Published online Feb 21, 2010. doi: 10.3748/wjg.v16.i7.837
Revised: December 23, 2009
Accepted: December 30, 2009
Published online: February 21, 2010
AIM: To investigate the pharmacological effect of JCM-16021, a Chinese herbal formula, and its underlying mechanisms.
METHODS: JCM-16021 is composed of seven herbal plant materials. All raw materials of the formula were examined according to the quality control criteria listed in the Chinese Pharmacopeia (2005). In a neonatal maternal separation (NMS) model, male Sprague-Dawley rats were submitted to daily maternal separation from postnatal day 2 to day 14, or no specific handling (NH). Starting from postnatal day 60, rats were administered JCM-16021 (2, 4, 8 g/kg per day) orally twice a day for 28 d. Pain threshold pressure and electromyographic activities of external oblique muscles in response to colorectal distention recorded with a Power Lab System (AD Instruments International), were tested as pain indices. Changes in serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) concentrations in the colon of rats were analyzed; the enterochromaffin cell numbers and serotonin transporter in the colon of rats were also evaluated with an immunohistochemistry method.
RESULTS: NMS treatment significantly reduced pain threshold pressure (37.4 ± 1.4 mmHg), as compared to that of NH rats (57.7 ± 1.9 mmHg, P < 0.05). After JCM-16021 treatment, the pain threshold pressure significantly increased when compared to that before treatment (34.2 ± 0.9 mmHg vs 52.8 ± 2.3 mmHg in the high dose group, 40.2 ± 1.6 mmHg vs 46.5 ± 1.3 mmHg in the middle dose group, and 39.3 ± 0.7 mmHg vs 46.5 ± 1.6 mmHg in the low dose group, P < 0.05). Also JCM-16021 significantly and dose-dependently decreased electromyographic activity to the graded colorectal distension (CRD), (the mean ΔAUC values were: 0.17 ± 0.03, 0.53 ± 0.15, 1.06 ± 0.18, 1.22 ± 0.24 in the high dose group; 0.23 ± 0.04, 0.68 ± 0.17, 1.27 ± 0.26, 1.8 ± 0.3 in the middle dose group; and 0.29 ± 0.06, 0.8 ± 0.16, 1.53 ± 0.24, 2.1 ± 0.21 in the low dose group for the pressures 20, 40, 60, 80 mmHg), as compared to the NMS vehicle group. The mean ΔAUC values were: 0.57 ± 0.12, 1.33 ± 0.18, 2.57 ± 0.37, 3.08 ± 0.37 for the pressures 20, 40, 60, 80 mmHg (P < 0.05). JCM-16021 treatment significantly reduced the 5-HT concentrations (from high, middle and low dosage groups: 60.25 ± 5.98 ng/100 mg, 60.32 ± 4.22 ng/100 mg, 73.31 ± 7.65 ng/100 mg), as compared to the NMS vehicle groups (93.11 ± 9.85 ng/100 mg, P < 0.05); and increased the 5-HIAA concentrations (after treatment, from high, middle and low dosage groups: 54.24 ± 3.27 ng/100 mg, 50.34 ± 1.26 ng/100 mg, 51.37 ± 2.13 ng/100 mg) when compared to that in the NMS vehicle group (51.75 ± 1.98 ng/100 mg, P < 0.05); but did not change the enterochromaffin cell numbers in the colon of rats. In addition, NMS rats had higher SERT expression (n = 10) than NH rats (n = 8, P < 0.05). JCM-16021 treatment significantly decreased SERT expression when compared to the NMS group (P < 0.01-0.001).
CONCLUSION: JCM-16021 can attenuate visceral hypersensitivity, and this analgesic effect may be mediated through the serotonin signaling pathway in the colon of rats.
- Citation: Bian ZX, Zhang M, Han QB, Xu HX, Sung JJ. Analgesic effects of JCM-16021 on neonatal maternal separation-induced visceral pain in rats. World J Gastroenterol 2010; 16(7): 837-845
- URL: https://www.wjgnet.com/1007-9327/full/v16/i7/837.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i7.837
Irritable bowel syndrome (IBS) is characterized by chronic abdominal pain and altered bowel movements such as diarrhea and constipation[1,2]. Although conventional therapies (e.g. laxatives, antidepressants, antispasmodics, and bulking agents) are used to relieve the symptoms of IBS, the overall efficacy of these agents is poor[3,4]; and these agents have not been proven to be more effective than placebo in providing overall relief of symptoms in randomized, controlled clinical trials. Therefore, increasing numbers of IBS sufferers are seeking help from complementary and alternative medicines. Recently, our research group showed that JCM-16021, an herbal formula composed of seven herbs, can relieve symptoms in IBS patients[5]. In this randomized, double-blinded, and placebo-controlled trial, 84 diarrhea-predominant IBS patients received treatment (28 patients in each arm). At the end of the 8 wk treatment, 52% of participants in the JCM-16021 plus Holopon (hyoscine methobromide)-placebo group (Group A), 32% in the Holopon plus herbal-placebo group (Group B), and 42.7% in the double placebo group (Group C) experienced overall symptom improvements. Patients in Group A had the highest percentage improvement (Group A vs Group B vs Group C: 52% vs 32% vs 42.7%), but the mechanism of this effect remains unclear.
Serotonin (5-HT), an important neurotransmitter and paracrine signaling molecule, alters visceral perception and motor function by influencing the sympathetic, parasympathetic, and enteric nervous systems[6]. The majority of 5-HT is synthesized and stored in enterochromaffin (EC) cells in the gastrointestinal tract. Previous studies have shown that the changes in EC cells and the increased 5-HT concentrations in the human colon are associated with the generation of IBS symptoms and in other gastrointestinal functional disorders[7-10]. Furthermore, novel serotonergic agents, such as the 5-HT3 antagonist alosetron and the 5-HT4 agonist tegaserod, have significant impacts on IBS symptoms through their visceral analgesic properties and diverse effects on motor functions in the lower gastrointestinal tract[11]. Therefore, the 5-HT signaling pathway represents a promising target for IBS treatment.
A neonatal maternal separation (NMS)-induced visceral hyperalgesia rat model was previously established[12]. Because its characteristics mimic the symptoms of IBS patients, it is often used to study the mechanism of visceral hyperalgesia and to evaluate the pharmacological effects of potential IBS therapies[13,14].
Considering the effects of JCM-16021 in IBS patients and the function of serotonin in visceral hyperalgesia, this study aimed to investigate the analgesic effect of JCM-16021 on NMS-induced visceral hyperalgesia in rats, and its potential underlying mechanism. We hypothesized that JCM-16021 could attenuate visceral pain through the 5-HT signaling pathway in the colon of rats. These results were previously presented as a poster at the 16th United European Gastroenterology Week in October 2008 in Vienna, Austria[15].
JCM-16021 is composed of seven plant materials, which are listed in Table 1. Purchasing, authentication and quality control of all seven herbs were performed based on the requirements of the Chinese Pharmacopoeia[16]. The authenticated voucher specimens (the voucher numbers are CMED-0043-02, CMED-0018-17, CMED0024-02, CMED-0044-02, CMED-0180-02, CMED-0179-02, CMED-0118-02) were stored in the Research Laboratory, Hong Kong Jockey Club Institute of Chinese Medicine, Hong Kong, China.
| Composition and samples | Atractylone1 | Gallic acid1 | Corilagin2 | Paeoniflorin3 | Magnolol4 | Honokiol5 | Tetrahydropalmatine6 | Quercitrin7 | Heavy metal & pesticide residues |
| Fructus Terminaliae Chebulae (Terminalia chebula Retz.) 9% | + | 2.18% | Pass | ||||||
| Radix Paeoniae Lactiflorae (Paeonia lactiflora Pall.) 14% | 0.45% | Pass | |||||||
| Cortex Magnoliae Officinalis (Magnolia officinalis Rehd. et Wils.) 9% | 1.76% | 0.89% | Pass | ||||||
| Rhizoma Corydalis Yanhusuo (Corydalis yanhusuo W. T. Wang) 14% | 0.10% | Pass | |||||||
| Herba Polygoni Chinensis (Polygonum chinense L) 18% | 0.04% | Pass | |||||||
| Rhizoma Atractylodis Macrocephalae (Atractylodes macrocephala Koidz) 18% | + | Pass | |||||||
| Semen coicis Lachryma-jobi [Coix lacryma-jobi L. var. ma-yuan (Roman.) Stapf] 18% | Pass | ||||||||
| Final product | - | + | 2.43% | 0.70% | ND | ND | 0.01% | ND | Pass |
Chloral hydrate was purchased from Fluka. Hematoxylin, 5-HT, and 5-HIAA were purchased from Sigma (Sigma-Aldrich Co., St. Louis, MO, USA). Holopon (hyoscine methobromide, 99%) was purchased from GSK Hong Kong.
JCM-16021 was prepared in the form of granules as follows: mixed medicinal materials weighing approximately 110 g (equal to the total amount of raw materials in one day’s dosage of JCM-16021 formula for IBS patients) were macerated for 30 min, subsequently decocted for 60 min three times, and rinsed 10 times (v/w) with distilled water. The filtrates were combined and dried in a vacuum at 40°C. A water-soluble pale yellow powder, approximately 22 g, was obtained. To ensure the quality of the final product, all raw materials were examined according to the quality control criteria listed in the Chinese Pharmacopeia 2005[16]. As recommended by the Chinese Pharmacopeia 2005[16], paeoniflorin, magnolol, honokiol, and tetrahydropalmatine were selected as the chemical markers for Radix Paeoniae Lactiflorae, Cortex Magnoliae Officinalis, and Rhizoma Corydalis Yanhusuo, respectively. Corilagin, a constituent of Fructus Terminaliae Chebulae was also selected since it is a major component of the final product. Quercitrin was used as the chemical marker of Herba Polygoni Chinensis. In addition, gallic acid and atractylone were qualitatively checked in Fructus Terminaliae Chebulae and Rhizoma Atractylodis Macrocephalae, respectively. Heavy metals and pesticide residues were monitored to ensure safety.
Primiparous timed-pregnant Sprague-Dawley female rats were obtained from the Laboratory Animal Services Centre, The Chinese University of Hong Kong, on gestational day 13-14. Dams were housed individually in macrolon cages and maintained in rooms with temperature kept at 23 ± 2°C and an alternating 12: 12 h light-dark cycle. All of the experimental protocols were carried out with the approval of the Committee on Use of Human-Animal Subjects in Teaching and Research of the Hong Kong Baptist University and according to the Regulations of the Department of Health, Hong Kong, China.
The neonatal maternal separation (NMS) rat model was established based on a previous report[12]. Briefly, pups in the NMS group were separated from their mothers and placed into individual cages in another room 180 min daily from postnatal day 2 to day 14, whereas normally-handled (NH) pups remained undisturbed in their home cage with the dam. All pups were weaned on postnatal day 22, and only male pups were used in the present study to avoid hormonal cycle induced variations. Male rats on postnatal day 60 were used in a series of three experiments.
This experiment involved three sets of studies: The first series of experiments aimed to evaluate the pharmacological effects of JCM-16021 on visceral pain by assessing changes in pain threshold pressure before and after JCM-16021 treatment. Six groups of rats were used. Group 1 (n = 10) with NH rats and Group 2 (n = 10) with NMS rats were given distilled water as a control. Groups 3, 4 and 5 (n = 10, 9, 9) with NMS rats received JCM-16021 at 8, 4 and 2 g/kg per day, respectively. Group 6 received 0.3 mg/kg per day Holopon (hyoscine methobromide, 99%, GSK Hong Kong) as an active control (n = 8). All pain threshold pressure detection tests were conducted in the morning between 9 am and 12 pm.
The second series of experiments aimed to test the analgesic effect of JCM-16021 through assessing electromyographic (EMG) activities of the left external abdominal oblique muscles to colorectal distension (CRD) before and after treatment with JCM-16021. Grouping (n = 7-10) was the same as that in the first series, with surgeries performed on treatment day 23, and EMG recording conducted on treatment day 28. The pain threshold test was not performed in this set of rats.
A third series of experiments with five groups (46 rats, n = 8-10 each group) of rats aimed to test the effects of JCM-16021 on the concentration of 5-HT, 5-hydroxyindoleacetic acid (5-HIAA), EC cell number and expression of serotonin transporter (SERT). Group 1 with NH rats and Group 2 with NMS rats received distilled water as a control. Groups 3, 4 and 5 with NMS rats received JCM-16021 at dosages of 8, 4, and 2 g/kg per day respectively. When the treatment course finished, the rats were deeply anesthetized with an overdose of midazolam hydrochloride, and 4 cm of the colon (5-6 cm from the anus) was harvested immediately. A piece of the colon was immediately fixed in 4% neutral-buffered paraformaldehyde for immunostaining. The rest was frozen with liquid nitrogen, and stored at -80°C for later analysis of 5-HT/5-HIAA content.
Starting at postnatal day 60 (body weight around 250 g), rats were daily treated orally with different dosages of JCM-16021 (8, 4 and 2 g/kg per day body weight, in 10 mL/kg distilled water), Holopon at a dosage of 0.3 mg/kg per day body weight, or vehicle (distilled water at 10 mL/kg body weight). The dosage of JCM-16021 was set according to the clinical trial and 4 g/kg per day was determined to be equivalent to the clinical dosage[5].
AWR tests were performed as previously described[17]. Briefly, a flexible latex balloon (medical finger glove, 4 cm long, 2.3 cm diameter flaccid) was inserted into the rat colon. Rats were allowed to adapt in a transparent box alone for 30 min after insertion of the colorectal balloon. CRD was then applied in increments of 5 mmHg, maintained for 2 s at each step to observe. The pain threshold pressure was defined as the intensity of CRD that induced a sudden and persistent abdominal muscle contraction in rats with abdomen lift off the platform. The experiments were repeated three times with at least 5 min intervals for recovery. During the test, the observers were blinded to the treatment groups of the rats.
To measure rat’s visceral sensitivity, the visceral motor response (VMR) to CRD was studied by recording the EMG as previously described with modification[12,18]. Briefly, a pair of Teflon-coated stainless wires (Cooner Wire, Chatsworth, CA) was surgically implanted into the left external abdominal oblique muscles on treatment day 23 and EMG recording tests were conducted on treatment day 28. On the test day, animals were subjected to CRD. A flexible latex balloon (medical finger glove, 4 cm long, 2.3 cm diameter flaccid) tied around a urethral catheter (3 mm diameter) was lubricated with liquid paraffin oil and inserted intrarectally in its descending colon with the distal tip 1 cm from the anal verge and secured to the base of the tail under short ether anesthesia. CRD was initiated by Barostat (Distender Series II R, G&J electronics, Canada). The EMG recording signal was amplified and filtered (50-5000 Hz) by Power Lab System (AD Instruments International). Three cycles of graded CRD (20, 40, 60, and 80 mmHg; 20 s duration; 2 min inter-stimulus interval) were applied to each rat. During the five day recovery from surgery, the treatments were continued. The overall effect of any given reagents was determined by calculating the changes of the area under the curve (AUC) of the raw EMG amplitude response after treatment, based on the formula ΔAUC% baseline = (AUC during CRD - AUC before CRD)/AUC before CRD.
5-HT and 5-HIAA concentrations in the colon were analyzed following a procedure with a slight modification[19]. Fluorescence of the handled sample was measured at an activation wavelength of 365 nm and an emission wavelength of 470 nm.
Immunohistochemical detection of EC cells in the colon of rats was performed using a routine streptavidin-biotin peroxidase technique employing Chr-A antibody (1:250, Santa Cruz Biotechnology, Santa Cruz, CA, USA)[20] and mouse monoclonal anti-SERT antibody (1:250, Advanced targeting system, AB-N09) as previously described[19]. The immunoreaction products were observed under a light NIKON microscope equipped with a NIKON color digital camera system. A NIKON 20 × objective was used to collect images of colon sections. The mean densities of the positive immunoreaction of serotonin transporter receptors and the positive cell numbers of Chr-A in at least 6 serial slides from the colonic sections of each rat were analyzed.
All data are expressed as mean ± SE. The changes in visceral pain threshold pressure were analyzed by comparing the values before and after treatment for each group using a paired t-test, and the differences between before and after treatment in a group using a one-way analysis of variance (ANOVA). EMG activity data were analyzed by one-way ANOVA between different groups to determine whether the overall change was significant, in a similar way to the analysis of the changes of concentrations of 5-HT and 5-HIAA, EC cell numbers and SERT in rat colon. P < 0.05 was considered statistically significant.
To ensure the quality of JCM-16021, eight chemical markers were qualitatively and quantitatively tracked from the raw materials to the final product, and heavy metals and pesticide residues were also examined. The results are listed in Table 1[5].
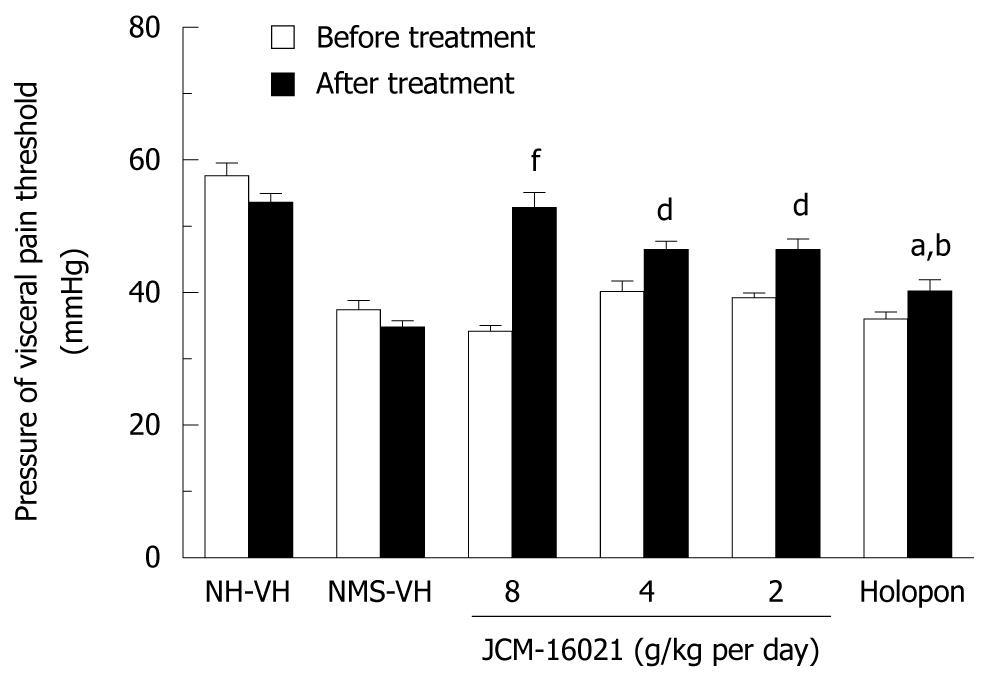
As shown in Figure 1, there is a significant decrease in pain threshold pressure in the NMS vehicle rats before the treatment, when comparing with that of the NH vehicle group before the treatment (P < 0.001). The pain threshold pressure values were 37.4 ± 4.4 mmHg and 57.7 ± 5.9 mmHg in NMS vehicle and NH vehicle groups before treatment, respectively.
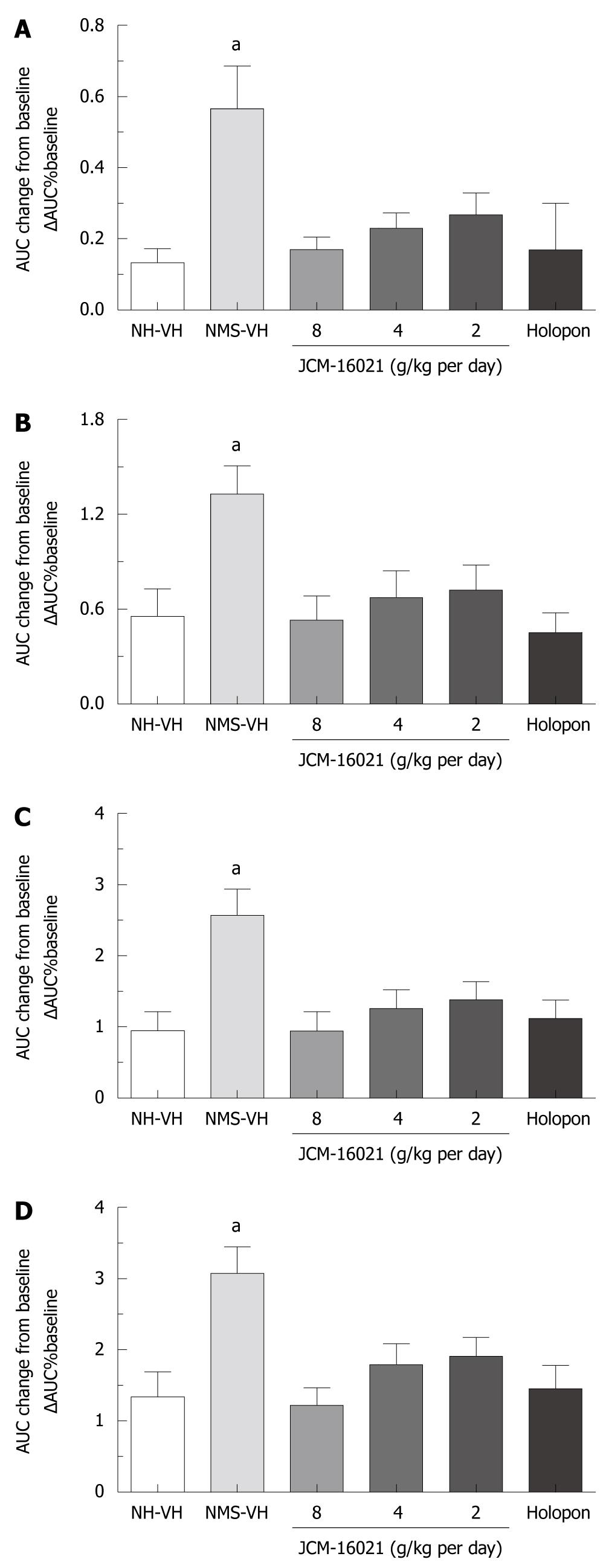
In EMG tests, the visceromotor response to CRD, which was reflected as AUC changes over the baseline in the NMS vehicle group after vehicle treatment (0.57 ± 0.12, 1.33 ± 0.18, 2.57 ± 0.37, 3.08 ± 0.37 under the pressures 20, 40, 60, 80 mmHg) was significantly increased compared to that of the NH vehicle group after vehicle treatment (0.11 ± 0.04, 0.58 ± 0.19, 0.96 ± 0.3, 1.39 ± 0.39 under the pressures 20, 40, 60, 80 mmHg, Figure 2) (P < 0.05). These results indicate that NMS induces allodynia (20 mmHg) and visceral hyperalgesia (40-80 mmHg) in rats.
As shown in Figure 1, JCM-16021 can significantly reduce the pain threshold pressure in three dosage groups (from high dose to low dose: 52.8 ± 2.3 mmHg, 46.5 ± 1.3 mmHg, and 46.5 ± 1.6 mmHg) comparing with that of the NMS vehicle group (34.8 ± 0.9 mmHg, P < 0.05). After treatment the pain threshold pressure in three JCM-16021 groups also significantly decreased when compared to that before treatment (from high dose to low dose: 52.8 ± 2.3 mmHg vs 34.2 ± 0.9 mmHg, 46.5 ± 1.3 mmHg vs 40.2 ± 1.6 mmHg and 46.5 ± 1.6 mmHg vs 39.3 ± 0.7 mmHg, P < 0.01). Holopon also had a similar analgesic effect to JCM-16021 when comparing the pain threshold pressure values either with that of NMS vehicle group or the value before the Holopon treatment.
In the EMG test, as shown in Figure 2A-D, the EMG activity to the graded CRD, which was reflected as AUC changes over the baseline, significantly and dose-dependently decreased after JCM-16021 treatment compared to that of the NMS vehicle group (P < 0.05). The mean ΔAUC significantly fell in the high dose group (0.17 ± 0.03, 0.53 ± 0.15, 1.06 ± 0.18, 1.22 ± 0.24 for the pressures 20, 40, 60, 80 mmHg), middle dose group (0.23 ± 0.04, 0.68 ± 0.17, 1.27 ± 0.26, 1.8 ± 0.3 for the pressures 20, 40, 60, 80 mmHg), and low dose group (0.29 ± 0.06, 0.8 ± 0.16, 1.53 ± 0.24, 2.1 ± 0.21 for the pressures 20, 40, 60, 80 mmHg), compared to that of the NMS vehicle group. The mean ΔAUC values were: 0.57 ± 0.12, 1.33 ± 0.18, 2.57 ± 0.37, 3.08 ± 0.37 for the pressures 20, 40, 60, 80 mmHg, P < 0.05. Also Holopon significantly reduced the EMG activity compared to the NMS vehicle group (P < 0.05).
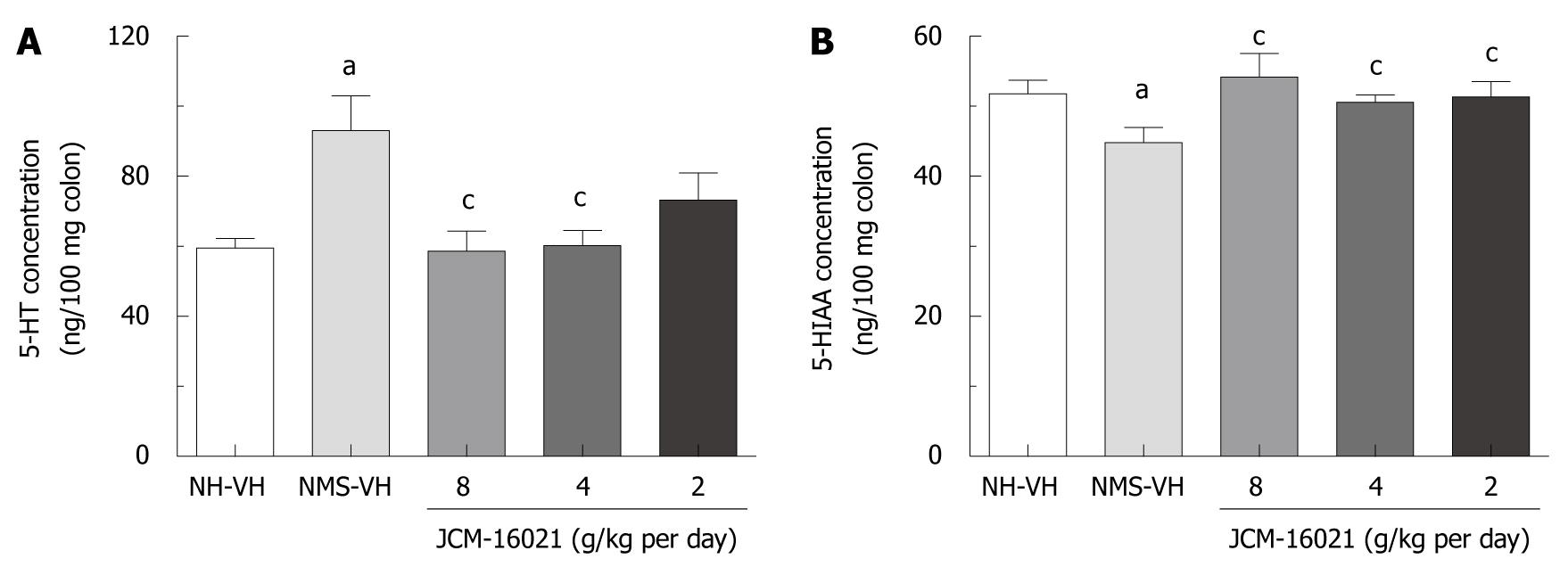
As shown in Figure 3A, 5-HT concentration in NMS vehicle groups (n = 9, 93.11 ± 9.85 ng/100 mg) was significantly higher than that in NH vehicle groups (n = 8, 59.53 ± 7.57 ng/100 mg, P < 0.01). JCM-16021 treatment at the high and middle dosage, but not the low dosage, significantly decreased the 5-HT concentration in the colon of rats (P < 0.01), when compared to the NMS vehicle group. After treatment with JCM-16021, the 5-HT concentrations in high, middle and low dosage groups (n = 9, 10, 10) were 60.25 ± 5.98 ng/100 mg, 60.32 ± 4.22 ng/100 mg, 73.31 ± 7.65 ng/100 mg, respectively. Further, although there was no significant difference in 5-HT concentration between the low dosage group and NMS vehicle group, the value was still lower than that of the NMS-VH group (93.11 ± 9.85 ng/100 mg). Clearly, there is a tendency that JCM-16021 treatment could reduce the 5-HT concentration.
NMS treatment significantly decreased 5-HIAA concentration in the colon of rats. 5-HIAA concentration in NMS vehicle groups (n = 9, 44.86 ± 2.13 ng/100 mg) was significantly higher than that in NH vehicle groups (n = 8, 51.75 ± 1.98 ng/100 mg, P < 0.05). JCM-16021 treatment significantly increased the 5-HIAA concentration in the colon of rats when compared with that of the NMS control group (P < 0.05). After treatment with JCM-16021, the 5-HIAA concentrations were 54.24 ± 9.81 ng/100 mg in the high dosage group, 50.61 ± 1.26 ng/100 mg in the middle dosage group, and 51.37 ± 2.13 ng/100 mg in the low dosage group (Figure 3B).
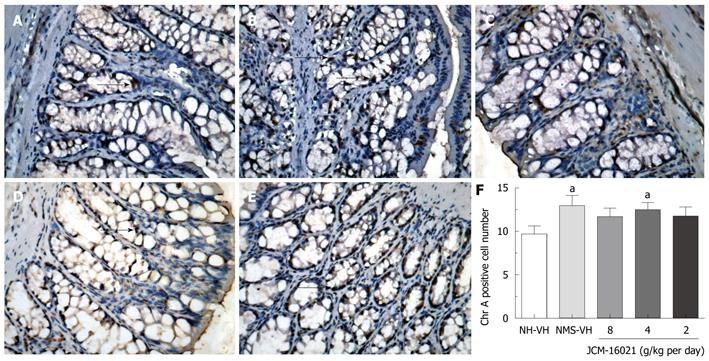
As shown in Figure 4, EC cell number in NMS vehicle groups (n = 9, 12.97 ± 1.17) was significantly higher than that in NH vehicle groups (n = 8, 9.70 ± 0.92, P < 0.05). JCM-16021 treatment did not significantly change the EC cell number in the three dose groups.
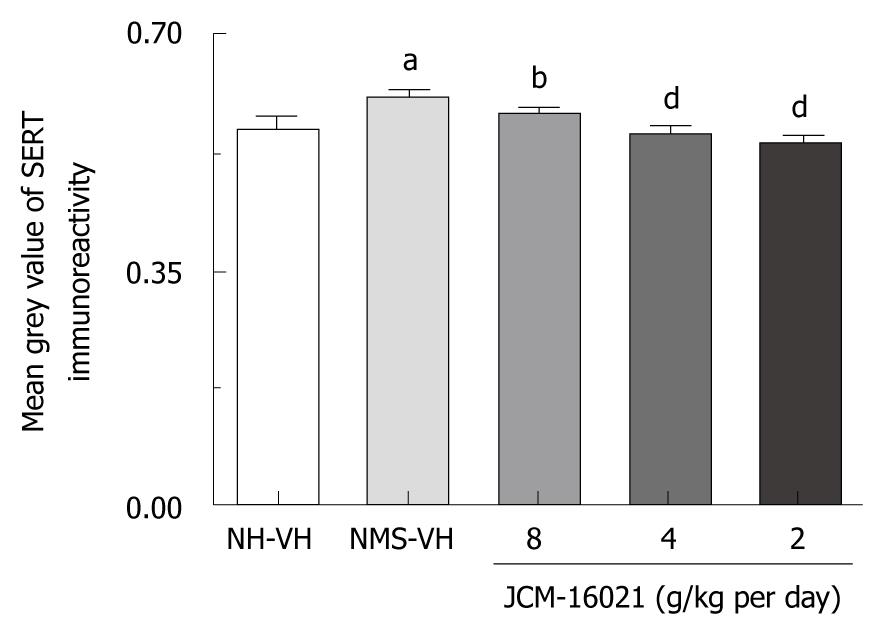
As shown in Figure 5, NMS rats had higher SERT expression (0.61 ± 0.03, n = 10) than NH rats (0.56 ± 0.05, n = 8, P < 0.05). JCM-16021 treatment at different dosages (n = 10 in each group) significantly decreased SERT expression in the colon. The mean gray indexes among JCM-16021 groups were 0.59 ± 0.03 in the high dose group, 0.54 ± 0.02 in the middle dose group and 0.53 ± 0.03 in the low dose group (P < 0.01-0.001).
This study demonstrated that JCM-16021 can dose-dependently attenuate the visceromotor response to CRD in NMS rats. Moreover, it decreases 5-HT concentration and increases 5-HIAA concentration in the colon of rats. These findings indicate that JCM-16021 has an analgesic effect on visceral hyperalgesia, and this effect may be mediated through the serotonin signaling pathway in the colon of rats.
Chronic visceral hyperalgesia is an important and characteristic feature of IBS and other functional bowel disorders[21]. In order to investigate the mechanism of visceral hyperalgesia, animal models have been developed, such as the early life colon irritation model[17], neonatal maternal separation model[12], and adult repeated stress model in rodents[22]. The current study showed that NMS induced a lower pain threshold pressure than that seen in NH rats, and increased EMG activity in response to CRD, thus confirming that NMS induces visceral hyperalgesia in adulthood[12]. Further, our results also showed that even in 20 mmHg CRD stimulation, NMS rats still have significant EMG changes compared to NH rats, thus NMS induces not only visceral hyperalgesia, but also allodynia in adulthood. Our results also showed that JCM-16021 increased the pain threshold pressure with a dose-related effect, and dose-dependently reduced the EMG activity to CRD in NMS rats. Therefore, these results indicate that JCM-16021 has an analgesic effect which can attenuate allodynia and visceral pain in NMS rats. Interestingly, the results from the AWR test showed that there is no dose-related response, but the results from the EMG test did show a dose-dependent response. The difference may originate from the objectivity of the two pain indexes; the EMG data, as a quantitative value, is more reliable than that of pain threshold pressure.
As for the mechanism of visceral hyperalgesia, it is believed that the up-regulation of visceral pain perception results, at least in part, from profound and long-lasting changes in the development of the central nervous system, including systems that regulate stress responsiveness[23,24]. With regard to the 5-HT effect in visceral hyperalgesia, previous data is not consistent. Our previous study reported that the amount of colonic 5-HT in rats with visceral hyperalgesia induced by mechanical colorectal irritation significantly increased with postnatal day[19]. Another study showed that serotonin is actively involved in pathophysiological processes of visceral hyperalgesia because serotonin concentration is significantly decreased in the spinal cord and but not in the colon; and 5-HT significantly increased in the colons of rats after CRD[13]. It is well known that 5-HT in the gastrointestinal tract is generally believed to be one of the most important mediators and regulators of bowel sensation and motility[9,25]. The current study found that 5-HT concentrations in the colons of the NMS vehicle group were significantly higher than that in the NH vehicle group supporting the concept that 5-HT is an important mediator involved in NMS-induced visceral hyperalgesia.
Our data also showed that JCM-16021 not only significantly reduces the 5-HT concentration but also significantly increases the 5-HIAA concentration. Serotonin, as an important gastrointestinal signaling molecule, is synthesized from the amino acid tryptophan via a short metabolic pathway consisting of two enzymes: tryptophan hydroxylase and amino acid decarbocarboxylase. Recent studies have demonstrated that distinct changes in intestinal EC cell number and 5-HT content have significant relationships with symptoms in IBS patients[26,27]. Therefore, the serotonin signaling pathway has been proposed as a therapeutic target to improve the symptoms of IBS[11]. Our current study found that NMS not only increases 5-HT concentration but also decreases the levels of its metabolite 5-HIAA in the colon of rats. After JCM-16021 treatment, the 5-HT concentration was decreased while 5-HIAA concentration increased. 5-HIAA is a major product of 5-HT breakdown, which is excreted in the urine. The increase in 5-HIAA indicates that more 5-HT was broken down after JCM-16021 treatment. Therefore, the data indicate that JCM-16021 affected 5-HT action and its metabolism in the colon.
It is well-known that a large proportion of 5-HT in the body is found in the gastrointestinal tract, and is primarily contained within EC cells[26]. This study found that EC cell number in NMS control rats is higher than that in NH control rats. It is possible that NMS induces hyperplasia of EC cells, thus NMS rats have higher concentrations of 5-HT in their colons compared to NH rats. Our results also showed that JCM-16021 cannot significantly change EC cell number in the colon of rats. This suggests that the hyperplasia of EC cells may be a permanent change similar to the elevated activation of the cingulate cortex and sensitization of the ascending pathway involving the spinal cord and the thalamo-cortico-amygdala pathway[24]. Therefore, EC cell number was not changed with treatment.
SERT is necessary for termination of serotonergic action in the colon. After the release by EC cells, serotonin is taken up again from the mucosa into the nerve fibers[11]. Altered SERT expression and function could contribute to the abdominal hypersensitivity and abnormal colonic motility associated with IBS and IBD[27]. A previous report showed 5-HT and mucosal SERT are both decreased in ulcerative colitis, diarrhea-predominant IBS and constipation-predominant IBS[28]. Our study showed that NMS rats with vehicle have significantly increased SERT expression in the colon with increased concentration of 5-HT, compared with NH rats with vehicle. The increase in SERT expression could be due to an adaptive response to improve disturbed gut function and ameliorate symptoms; thus increased 5-HT could be terminated quickly under different stimulations. After treatment with JCM-16021, SERT expressions were decreased along with 5-HT content. Such decreases could result in the inhibition of SERT function. It is reported that inhibition of SERT function leads to decreased transiency in the gut and lower sensitivity[27,29,30]. Our data showed that JCM-16021 reduced SERT expression in the colon of rats, indicating that JCM-16021 inhibits SERT function so as to induce lower sensitivity.
In summary, the present findings provide evidence for the analgesic effect of JCM-16021 on visceral hyperalgesia in rats. This effect may be mediated through changes in the synthesis and metabolism of 5-HT in the colons of rats.
Increasing numbers of irritable bowel syndrome (IBS) sufferers are seeking help from complementary and alternative medicines because conventional therapies have not been proven to be more effective than placebo in providing overall relief of symptoms in randomized, controlled clinical trials. The 5-HT signaling pathway represents a promising target for IBS treatment. JCM-16021 improved the symptoms of IBS patients but the mechanism is unknown.
A neonatal maternal separation (NMS)-induced visceral hyperalgesia rat model is often used to study the mechanism of IBS and to evaluate the pharmacological effects of potential IBS therapies. This study aimed to investigate the analgesic effect of JCM-16021 on NMS-induced visceral hyperalgesia in rats, and its potential underlying mechanism.
Recent studies have highlighted the role of serotonin (5-HT) in the generation of IBS symptoms and in other gastrointestinal functional disorders. Furthermore, novel serotonergic agents, such as the 5-HT3 antagonist alosetron and the 5-HT4 agonist tegaserod, have significant impacts on IBS symptoms through their visceral analgesic properties and diverse effects on motor functions in the lower gastrointestinal tract. This is the first study to report that the analgesic effect of JCM-16021, a Chinese herbal formula, on visceral hyperalgesia in rats may be mediated through changes in the synthesis and metabolism of 5-HT in the colons of rats.
This study provides direct evidence for the analgesic effect of JCM-16021 on visceral hyperalgesia in rats. JCM-16021 might become a reliable therapy to relieve the symptoms of IBS patients.
This is an interesting paper showing the analgesic effect of a Chinese Medicine herb, JCM-16021 on maternal separation stress induced visceral hypersensitivity in male rats. Together with their previous data in IBS patients, this additional preclinical work suggests that JCM-16021 may be of therapeutic interest for IBS.
Peer reviewer: Million Mulugeta, DVM, PhD, Professor, Department of Medicine, Division of Digestive Diseases, UCLA, David Geffen School of Medicine, Los Angeles, CA 90073, United States
S- Editor Wang JL L- Editor O’Neill M E- Editor Ma WH
| 1. | Somers SC, Lembo A. Irritable bowel syndrome: evaluation and treatment. Gastroenterol Clin North Am. 2003;32:507-529. |
| 2. | Tillisch K, Chang L. Diagnosis and treatment of irritable bowel syndrome: state of the art. Curr Gastroenterol Rep. 2005;7:249-256. |
| 3. | Camilleri M. Management of the irritable bowel syndrome. Gastroenterology. 2001;120:652-668. |
| 5. | Sung JJY, Bian ZX, Wu JCY, Ziea ET, Suen BY, Leung WK. Herbal medicine versus hyosine in the treatment of irritable bowel syndrome: A double-blinded randomized study. Gut. 2007;56:A338. |
| 6. | Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13 Suppl 2:15-30. |
| 7. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. |
| 8. | Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188-194. |
| 9. | Crowell MD, Shetzline MA, Moses PL, Mawe GM, Talley NJ. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs. 2004;5:55-60. |
| 10. | Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067-1076. |
| 11. | Camilleri M. Serotonergic modulation of visceral sensation: lower gut. Gut. 2002;51 Suppl 1:i81-i86. |
| 12. | Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307-G316. |
| 13. | Ren TH, Wu J, Yew D, Ziea E, Lao L, Leung WK, Berman B, Hu PJ, Sung JJ. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2007;292:G849-G856. |
| 14. | Zhang XJ, Li Z, Leung WM, Liu L, Xu HX, Bian ZX. The analgesic effect of paeoniflorin on neonatal maternal separation-induced visceral hyperalgesia in rats. J Pain. 2008;9:497-505. |
| 15. | Bian ZX, Zhang M, Xu HX, Sung JJY. Chinese medicine formula JCM-16021 attenuates visceral pain of neonatal maternal separation rats via serotonergic pathway. Gut. 2008;57:A153. |
| 16. | Pharmacopoeia Commission of the Ministry of Public Health of PRC. Chinese Pharmacopoeia: Chemical Industry Press 2005; . |
| 17. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. |
| 18. | Li Z, Zhang XJ, Xu HX, Sung JJ, Bian ZX. Intracolonical administration of protease-activated receptor-2 agonists produced visceral hyperalgesia by up-regulating serotonin in the colon of rats. Eur J Pharmacol. 2009;606:199-204. |
| 19. | Tian XY, Bian ZX, Hu XG, Zhang XJ, Liu L, Zhang H. Electro-acupuncture attenuates stress-induced defecation in rats with chronic visceral hypersensitivity via serotonergic pathway. Brain Res. 2006;1088:101-108. |
| 20. | Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120-1130. |
| 21. | Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19:62-88. |
| 22. | Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42-G53. |
| 23. | Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81-103. |
| 24. | Chung EK, Zhang X, Li Z, Zhang H, Xu H, Bian Z. Neonatal maternal separation enhances central sensitivity to noxious colorectal distention in rat. Brain Res. 2007;1153:68-77. |
| 25. | Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397-414. |
| 26. | Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651-1659. |
| 27. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. |
| 28. | Coates MD, Johnson AC, Greenwood-Van Meerveld B, Mawe GM. Effects of serotonin transporter inhibition on gastrointestinal motility and colonic sensitivity in the mouse. Neurogastroenterol Motil. 2006;18:464-471. |
| 29. | Wang SH, Dong L, Luo JY, Gong J, Li L, Lu XL, Han SP. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041-6047. |
| 30. | O'Mahony S, Chua AS, Quigley EM, Clarke G, Shanahan F, Keeling PW, Dinan TG. Evidence of an enhanced central 5HT response in irritable bowel syndrome and in the rat maternal separation model. Neurogastroenterol Motil. 2008;20:680-688. |