikai Niigata Second Hospital, Teraji 280-7, Niigata, Niigata 950-1104, Japan
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
| 1. | Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633-649. |
| 2. | Briasoulis E, Liakakos T, Dova L, Fatouros M, Tsekeris P, Roukos DH, Kappas AM. Selecting a specific pre- or postoperative adjuvant therapy for individual patients with operable gastric cancer. Expert Rev Anticancer Ther. 2006;6:931-939. |
| 3. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. |
| 4. | Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Saletti P, Köberle D, Borner MM, Rufibach K, Maibach R. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25:3217-3223. |
| 5. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. |
| 6. | Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma. First English Edition. Tokio: Kanehara & Co. Ltd 1995; . |
| 7. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma-2nd English Edition-. Gastric Cancer. 1998;1:10-24. |
| 8. | Siewert JR, Böttcher K, Roder JD, Busch R, Hermanek P, Meyer HJ. Prognostic relevance of systematic lymph node dissection in gastric carcinoma. German Gastric Carcinoma Study Group. Br J Surg. 1993;80:1015-1018. |
| 9. | Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, Van de Velde CJ. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441-1447. |
| 10. | Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059-1064. |
| 11. | Janunger KG, Hafström L, Glimelius B. Chemotherapy in gastric cancer: a review and updated meta-analysis. Eur J Surg. 2002;168:597-608. |
| 12. | Lim L, Michael M, Mann GB, Leong T. Adjuvant therapy in gastric cancer. J Clin Oncol. 2005;23:6220-6232. |
| 13. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. |
| 14. | Boige V, Pignon J, Saint-Aubert B, Lasser P, Conroy T, Bouché O, Segol P, Bedenne L, Rougier P, Ychou M. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD 07-FFCD 9703 trial [Abstract]. Proc Am Soc Clin Oncol. 2007;25:4510. |
| 15. | Biffi R, Chiappa A, Luca F, Pozzi S, Lo Faso F, Cenciarelli S, Andreoni B. Extended lymph node dissection without routine spleno-pancreatectomy for treatment of gastric cancer: low morbidity and mortality rates in a single center series of 250 patients. J Surg Oncol. 2006;93:394-400. |
| 16. | Degiuli M, Sasako M, Calgaro M, Garino M, Rebecchi F, Mineccia M, Scaglione D, Andreone D, Ponti A, Calvo F. Morbidity and mortality after D1 and D2 gastrectomy for cancer: interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol. 2004;30:303-308. |
| 17. | Sierra A, Regueira FM, Hernández-Lizoáin JL, Pardo F, Martínez-Gonzalez MA, A-Cienfuegos J. Role of the extended lymphadenectomy in gastric cancer surgery: experience in a single institution. Ann Surg Oncol. 2003;10:219-226. |
| 18. | Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530. |
| 19. | Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069-2077. |
| 20. | Braga M, Zuliani W, Foppa L, Di Carlo V, Cristallo M. Food intake and nutritional status after total gastrectomy: results of a nutritional follow-up. Br J Surg. 1988;75:477-480. |
| 21. | Cascinu S, Labianca R, Barone C, Santoro A, Carnaghi C, Cassano A, Beretta GD, Catalano V, Bertetto O, Barni S. Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J Natl Cancer Inst. 2007;99:601-607. |
| 22. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. |
| 23. | D'Ugo DM, Coppola R, Persiani R, Ronconi P, Caracciolo F, Picciocchi A. Immediately preoperative laparoscopic staging for gastric cancer. Surg Endosc. 1996;10:996-999. |
| 24. | Lehnert T, Rudek B, Kienle P, Buhl K, Herfarth C. Impact of diagnostic laparoscopy on the management of gastric cancer: prospective study of 120 consecutive patients with primary gastric adenocarcinoma. Br J Surg. 2002;89:471-475. |
| 25. | Hünerbein M, Rau B, Hohenberger P, Schlag PM. The role of staging laparoscopy for multimodal therapy of gastrointestinal cancer. Surg Endosc. 1998;12:921-925. |
| 26. | Nakagawa S, Nashimoto A, Yabusaki H. Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer. 2007;10:29-34. |
| 27. | Gretschel S, Siegel R, Estévez-Schwarz L, Hünerbein M, Schneider U, Schlag PM. Surgical strategies for gastric cancer with synchronous peritoneal carcinomatosis. Br J Surg. 2006;93:1530-1535. |
| 28. | Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, Noh SH. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005;103:2383-2390. |
| 29. | Lim JS, Kim MJ, Yun MJ, Oh YT, Kim JH, Hwang HS, Park MS, Cha SW, Lee JD, Noh SH. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol. 2006;7:249-256. |
| 30. | Hartgrink HH, van de Velde CJ, Putter H, Songun I, Tesselaar ME, Kranenbarg EK, de Vries JE, Wils JA, van der Bijl J, van Krieken JH. Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol. 2004;30:643-649. |
| 31. | Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953-3958. |