Published online Feb 21, 2010. doi: 10.3748/wjg.v16.i7.862
Revised: October 2, 2009
Accepted: October 9, 2009
Published online: February 21, 2010
AIM: To evaluate changes in colorectal cancer (CRC) survival over the last 20 years.
METHODS: We compared two groups of consecutive CRC patients that were prospectively recruited: Group I included 1990 patients diagnosed between 1980 and 1994. Group II included 871 patients diagnosed in 2001.
RESULTS: The average follow up time was 21 mo (1-229) for Group I and 50 mo (1-73.4) for Group II. Overall median survival was significantly longer in Group II than in Group I (73 mo vs 25 mo, P < 0.001) and the difference was significant for all tumor stages. Post surgical mortality was 8% for Group Iand 2% for Group II (P < 0.001). Only 17% of GroupI patients received chemotherapy compared with 50% of Group II patients (P < 0.001).
CONCLUSION: Survival in colorectal cancer patients has doubled over the past 20 years. This increase seems to be partly due to the generalization in the administration of chemotherapy and to the decrease of post surgical mortality.
- Citation: Bujanda L, Sarasqueta C, Hijona E, Hijona L, Cosme A, Gil I, Elorza JL, Asensio JI, Larburu S, Enríquez-Navascués JM, Jover R, Balaguer F, Llor X, Bessa X, Andreu M, Paya A, Castells A, Association GOGOTSG. Colorectal cancer prognosis twenty years later. World J Gastroenterol 2010; 16(7): 862-867
- URL: https://www.wjgnet.com/1007-9327/full/v16/i7/862.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i7.862
Colorectal cancer (CRC) is the second most common form of cancer and the second leading cause of cancer death in both men and women in most developed countries including Spain. Mortality has increased by an annual average of 2.6% for men and 0.8% for women since 1975, without variations[1]. It is estimated that CRC caused 11 900 deaths in Spain in 2000, which represents 11% of the total deaths from cancer in men and 15% of those in women[1].
The primary treatment for this condition is surgical resection. Despite resection of all macroscopic tumors, patients whose primary tumor has penetrated the serosa or that have regional lymph node metastases at the time of surgery have high recurrence rates. An effective adjuvant program to eradicate microscopic tumor foci is clearly needed for such high-risk patients[2]. A major advance in adjuvant treatment of CRC came with trials that explored the combination of 5-fluorouracil and levamisole or leucovorin (FL). Therapy with FL reduced the overall death rate by 33% relative to surgery alone in patients with stage III disease. During the last few years, sequential advances in chemotherapy after surgical resection (adjuvant chemotherapy) have had an irrefutable and substantial benefit[3].
The aim of the present study was to evaluate CRC survival over the last 20 years.
A total of 1990 patients patients diagnosed with CRC between 1980 and 1994 and 2001 were included in a prospective and consecutive manner. Patients were enrolled in two time periods: Group I included 1119 patients recruited between 1980 and 1994. Group II included 871 patients recruited during 2001. Patients were recruited as part of the EPICOLON project[4,5]. Age, sex, tumor features (location, TNM stage, differentiation) and type of chemotherapy administered, if any, was collected for all patients. Stage was defined according to the 4th Edition of the TNM classification[6].
EPICOLON was a prospective, multicenter, nationwide study that was set up to record consecutive cases of CRC in 25 hospitals in Spain over one year. The initial aim of the study was to determine the incidence and characteristics of familial forms of CRC in Spain.
Patients went through periodical follow up in medical consultations or by telephone. Patients with familial adenomatous polyposis or inflammatory bowel disease were excluded.
In order to evaluate survival trends, Group I was divided in three subgroups: patients diagnosed between 1980 and 1985 (343); patients diagnosed between 1986 and 1990 (392); and patients diagnosed between 1991 and 1994 (384).
Death during the first 30 d post surgery was considered postoperative mortality.
All patients provided written informed consent before enrollment in the study. The study was approved by the Institutional Review Board or Ethics Committee at each center and complied with the provisions of the Good Clinical Practice guidelines.
Continuous variables are defined with (mean ± SD). Discrete variables are defined by absolute and relative frequencies. The function of survival is calculated by the Kaplan Meier estimator. The accumulated probability of survival is presented at 3, 5 and 10 years of monitoring. The Log-Rank test is used to evaluate the differences in survival between the different categories of independent variables. Multivariable models are constructed using Cox regression analysis and the independent effect of each variable on mortality is estimated using Relative Risks (Hazard Ratio) and the respective confidence intervals of 95%. The SPSS v.15 program was used for analysis.
The average follow up time was 21 mo for Group I and 50 mo for Group II. Average follow up for deceased patients was 12 mo for Group I and 16 mo for Group II. For patients still alive, follow up was 67 mo for Group I and 60 mo for Group II.
The characteristics of the patients are described in Table 1. Both groups were similar regarding tumor stages at diagnose and tumor location. Significant differences were observed in sex and age: the average age of Group I was 66.7 years compared to 69 years in Group II; the percentage of men was 61% and 57%, respectively. Slight differences were also observed in tumor differentiation: Group I had 4% of poorly differentiated tumors and Group II 8%. The greatest differences were found in the proportion of patients that received chemotherapy: 17% in Group I and 47% in Group II.
| Group I (n = 1119) | Group II (n = 871) | P value | |
| Age mean (SD) | 66.7 (12.4) | 69.1 (11.5) | 0.000 |
| Sex | 0.027 | ||
| Males | 638 (57) | 531 (61) | |
| Females | 481 (43) | 340 (39) | |
| Tumor localization | |||
| Distal to splenic flexure | 831 (75) | 644 (74) | |
| Proximal to splenic flexure | 272 (25) | 227 (26) | |
| TNM | 0.11 | ||
| I | 190 (17) | 122 (14) | |
| II | 403 (36) | 348 (40) | |
| III | 302 (27) | 244 (28) | |
| IV | 224 (20) | 157 (18) | |
| Tumor differentiation | 0.000 | ||
| Poor | 45 (4) | 70 (8) | |
| Well-moderate | 1074 (96) | 801 (92) | |
| Receiving chemotherapy | 190 (17) | 409 (46.9) | 0.000 |
The probability of survival at 3 and 5 years was approximately 20% higher in Group II than in Group I . Three year survival was 44% for Group I and 65% for Group II. Five year survival was 35% for Group I and 57% for Group II.
Overall median survival was significantly longer in Group II than in Group I (73 mo vs 25 mo, P < 0.001). Three years after cancer diagnosis, 21% more patients were alive in Group II than in Group I.
Multivariate analysis showed 4 independent factors associated with a higher mortality risk (Table 2).
| Hazard ratio (95% CI) | |
| Stage | |
| II vs I | 1.6 (1.2–2.1) |
| III vs I | 3.5 (2.7–4.6) |
| IV vs I | 13.7 (10.4-18.0) |
| Group | |
| Ivs II | 2.0 (1.7–2.4) |
| Chemotherapy | |
| No vs Yes | 1.6 (1.3–1.9) |
| Grade | |
| I, II vs III | 1.5 (1.1–1.9) |
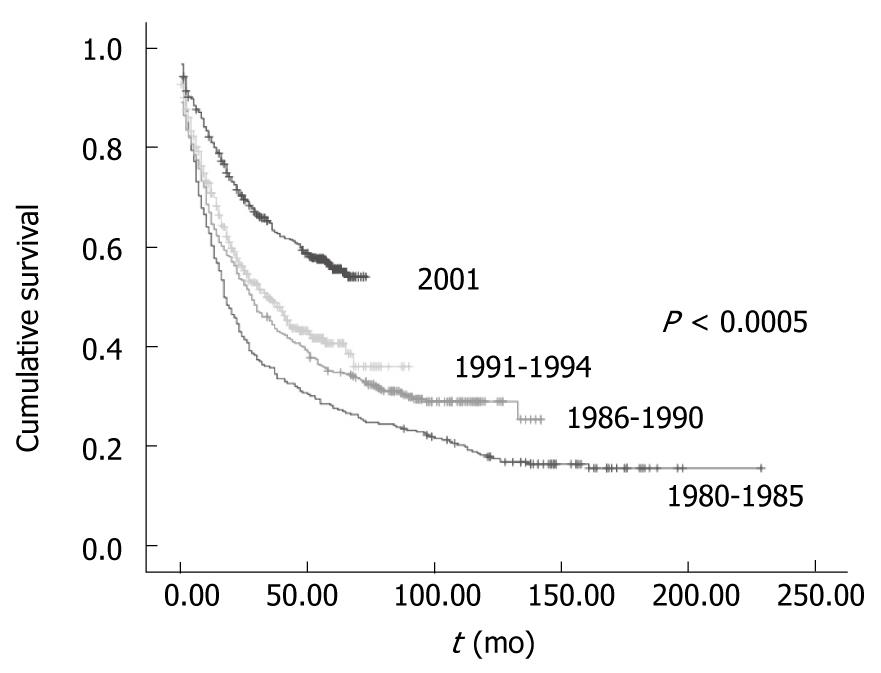
Survival in Group I: In Group I survival increased over the years in parallel to chemotherapy use (Figure 1). Ten percent of patients (35 patients) received chemotherapy between 1980 and 1985, 16% (63) in the period between 1986 and 1990 and 24% (92) in the period between 1991 and 1994. The median survival was 17 mo for the period between 1980 and 1985, 28 mo for the period between 1986 and 1990 and 34 mo for the period between 1991 and 1994 (Figure 1).
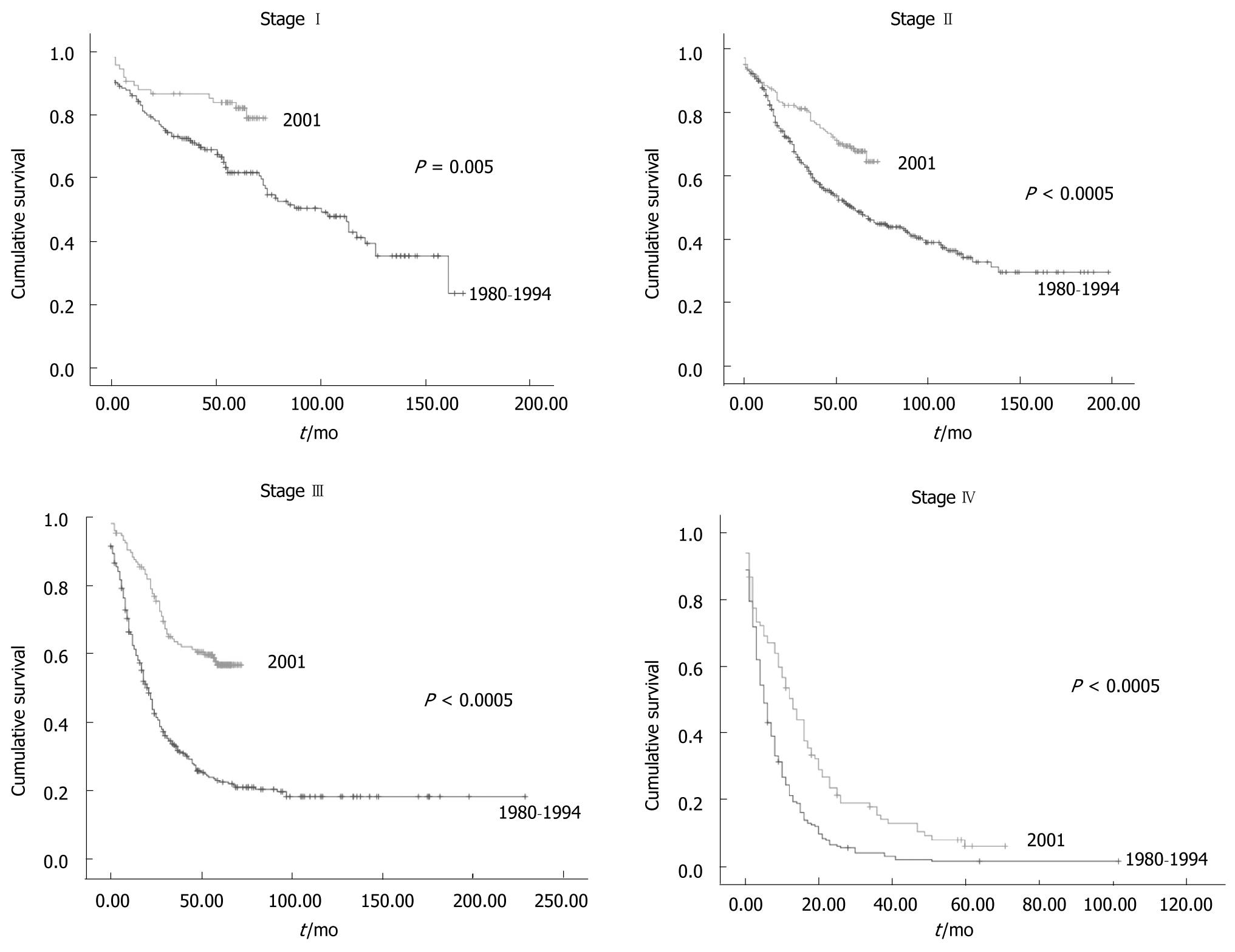
Survival was greater for group II than for group I for all tumor stages (Figure 2). Postoperative mortality was 8% for Group Iand 2% for Group II (Table 3). However, when patients in Group Iwho had died within 30 d of emergency surgery were excluded the postoperative mortality rate was equal to that in Group II. Postoperative mortality in patients operated electively in Group I was 3% (5 patients) for stageI , 2% (10 patients) for stage II, 3% (10 patients) for stage III and 4% (10 patients) for stage IV. These data were similar to Group II. Twenty six percent more patients received chemotherapy in Group II than in Group I for stage III CRC For the other tumor stages the percentage of patients that received chemotherapy was also greater in Group II, but to a smaller degree than stage III: 3% for stageI , 14% for stage II and 11% for stage IV.
| Stage I | Stage II | Stage III | Stage IV | |||||
| Group I (n = 190) | Group II (n = 122) | Group I (n = 403) | Group II (n = 348) | Group I (n = 302) | Group II (n = 244) | Group I (n = 224) | Group II (n = 157) | |
| Age | 66.7 | 70 | 66.7 | 70 | 66.7 | 70 | 67.5 | 70 |
| Rectal localization | 93 (49) | 65 (44) | 121 (30) | 115 (33) | 103 (34) | 81 (33) | 67 (30) | 49 (31) |
| Postoperative mortality | 17 (9) | 2 (2) | 20 (5) | 7 (2) | 21 (7) | 5 (2) | 29 (13) | 6 (4) |
| Chemotherapy and/or radiotherapy | 10 (5) | 10 (8) | 48 (12) | 90 (26) | 91 (30) | 137 (56) | 54 (24) | 55 (35) |
The last few years have seen great advances in treatment of CRC. The survival has increased substantially. In our study survival rate almost doubled over the last 20 years, from 35% to 57% at 5 years. Determining factors in this increased survival have been the generalization of chemotherapeutic agents and surgical advances, especially pertaining to rectal cancer.
The first chemotherapeutical agent used in the treatment of CRC was fluorouracil (FL) in 1958[7]. Since then and up to the last decade, FL has been the only drug used in CRC treatment. In 1988 a meta-analysis on the effect of FL showed a 10% reduction in the risk of death and an increase of 2.3% in survival after 5 years. In a subgroup of patients the risk of death was reduced by 17% and global survival after 5 years improved up to 34%[7]. Based on the results of this and other studies, the National Cancer Institute and the American Society of Clinical Oncology recommended FL-based post surgical treatment in their annual conference in 1997. In stage III disease, FL increases overall five year survival from about 51% to 64%[8]. The use of adjuvant FL in patients with stage II disease is controversial. FL in advanced CRC prolongs median survival by approximately 5 mo (6 mo without treatment to 11 mo with FL)[9].
The US Food and Drug Administration (FDA) has approved a host of new chemotherapeutic agents for advanced colon cancer and for relapsing disease. Since the year 2000, irinotecan was approved as a first line treatment in metastatic CRC. In 2002, oxaliplatin was approved for use along with other drugs as adjuvant treatment of relapsing CRC. Sequential exposure to various combinations of FL, irinotecan and oxaliplatin extends median overall survival by approximately 20 mo[10]. The FDA approved capecitabine in 2005. Bevacizumab, a monoclonal humanized antibody united with the vascular endothelial growth factor (VEGF) was approved by the FDA for metastatic colon cancer in 2005. The availability of these new agents has resulted in a sustained increase in the use of chemotherapy for CRC patients from 17% in the period of 1980 to 1994 to 50% in 2001. This has been key to the increase of survival, fundamentally in stage III and slightly less in the other CRC stages.
The other important factor that has contributed to an increased survival has been surgical improvements, specifically in the rectal area. Surgery-related mortality has consistently decreased over the years. For example, a Belgian study showed a decrease in surgical mortality from 20.1% between 1973 and 1979 to 7.8% between 1980 and 1986[11]. In our hospital, mortality decreased from 9.1% in 1981 to 8% in 1991[12]. In the last decade, the postoperative mortality for CRC varied between 1% and 9.9%[12-18]. Emergency surgery for CRC is associated with a high postoperative morbidity and mortality[14,18,19]. In rectal cancer, the number of abdomino-perineal resections has decreased from 26.3% in 1981 to 17.2% in 1991[12]. Between 1987 and 1992, the mortality in the Istituto di Patología Speciale Chirurgica of the University of Bologna was 7.6% in anterior resections and 14.2% in abdomino-perineal resections[20]. Our study also observed a significant decrease in the percentage of abdomino-perineal resections, from 30% in 1980 to 18% in 1994. The use of mechanical sutures in rectal cancer operations has allowed a higher rate of sphincter preservation after low anterior resection[21]. On the other hand, the number of anterior rectal resections has increased due to the use of mechanical sutures in the middle third of the rectum.
The availability since 1994 of self-expanding stents for obstructive colorectal cancer[22] has resulted in a dramatic decrease in the number of urgent palliative surgeries in patients with metastases and a subsequent decrease in the associated postoperative mortality in our study and others[23].
Other factors that we have not analyzed and may also have played a significant role in the increased survival include the use of new antibiotics, improvements in the pre- and post-surgical care of patients, the improved application of radiotherapy and chemotherapy in rectal cancer, the surgical treatment of metastases and the improvement in bowel cleansing. Another limitation of our study is that the Group II cohort was not entirely similar to that of Group I , however, it was a cohort suitable for comparing with Group I (20 years earlier) and showing the differences in different variables such as survival, postoperative mortality or the application of chemotherapy-radiotherapy. In both groups of patients data were collected consecutively and protocols followed strictly.
In conclusion, we observed the survival has increased steadily over the years and is now almost double that 20 years ago. This increase has been in parallel with an increase in the administration of adjuvant chemotherapy and a decline in postoperative mortality.
Colorectal cancer (CRC) is the second leading cause of cancer mortality. In the last two decades major advances have occurred in treatment. Adjuvant chemotherapy has extended its indications and incorporated new drugs. Surgery outcomes have also significantly improved, with less emergency operations, lower postoperative mortality and a decrease in the number of abdomino-perineal resections. There are few studies geared at analyzing these factors and their influence on CRC survival looking at a 20 year time span.
Numerous clinical trials have shown that chemotherapy and surgical advances have dramatically improved the prognosis in patients with CRC. Few studies outside the mentioned clinical trials have examined the impact of these advances on clinical outcome over the past two decades.
This study has quantified the effect of new adjuvant chemotherapy and improved surgical techniques on the prognosis of patients with CRC.
These data show that the increased use of chemotherapy for CRC and better surgical techniques are directly linked to improved survival.
This article compares survival differences between two cohorts of patients operated by colorectal cancer from 1980 to 1994 and 2001. It emphasizes the importance of the highest percentage of patients receiving chemotherapy and better postsurgical mortality in the last group doubled over the last 20 years.
Peer reviewer: Omar Vergara-Fernandez, MD, Departments of Surgery, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Vasco de Quiroga No. 15, Col. Seccion XVI. Deleg. Tlalpan,CP 14000, México
S- Editor Tian L L- Editor O’Neill M E- Editor Tian L
| 1. | Department of Health, Government of Spain. La situación del cáncer en España. Madrid. 2005;. |
| 2. | Bresalier RS; Malignant neoplasms of the large intestine. In: Feldman M, Friedman LS, editors. Sleisenger & Fordtran’s gastrointestinal and liver disease: pathophysiology, diaÂgnosis, management. Sleisenger. 7 th ed. Philadelphia, Pennsylvania: Saunders, 2002: 2215-2261. . |
| 3. | Allegra C, Sargent DJ. Adjuvant therapy for colon cancer--the pace quickens. N Engl J Med. 2005;352:2746-2748. |
| 4. | Piñol V, Castells A, Andreu M, Castellví-Bel S, Alenda C, Llor X, Xicola RM, Rodríguez-Moranta F, Payá A, Jover R, Bessa X; Gastrointestinal Oncology Group of the Spanish Gastroenterological Association. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA. 2005;293:1986-1994. |
| 5. | Rodríguez-Moranta F, Castells A, Andreu M, Piñol V, Castellví-Bel S, Alenda C, Llor X, Xicola RM, Jover R, Payá A, Bessa X, Balaguer F, Cubiella J, Argüello L, Morillas JD, Bujanda L; Gastrointestinal Oncology Group of the Spanish Gastroenterological Association. Clinical performance of original and revised Bethesda guidelines for the identification of MSH2/MLH1 gene carriers in patients with newly diagnosed colorectal cancer: proposal of a new and simpler set of recommendations. Am J Gastroenterol. 2006;101:1104-1111. |
| 6. | TNM Atlas. Ilustrated Guide to the TNM/pTNM Classification of Malignant Tumors, 4.ª ed. Berlín: Springer Verlag 1997; . |
| 7. | Curreri AR, Asnsfield FJ, McIver FA, Waisman HA, Heidelberger C. Clinical studies with 5-fluorouracil. Cancer Res. 1958;18:478-484. |
| 8. | Buyse M, Zeleniuch-Jacquotte A, Chalmers TC. Adjuvant therapy of colorectal cancer. Why we still don't know. JAMA. 1988;259:3571-3578. |
| 9. | Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, Benedetti J, Francini G, Shepherd LE, Francois Seitz J. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797-1806. |
| 10. | Mayer RJ. Two steps forward in the treatment of colorectal cancer. N Engl J Med. 2004;350:2406-2408. |
| 11. | Canivet JL, Damas P, Desaive C, Lamy M. Operative mortality following surgery for colorectal cancer. Br J Surg. 1989;76:745-747. |
| 12. | Ruiz Montesinos I, Elorza Orúe JL, Alcón Caracena A, Olaizola Ayerdi A, Irazusta Goena M, Álvarez Caperochipi FJ. Evolución del carcinoma colorrectal a lo largo de una década. Cir Esp. 1997;61:273-277. |
| 13. | Semmens JB, Platell C, Threlfall TJ, Holman CD. A population-based study of the incidence, mortality and outcomes in patients following surgery for colorectal cancer in Western Australia. Aust N Z J Surg. 2000;70:11-18. |
| 14. | Nickelsen TN, Jørgensen T, Kronborg O. Thirty-day mortality after surgery for colorectal cancer in Denmark. Colorectal Dis. 2005;7:500-506. |
| 15. | Glen P, Simpson MF, Donnelly L, Leonard S, Macdonald A. Thirty-day mortality from colorectal cancer surgery within a deprived population. Colorectal Dis. 2005;7:193-195. |
| 16. | Alves A, Panis Y, Mathieu P, Mantion G, Kwiatkowski F, Slim K. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg. 2005;140:278-283, discussion 284. |
| 17. | Ong ES, Alassas M, Dunn KB, Rajput A. Colorectal cancer surgery in the elderly: acceptable morbidity? Am J Surg. 2008;195:344-348; discussion 348. |
| 18. | Kelz RR, Gimotty PA, Polsky D, Norman S, Fraker D, DeMichele A. Morbidity and mortality of colorectal carcinoma surgery differs by insurance status. Cancer. 2004;101:2187-2194. |
| 19. | Itani KM, Denwood R, Schifftner T, Joehl RJ, Wright C, Henderson WG, DePalma RG. Causes of high mortality in colorectal surgery: a review of episodes of care in Veterans Affairs hospitals. Am J Surg. 2007;194:639-645. |
| 20. | Boschi L, Lecce F, Bazzocchi F, Del Gaudio A. [Surgical treatment of rectal cancer: comparison of anterior resection and abdominoperineal excision]. Minerva Chir. 1995;50:831-834. |
| 21. | Crucitti F, Sofo L, Doglietto GB, Bellantone R, Perri V, Zucchetti F. [Mechanical sutures in surgery of cancer of the rectum (a personal contribution)]. Chir Ital. 1983;35:823-830. |
| 22. | Tejero E, Mainar A, Fernández L, Tobío R, De Gregorio MA. New procedure for the treatment of colorectal neoplastic obstructions. Dis Colon Rectum. 1994;37:1158-1159. |
| 23. | Tilney HS, Lovegrove RE, Purkayastha S, Sains PS, Weston-Petrides GK, Darzi AW, Tekkis PP, Heriot AG. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc. 2007;21:225-233. |