Published online Feb 21, 2010. doi: 10.3748/wjg.v16.i7.818
Revised: October 6, 2009
Accepted: October 13, 2009
Published online: February 21, 2010
Pancreatic cancer is associated with a poor prognosis, and surgical resection remains the only chance for curative therapy. In the absence of metastatic disease, which would preclude resection, assessment of vascular invasion is an important parameter for determining resectability of pancreatic cancer. A frequent error is to misdiagnose an involved major vessel. Obviously, surgical exploration with pathological examination remains the “gold standard” in terms of evaluation of resectability, especially from the point of view of vascular involvement. However, current imaging modalities have improved and allow detection of vascular invasion with more accuracy. A venous resection in pancreatic cancer is a feasible technique and relatively reliable. Nevertheless, a survival benefit is not achieved by curative resection in patients with pancreatic cancer and vascular invasion. Although the discovery of an arterial invasion during the operation might require an aggressive management, discovery before the operation should be considered as a contraindication. Detection of vascular invasion remains one of the most important challenges in pancreatic surgery. The aim of this article is to provide a complete review of the different imaging modalities in the detection of vascular invasion in pancreatic cancer.
- Citation: Buchs NC, Chilcott M, Poletti PA, Buhler LH, Morel P. Vascular invasion in pancreatic cancer: Imaging modalities, preoperative diagnosis and surgical management. World J Gastroenterol 2010; 16(7): 818-831
- URL: https://www.wjgnet.com/1007-9327/full/v16/i7/818.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i7.818
The incidence of pancreatic cancer has gradually increased over the 20th century and in the early years of this century[1,2]. Cancer of the pancreas is the sixth most common cancer and fourth cause of death from cancer (22% of deaths among gastrointestinal cancers)[1-3].
Pancreatic cancer is associated with a poor prognosis, with less than 5% of patients surviving 5 years after the diagnosis[4]. Surgical resection remains the only chance for curative therapy in these patients[5-7]. Accurate preoperative staging of pancreatic cancer is essential to avoid unnecessary surgery in those with unresectable disease and, at the same time, in order not to deny the opportunity for cure in patients with resectable disease[5,6,8].
Only 16% of patients initially present a disease confined to the pancreas (stage I)[6,7]. Thus, of patients seen, 85%-90% have surgically unresectable tumors at the time of diagnosis[6,7,9-11].
There is no evidence-based consensus on the optimal preoperative imaging assessment of patients with suspected pancreatic cancer[6,8,12].
The criteria of unresectability are numerous[7,13-23]. However, in the absence of metastatic disease which precludes resection, assessment of vascular invasion is an important parameter for determining resectability for pancreatic cancer[5]. A frequent error is to misdiagnose an involved major vessel[11]. Vascular invasion is a relatively frequent discovery in pancreatic cancer; found in 21%-64% of patients, depending on the population studied[7,24].
From the point of view of arterial vessels, a tumoral infiltration of a large trunk (celiac axis, superior mesenteric artery, or hepatic artery) must be carefully analyzed because it constitutes a contraindication to surgery[25-27]. However, isolated involvement of smaller branches such as the gastro-duodenal artery will not preclude surgical resection[25]. The superior mesenteric vessels are the most frequently involved vessels in this cancer, due to their intimate relationship with the head, the uncinate process, and body of the pancreas[25,28].
Limited venous invasion does not represent an absolute contraindication for surgery[4,26,27,29-31]. Obviously, surgical exploration with pathological examination remains the “gold standard” in terms of evaluation of resectability, especially from the point of view of vascular involvement. However, current imaging modalities have improved and allow detection of vascular invasion with more accuracy. Detection is the key to the surgeon’s preoperative planning, because the posterior and lateral surfaces of the portal and superior mesenteric vein can be evaluated only after the surgical procedure is well advanced[14]. Thus, the management of a suspicious tumoral adhesion to a vessel is one of the most important challenges in a Whipple type procedure.
In this review, the current imaging modalities for assessing vascular involvement of pancreatic cancer will be discussed. Subsequently, the management and outcome of vascular invasion in patients with pancreatic cancer will also be reviewed briefly.
Computer tomography (CT) gives information about localization, size and extension of tumor[8,18], while being non-invasive[32]. A recent meta-analysis showed CT to be 91% sensitive and 85% specific for tumoral detection[33]. Phoa et al[34] showed that, with regard to tumor convexity towards a vessel, Grades D (concave contour of the tumor towards vessel) or E (circumferential involvement of vessel) have a risk of invasion of 88%; and a possibility of resection of 7% for the type D and of 0% for the type E[35]. Loyer et al[35] found that Grades A (fat plane separating the tumor from the vessel) and B (normal pancreatic tissue between tumor and vessel) had a resection rate of 95%, therefore these two grades are factors of better prognosis.
On the other hand, the length of tumor contact with the vessel (if it is greater than 5 mm) is a relatively good predictive factor for vascular invasion (78% for portal vein and 81% for superior mesenteric vein)[34].
A circumferential contact of more than 180 degrees has been shown to have a good correlation with unresectability[34,36,37]. For this criterion, Lu et al[38] found a sensitivity of 84%, a specificity of 98%, a positive predictive value (PPV) of 95%, and a negative predictive value (NPV) of 93%, for unresectability. Furthermore, Phoa et al[34] reported a sensitivity of 60%, and a specificity of 90%, if tumor convexity Grades D or E were combined with circumferential involvement of > 90 degrees. In addition, a strongly narrowed vessel also has an important risk of being invaded[34,36], but prudence is essential, especially for a vein, due to the mass effect of the tumor without the presence of vascular invasion[10,39,40]. In addition, an artery may be completely invaded, with no apparent change in vessel caliber[36,39].
Concerning the irregularity of the vascular wall, Li et al[36] reported a sensitivity and a specificity of 45% and 99%, respectively, for tumor detection in arteries, and 63% and 100% in the case of veins.
Regarding the rare superior mesenteric vein teardrop sign, Hough et al[41] found a sensitivity of this CT sign of 91% and a specificity of 98%; similar findings were reported in other series[36].
Consequently, Li et al[36] reported that the CT criteria for arterial invasion might be: an arterial embedment in tumor, or the combination of tumor involvement of more than one-half of the circumference of the arteries with artery wall irregularity or with artery stenosis (sensitivity of 79%, specificity of 99%). The criteria for venous invasion might be venous occlusion, tumor involvement of more than one-half of the circumference of the veins, vein wall irregularity, vein caliber stenosis, and teardrop superior mesenteric vein sign (sensitivity of 92%, specificity of 100%).
From the point of view of the detection of vascular invasion, many studies have evaluated CT (Table 1). CT has improved much these last years. Technology has developed multi-slice with 4-64 detector rows, allowed thin-sections and dual-phase, with faster time of acquisition, and numerous possibilities of image post-processing (3D reconstructions, multiplanar reconstructions)[19,29,40,42-45].
| Studies (yr) | n | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| Megibow et al[24] (1995) | 118 | 47 | 69 | 89 | 28 |
| Raptopoulos et al[208] (1997) | 82 | NA | NA | NA | 96 |
| Sugiyama et al[91] (1997) | 73 | 651 | 77 | NA | NA |
| McCarthy et al[16] (1998) | 67 | NA | NA | 55/942 | 95/94 |
| Diehl et al[209] (1998) | 89 | 86 | NA | NA | NA |
| Böttger et al[10] (1998) | 255 | 22.23 | 96.4 | 72.7 | 74.1 |
| Sugiyama et al[88] (1999) | 91 | 644 | 79 | NA | NA |
| Nakao et al[105] (1999) | 55 | 82.15 | 74.1 | 76.7 | 80 |
| Pietrabissa et al[130] (1999) | 50 | 82 | 53 | NA | NA |
| Gress et al[89] (1999) | 151 | 15 | 100 | 100 | 60 |
| Squillaci et al[69] (2003) | 50 | 97 | 100 | 100 | 95 |
| House et al[210] (2004) | 115 | 85-876 | 95-99 | 83-93 | 92-98 |
| Soriano et al[8] (2004) | 62 | 67 | 94 | 89 | 80 |
| Li et al[36] (2005) | 54 | 92/797 | 100/99 | NA | NA |
| Buchs et al[98] (2007) | 153 | 54.58 | 91.2 | 66.7 | 86.1 |
Fourteen years ago, Yoshimi et al[46] reported one of the first cases of 3D vascular reconstruction, allowing the evaluation of portal invasion with a higher accuracy than angiography alone. Currently, pancreatic section thickness of 1 mm is obtained in approximately 20 s, allowing true volume acquisition, with vascular details better than angiography[28,47,48] useful when assessing vascular invasion[44]. Furthermore, CT angiography allows anatomical study of small pancreatic vessels with a remarkable degree of accuracy[49,50].
Moreover, dilation of the peri-pancreatic veins with no visualization of inferior branches on CT suggests tumor invasion of peri-pancreatic tissue[50].
Several studies have highlighted the importance of the moment of image acquisition. With regard to the pancreas, it seems that a portal venous phase (60 s after intravenous administration of iodinated contrast medium) or that a pancreatic phase (40-70 s) provides more information than an arterial phase (18 s) or that of a hepatic phase (70 to 100 s)[19,29,51-54]. McNulty et al[51] reported that an arterial phase can be reserved for patients in whom CT angiography is required.
Lastly, Imbriaco et al[55] showed that dual-phase helical CT (arterial: 20 s, and pancreatic late: 70 s) was interesting but was comparable with single-phase helical CT (pancreatic early: 50 s).
In conclusion, CT is the assessment of choice in first intention, permitting in one non-invasive examination a TNM staging evaluation.
From the vascular point of view, many criteria exist (especially circumferential involvement of vessel of more than 180 degrees, radiological absence of a fat plane between tumor and vessel, vascular occlusion with collaterals, teardrop sign) which allow accuracy in diagnosing vascular invasion. Development of new radiological techniques (3D reconstructions, multiplanar reconstructions) has improved accuracy of assessment of vascular invasion.
MRI with cholangiopancreatography gives much information for the evaluation of primary tumor and metastatic dissemination, improved by the use of gadolinium or mangafodipir trisodium[1,13,47,56-58]. Currently, the use of MRI in an “all-in-one” staging method (MRI, coupled with angiography and cholangiopancreatography) is a subject under deliberation[58-60].
MRI criteria for vascular invasion are: (1) occlusion of the vessel, with or without collaterals, (2) tumoral infiltration of peri-vascular fat tissue, (3) circumferential contact of more than 180 degrees between the tumor and the vessel, and (4) mass effect along one side of the vessel for more than 2 cm[7,56,60,61].
As regards the detection of vascular invasion, MRI has an accuracy of approximately 94% for enhanced T1-weighted imaging[62]. Romijn et al[58] found in their study an accuracy of 81% with mangafodipir trisodium (definitely higher than MRI without contrast medium).
Other studies have attempted to analyze the performance of MRI in the detection of vascular invasion. They found a sensitivity of 47%-83%[24,60], a specificity of more than 95%[7,59], a PPV of more than 70%[7,8], and a NPV of 23%-96%[24,60].
Modern MRI technology makes it possible to obtain 3D reconstructions, facilitating the study of the peri-pancreatic vessels[61,63,64]. Some series have also demonstrated the adequate time for vascular pancreatic image acquisition: biphasic imaging at 15 and 45 s after arrival of contrast material (gadolinium) in the abdominal aorta[65].
Accuracy of MRI for vascular visualization is quite similar to that of CT[56,66,67]. It consequently seems logical to reserve this expensive and time-consuming technology for those patients not able to benefit from CT (allergy to iodine, renal insufficiency, pregnancy) or if CT findings are inconclusive[68].
Currently, conventional angiography is no longer part of the diagnostic protocol in most centers[13], because this examination does not permit the detection of the tumor itself[1], and can easily be replaced by other less invasive methods which give more information on tumoral extension.
On the other hand, preoperative arteriography may visualize vascular abnormalities (anatomical variations, acquired stenosis), allowing a possible modification of surgical strategy (revascularisation, replacement hepatic artery, embolization of an aneurism)[17,69,70].
With regard to vascular invasion, angiographic criteria are: (1) vascular stenosis or occlusion, with or without collaterals, (2) thrombosis of a vessel, (3) acute angle appearing in the venous wall, and (4) envelopment of the vessel within tumor[69,71-74].
In at least 20% of cases, angiography misses the vascular invasion[10], because it gives only information about the lumen of the vessel[72]. Angiography depends upon displacement of vessels and distortion of vascular contours unless clear vessel occlusion is present. Furthermore, the tumor may completely encase and invade the small amount of fat surrounding the vessel, and yet not cause a distortion of the contour of the vascular lumen, which is required for detection on angiography. This feature can be visualized during endoscopic ultrasonography or CT. Thus, angiography requires more extensive vascular involvement in order for it to be detected[5,74,75].
The results reported for detection of vascular invasion by pancreatic cancer using angiography are: a sensitivity between 21%[5,8] and more than 80%[10,76], a specificity between 50%[72] and 100%[8,69], a PPV more than 60%[5,72], and a NPV between 50%[72] and 83%[10]. Late angiographic times allow visualization of the portal vein, and possible invasions. In addition, it is possible to inject contrast medium directly into the portal vein by a transhepatic access, for example at the time of intravascular ultrasonography (see below).
In conclusion, studies show that angiography is paradoxically relatively poor in the detection of vascular invasion. On the other hand, it permits the visualization of arterial and venous anomalies, allowing a change in surgical strategy.
Abdominal US is often the first line examination for a patient presenting with jaundice and pain[13].
From the vascular point of view, US coupled with Doppler gives a reasonably reliable measure of vascular patency and can improve accuracy in assessing vascular invasion[13,77,78]. Its sensitivity ranges between 60%[79] and more than 90%[80]; its specificity has been reported to be higher than 90%[79,80], the PPV is higher than 90%[81], and the NPV is higher than 75%[82,83]. Very recently, authors reported US to be 93% accurate in detecting portal vein invasion, by using 3D vascular reconstruction technology[84].
Color Doppler sonographic criteria for vascular invasion are: (1) absence of hyperechoic tissue between the tumor and the vessel, (2) more than 2 cm continuity between tumor and vessel, (3) circumferential contact between the tumor and the vessel, (4) circumferential narrowing of vessel lumen, and (5) vascular occlusion or thrombosis[81-83,85-87].
In addition, perioperative US has been reported as 100% sensitive in identifying tumors, and 92% sensitive and specific in detecting portal invasion[88]. In 22% of patients with pancreatic neoplasms, US-Doppler makes it possible to modify therapeutic strategy[86].
In conclusion, US coupled with Doppler is a relatively accurate, cheap, and non-ionizing imaging modality for initial screening of patients with suspicion of tumors of the pancreas. However, US has demonstrated weakness in recognition of deeper localizations.
With regard to the detection of vascular invasion, studies have shown that US coupled with Doppler is a reliable method. However, these series evaluated almost exclusively the portal vein and its tributaries. Recent improvement in US imaging, allowing 3D reconstruction, offers new potential for this technology in the assessment of tumoral vascular involvement.
EUS is a relatively new technique, providing direct ultrasonic imaging of the pancreas through the gastrointestinal lumen[2,13]. However, the probes are expensive and EUS requires a trained endoscopist[13,63].
EUS has been shown to be accurate in diagnosing and staging pancreatic cancer[89], with the help of fine needle aspiration (FNA), with 96.6% sensitivity, 99.0% specificity, 96.2% NPV, and 99.1% PPV[90].
EUS criteria for vascular invasion are: (1) loss of the hyperechoic vessel wall/tumor interface, (2) direct visualization of tumor within the vessel lumen, (3) vascular encasement or occlusion, (4) non-visualization of a major vessel, in the presence of collaterals, (5) proximity of the tumor (< 3 mm) to the vessel, and (6) irregularity of the vascular wall[5,8,11,89,91-96].
Sugiyama et al[91] reported that EUS is more accurate than CT, US, and angiography for the detection of portal invasion; similar findings were shown in other series[97,98]. In addition, Brugge et al[93] showed that EUS was highly sensitive in the detection of portal and splenic vein invasions.
Arterial invasion is assessed with more difficulty by EUS[92,98-100]. Globally, the sensitivity is 50%-100%[92,95,101,102], the specificity 58%-100%[92,102], the PPV 28%-100%[92,96], and the NPV 18%-93%[89,94].
Very recently, Fritscher-Ravens et al[103] reported the use of 3D linear EUS in the assessment of vascular involvement with very interesting results compared with classical EUS. Linear 3D EUS enhanced the evaluation of vascular involvement of pancreatic lesions, especially in chronic pancreatitis.
In conclusion, it is appropriate to incorporate EUS in the preoperative assessment when there is suspicion of pancreatic cancer. From the point of view of the detection of vascular invasion, EUS has shown good accuracy, especially for venous invasion.
When a tumor appears to be contiguous with the portal vein or with the superior mesenteric vein, the diagnosis of vascular invasion can be difficult. Some limited reports have suggested that IVUS might allow the distinction between a simple compression by mass effect and invasion[71].
Moreover, IVUS makes it possible to detect intra-portal thrombus, sometimes missed by CT[71]. IVUS is performed either by a transhepatic access, or by a transmesenteric catheterization (during operative time)[71,104-108]. Complications are rare[72,104-106].
IVUS criteria for vascular invasion are: (1) obliteration of the echoic band of the portal vein by the hypoechoic tumor, (2) tumor mass blended with the venous wall, and (3) tumor protrusion into the vascular lumen[71,72,76,104-106,109].
One of the limitations of IVUS is the lack of specificity in the case of pancreatitis[71,105]. Moreover, IVUS has a limited penetration, allowing only localised investigations. Another weakness remains the lack of spatial orientation, making the interpretation of the images difficult[72,106].
There are few studies concerning IVUS in detection of vascular invasion in pancreatic cancer. Moreover, they report only portal and superior mesenteric vein results, not evaluating arterial invasion. The results are: sensitivity more than 95%[71,76], specificity more than 90%[71,76], PPV more than 90%[105], and NPV more than 95%[105].
Kaneko et al[109], has pioneered the use of IVUS in staging of pancreatic cancer, recently using 3D reconstructions of IVUS with a high degree of accuracy. Tezel et al[110] also reported that a contact of more than 18 mm between the tumor and the portal or the superior mesenteric vein was a factor of poor prognosis. The use of IVUS allows stent placement[111], a possibility in the palliative treatment of portal stenosis.
In conclusion, studies show that IVUS is probably superior to CT and portography for the detection of vascular invasion. However, data is available only for the portal vein and for the superior mesenteric vein. To our knowledge, there are no data concerning the utility of IVUS in detecting tumoral arterial invasion.
Because IVUS is expensive and invasive, Nakao et al[105] recommend performing this examination only in cases in which the distinction between compression and invasion cannot be made by conventional imaging techniques.
For almost 30 years[112], laparoscopic examination of the abdominal cavity has offered an excellent, although invasive, visualization of peritoneum and the liver[13,47,63,113,114].
From the vascular point of view, incision of the gastrohepatic omentum allows a direct access to the underlying vessels[47,115]. However, it seems certain that laparoscopy alone cannot detect vascular invasion, in particular mesenteric, without help of perioperative ultrasonography[116].
Currently, routine laparoscopy is not recommended in cases of cancer of the head of the pancreas, because it influences further surgical strategy in only 14%-19% of cases[116,117]. On the other hand, a study showed that in the case of cancer of the body or the tail of the pancreas, laparoscopy could avoid up to 50% of the operations, because of metastases not identified during staging[116].
Obviously, laparoscopy can also be used with a palliative aim (double derivations), if the tumor is unresectable[21,117-120]. Laparoscopy has its limits: it only allows visualization of the liver surface; impossibility of analyzing the retroperitoneum and its vessels; technical problems due to adherences[21,47,63,120,121].
LUS was subsequently developed, and this allows detailed study of the liver, the lymphatic area, and the corresponding vessels[47,121-126]. Vascular structures can be accurately visualized by LUS in approximately 95% of patients with tumors in the head of the pancreas[126].
LUS criteria for vascular invasion are: (1) loss of the hyperechoic vessel - tumor interface, (2) obliteration or thrombosis of a vessel, (3) a fixed stenosis, (4) vessel encasement by tumor encirclement and rigidity, and (5) presence of invading tumor within the vessel lumen[122,127-129].
There are numerous studies evaluating resectability by LUS, but to our knowledge few have focused on vascular invasion. They have found a sensitivity of more than 50%[129], a specificity of more than 80%[130], a PPV of 93%[127], and a NPV of 73%[128].
Despite these encouraging results, several authors do not recommend systematic use of laparoscopy or LUS. They prefer to recommend this technique for doubtful cases[21,121,131-133].
PET is a non-invasive imaging method, which gives information about cellular metabolic activity.
Currently, 18F-fluorodeoxyglucose (FDG) is injected and taken up preferentially by malignant tumors, and secondary localizations, rather than by healthy tissue[13,17,18,47,63,134-136]. The FDG is not metabolized and is trapped inside the cell[47], allowing it to be imaged in contrast to surrounding tissue[18].
PET is accurate in diagnosing small tumors (< 2 cm), as well as peritoneal implants and metastases[13,47,63,102,135,137-141]. In addition, PET is able to differentiate inflammatory pathologies from tumoral ones[47,135,139,142,143]. PET differentiates malignant and benign pathologies with a sensitivity of 85%-100% and a specificity of 67%-99%; often higher than that of CT[135,141,144-146].
In addition, false negatives exist in the case of strongly differentiated tumors, small periampullary tumors or in cases of hyperglycemia[63,135,146,147]. In the case of normo-glycemic patients, PET has a sensitivity for tumoral detection of 93%-98%[135,137,146,148,149], although in the case of hyperglycemic patients, this falls to 63%, or even less[135,137,146,149], in parallel with the NPV which falls from 96% to 38%[146].
Concerning lymphatic invasion, PET detection has proved poor, probably due to the proximity of regional lymph nodes to the primary tumor[102,134,135,137,150], and the lack of anatomic detail[13,18,139]. PET alone is unable to visualize vessels and cannot assess vascular invasion[63,135,151]. Thus, the association of PET with CT (PET/CT) seems promising[139,152].
Heinrich et al[139] showed recently that PET/CT has a PPV for the differentiation between a benign and a malignant pathology of 91%, whereas its NPV is 64%. PET/CT detects a cancer of the pancreas with a sensitivity of 93%, and is more specific than CT alone (69% vs 21%, respectively, P = 0.07). However, data are lacking regarding the assessment of vascular involvement. The use of multi-slice CT coupled with PET, and angio-CT protocols, might allow better visualization of the vessels.
Frequently, vascular invasion may be assessed only when the operation is already quite advanced (section of the pancreas, digestive transection)[22,27,153-156]. Palpation at the time of the Kocher maneuver (maneuver which permits exposure of structures behind duodenum and pancreatic head) is commonly performed to assess the relationship of a pancreatic head tumor to the superior mesenteric artery. However, if the tumor is large, if there is associated pancreatitis, or if the patient is undergoing reoperation, palpation is an inaccurate way to assess this critical tumor-vessel relationship prior to gastric and pancreatic transection[22].
The management of a suspicious tumoral adhesion to a vessel is one of the most important challenges in a Whipple procedure. In such a case, the surgeon is confronted with three options: (1) leave tumor attached to the vessel, resulting in a grossly positive margin of resection; (2) try to separate the tumor from the vessel, with a considerable hemorrhagic risk; and (3) or perform a partial or segmental resection of the portion of invaded vessel with reconstruction[22].
If the invasion of the superior mesenteric or portal vein is not in itself a criterion of unresectability[4,154,155,157], arterial invasion is a more controversial issue. Many authors regard this invasion as a contraindication to surgery[27,154,158], because of the high morbidity and mortality rates associated with arterial resection and reconstruction[159]. Furthermore, arterial invasion usually includes extensive involvement of the mesenteric neural plexus[160], rendering radical resection oncologically unsound because of the frequent finding of positive margins[154].
However, in many cases, the preoperative assessment cannot diagnose such an invasion. The surgeon must then adapt his surgical strategy. Fortner[161] recommended the resection of invaded arterial segment, if a reconstruction seemed possible.
From the arterial point of view, celiac or hepatic invasion, discovered during the operation, can be the object of a resection and a reconstruction, either by direct anastomosis, by interposition of a venous graft (for example reverse saphenous or internal jugular vein), or with a prosthesis[156,161-163]. An arterial graft (for example the splenic artery) can also be used[156,163]. These techniques seemed relatively reliable, with a mortality of 5%, in a recent study[164].
Regarding the modified Appleby’s operation (en-bloc resection of the celiac trunk with distal pancreatectomy and total gastrectomy) for advanced cancers of body and tail of the pancreas, several Japanese groups propose an extended resection of the celiac trunk, splenic artery, common hepatic artery, and/or superior mesenteric artery, resulting in 5-6 mo of average survival. Hepatic vascularization must be maintained and evaluated during the whole operation, and if necessary, compensated, in order to avoid an acute hepatic insufficiency[163,165-168].
Recently, Gagandeep et al[169] reported their experience using celiac axis resection for pancreatic cancer with a prolonged survival, and proposed the consideration of this technique for central and distal pancreatic cancer invading the celiac trunk.
Hirano et al[170] reported a high R0 resectability rate (91%) with distal pancreatectomy with en bloc celiac axis resection.
When the superior mesenteric artery is invaded, an arterial jejunal branch is isolated. Heparin is injected there, in order to allow the clamping of the superior mesenteric artery with full safety. The artery is then reconstructed either by direct anastomosis, or by anastomosis to the aorta[161].
In the case of an invasion of the hepatic artery, techniques of reconstruction require a venous graft (jugular, reverse saphenous, gonadic veins) or prosthesis, or an arterial graft (splenic, gastro-epiploic, gastro-duodenal)[22,163,171-173].
In some cases of cancer of the body of the pancreas, with invasion of the common hepatic artery and celiac trunk, Kondo et al[174] tried to embolize the hepatic artery, obtaining a collateral pathway from the superior mesenteric artery. This allowed a distal pancreatectomy with en bloc resection of the celiac trunk, without hepatic ischemia.
Other authors have described more traditional techniques of resection-reconstruction, using the gastro-duodenal artery[175]. Combined resection of the celiac trunk with a distal pancreatectomy has been found to improve the overall prognosis of patients with locally advanced cancer of the body and tail of the pancreas[176].
Contrary to arterial involvement, the invasion of the superior mesenteric vein or portal vein is not in itself a criterion of unresectability[4,154,155,157,177].
In uncommon cases, the pancreatic tumor infiltrates the anterior surface of the inferior vena cava. It is possible to excise the invaded part, and to replace it with a synthetic prosthesis. Often, autologous tissues are preferred (jugular, saphenous veins)[22].
When the portal vein is involved, it is legitimate to attempt a resection, especially if the vein is invaded by more than 2 cm, in order to obtain negative margins (Table 2)[4,178-180]. Portal invasion is not a predictor of aggressive tumor biology, but rather a reflection of tumor size and location[153,157,177,179]. Up to 50% of tumors thought to have vascular invasion intraoperatively have been found subsequently to have only inflammatory adhesions to the portal vein after histologic examination[157,181,182]. This finding underlines the difficulty in determining tumoral venous invasion before and during surgery, since peritumoral inflammation may simulate true tumor infiltration[178]. Very recently, Fukuda et al[183] reported that the depth of portal vein invasion significantly alters survival after curative pancreatic resection combined with portal vein resection. The survival rate was similar for patients with no portal invasion and those with superficial invasion. However, a deeper portal invasion was associated with a poorer survival rate, similar to that of patients undergoing non-curative resection.
| Studies (yr) | n | Mortality (%) | Survival at 1 year (%) | Median survival (mo) |
| Sindelar et al[159] (1989) | 20 | 201 | 50 | 12 |
| Tashiro et al[189] (1991) | 27 | 8.4 | 51.9 | NA |
| Ishikawa et al[74] (1992) | 35 | 5.7 | NA | 9+/-5 |
| Launois et al[193] (1993) | 9 | 0 | NA | 6.1 |
| Takahashi et al[156] (1994) | 79 | 16.5 | 17-61.52 | 6-14 |
| Allema et al[192] (1994) | 20 | 15 | 30% | 7 |
| Nakao et al[211] (1995) | 89 | 8 | 5.5-39.63 | NA |
| Nakao et al[190] (1995) | 104 | 8 | NA | NA |
| Roder et al[27] (1996) | 31 | 0 | 39 | 8 |
| Fuhrman et al[154] (1996) | 23 | 4 | NA | NA |
| Harrison et al[157] (1996) | 58 | 5 | 59 | 13 |
| Leach et al[196] (1998) | 31 | 0 | NA | 22 |
| Launois et al[188] (1999) | 14 | 0 | 23 | 5 |
| Bachellier et al[195] (2001) | 21 | 3.2 | NA | 13 |
| van Geenen et al[185] (2001) | 34 | 0 | 55 | 14 |
| Shibata et al[197] (2001) | 23 | 4 | 31 | 6.8-20.64 |
| Hartel et al[212] (2002) | 68 | 4 | 5 | NA |
| Aramaki et al[194] (2003) | 22 | 4.5 | NA | NA |
| Nakagohri et al[213] (2003) | 33 | 6 | 35-81 | 15 |
| Li et al[164] (2004) | 79 | 56 | NA | NA |
| Tseng et al[206] (2004) | 110 | 1 | 85 | 23.4 |
| Wagner et al[4] (2004) | 51 | 7.7 | NA | NA |
| Shimada et al[177] (2006) | 86 | 1 | 7 | 14 |
| Carrère et al[182] (2006) | 45 | 4.4 | 8 | 15 |
| Riediger et al[181] (2006) | 53 | 3.8 | 9 | NA |
| Fukuda et al[183] (2007) | 37 | 2.4 | 47.7 | NA |
The excision is done either by a segmentary resection, or by a tangential resection[22,184,185]. The reconstruction requires an end-to-end anastomosis either by direct suture or by using an interposition venous or prosthetic graft[22,74,154,156,157,161,162,184-189]. The technical limit of portal vein resection without graft is 4 cm in the hepatic hilus and 7 cm after pancreatic resection[189]. For minimal tumor invasion into the portal vein, autologous saphenous vein patch has been described[27]. Wide resection of the portal vein may require transection of the splenic vein. To avoid segmental portal hypertension, end-to-side reanastomosis of the splenic vein to the interposition graft is recommended[184].
If the portal clampage lasts longer than 30 min, it is recommended to clamp also the superior mesenteric artery, in order to prevent intestinal congestion[22,189]. If the portal clampage lasts longer than 60 min, it is necessary to consider a bypass between the superior mesenteric vein and femoral vein[189,190].
Resection of the portal vein is associated with a higher morbidity rate (bleeding, infections, cardiopulmonary complications), than when this is not performed[4,185,191]. In addition, Fuhrman et al[154] reported an operative time, an operative blood loss, and perioperative transfusion requirements of greater magnitude in patients who required venous resection. The mortality rate is also higher after portal vein resection but this value is not always significant[4,188,192,193]. These findings are not confirmed by other series[22,27,157,181,182,185,187,191,194-201]. Numerous authors have reported a mortality rate below 5%, similar to that of standard pancreatoduodenectomy[27,154,157,164,178,181,182,185,188,194-197].
In 62%-85% of cases, the vascular margins are found to be positive[27,31,185,192], explaining a very poor median survival. However, recently, Siriwardana et al[202] reported, in a systematic review of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer, a high rate (67.4%) of nodal involvement during the procedure. For the authors, this implied that by the time a pancreatic tumor involves the portal vein the risk of metastases is high, rendering the possibility of cure by surgery improbable[202].
If the tumor invades the superior mesenteric vein, it is not a criterion of unresectability. Various techniques exist to allow complete resection of the tumor, either by tangential excision, or by excision-reconstruction[153,155,161,162,185,197,203,204].
In conclusion, various studies show that venous resection in pancreatic cancer is a feasible technique and relatively reliable, at least with regard to mortality, but (importantly) at the price of a higher morbidity. However, a survival benefit is not achieved by curative resection in patients with pancreatic cancer and vascular invasion[205,206]. On the other hand, the discovery of an arterial invasion during the operation might require an aggressive management, using vascular reconstruction. Furthermore, neoadjuvant treatment (combination of 5-fluorouracil/cisplatin chemoradiation) showed only limited impact on survival but appeared to be associated with improved local control[207].
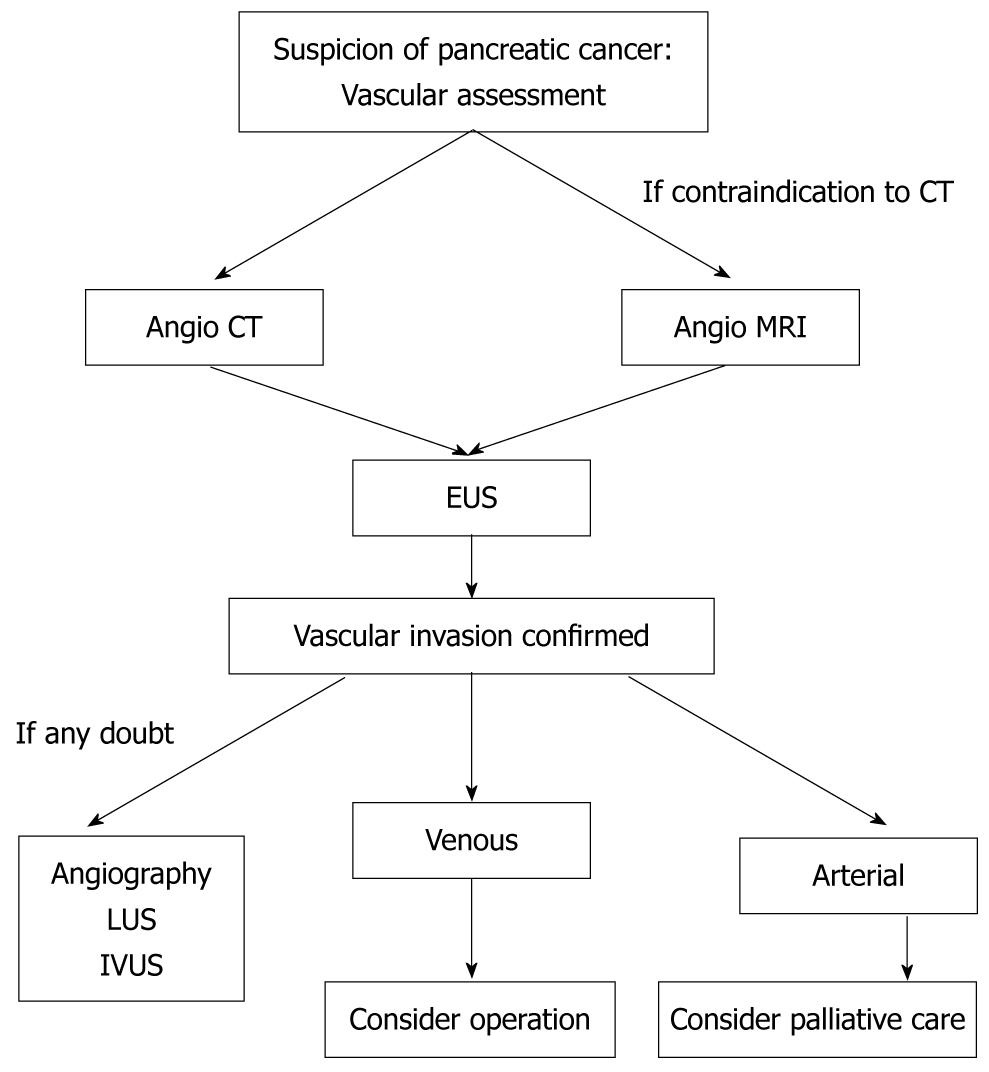
In the absence of metastatic disease, assessment of vascular invasion is a key aspect in the evaluation of resectability for pancreatic cancer. A frequent error is to misdiagnose an involved major vessel. Obviously, surgical exploration with pathological examination remains the “gold standard” in terms of evaluation of resectability, especially from the point of view of vascular involvement. However, current imaging modalities have improved and now allow detection of vascular invasion with more accuracy. Multi-slice CT has become the best imaging modality for this purpose, and the adjunction of PET might be a means to improve results further. EUS is useful, but it remains very operator-dependant. Data are still lacking for the exact role of MRI regarding this issue (Figure 1). Detection of vascular invasion remains one of the most important challenges in pancreatic surgery.
Peer reviewer: Georgios Papachristou, MD, Assistant Professor of Medicine, Division of Gastroenterology, Hepatology and Nutrition, UPMC Presbyterian, Mezzanine Level, C-Wing, 200 Lothrop Street , Pittsburgh, PA 15213, United States
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut. 2005;54 Suppl 5:v1-16. |
| 2. | Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455-465. |
| 3. | Connolly MM, Dawson PJ, Michelassi F, Moossa AR, Lowenstein F. Survival in 1001 patients with carcinoma of the pancreas. Ann Surg. 1987;206:366-373. |
| 4. | Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586-594. |
| 5. | Ahmad NA, Kochman ML, Lewis JD, Kadish S, Morris JB, Rosato EF, Ginsberg GG. Endosonography is superior to angiography in the preoperative assessment of vascular involvement among patients with pancreatic carcinoma. J Clin Gastroenterol. 2001;32:54-58. |
| 6. | DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D, Kopecky K. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753-763. |
| 7. | Arslan A, Buanes T, Geitung JT. Pancreatic carcinoma: MR, MR angiography and dynamic helical CT in the evaluation of vascular invasion. Eur J Radiol. 2001;38:151-159. |
| 8. | Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, Real MI, Gilabert R, Quintó L, Trilla A. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492-501. |
| 9. | Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248-257; discussion 257-260. |
| 10. | Böttger TC, Boddin J, Düber C, Heintz A, Küchle R, Junginger T. Diagnosing and staging of pancreatic carcinoma-what is necessary? Oncology. 1998;55:122-129. |
| 11. | Snady H, Bruckner H, Siegel J, Cooperman A, Neff R, Kiefer L. Endoscopic ultrasonographic criteria of vascular invasion by potentially resectable pancreatic tumors. Gastrointest Endosc. 1994;40:326-333. |
| 12. | Scaglione M, Pinto A, Romano S, Scialpi M, Volterrani L, Rotondo A, Romano L. Using multidetector row computed tomography to diagnose and stage pancreatic carcinoma: the problems and the possibilities. JOP. 2005;6:1-5. |
| 13. | Takhar AS, Palaniappan P, Dhingsa R, Lobo DN. Recent developments in diagnosis of pancreatic cancer. BMJ. 2004;329:668-673. |
| 14. | Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB Jr. MDCT in Pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol. 2004;182:419-425. |
| 15. | Saldinger PF, Reilly M, Reynolds K, Raptopoulos V, Chuttani R, Steer ML, Matthews JB. Is CT angiography sufficient for prediction of resectability of periampullary neoplasms? J Gastrointest Surg. 2000;4:233-237; discussion 238-239. |
| 16. | McCarthy MJ, Evans J, Sagar G, Neoptolemos JP. Prediction of resectability of pancreatic malignancy by computed tomography. Br J Surg. 1998;85:320-325. |
| 17. | Balci NC, Semelka RC. Radiologic diagnosis and staging of pancreatic ductal adenocarcinoma. Eur J Radiol. 2001;38:105-112. |
| 18. | Saisho H, Yamaguchi T. Diagnostic imaging for pancreatic cancer: computed tomography, magnetic resonance imaging, and positron emission tomography. Pancreas. 2004;28:273-278. |
| 19. | Catalano C, Laghi A, Fraioli F, Pediconi F, Napoli A, Danti M, Reitano I, Passariello R. Pancreatic carcinoma: the role of high-resolution multislice spiral CT in the diagnosis and assessment of resectability. Eur Radiol. 2003;13:149-156. |
| 20. | Fishman EK, Horton KM, Urban BA. Multidetector CT angiography in the evaluation of pancreatic carcinoma: preliminary observations. J Comput Assist Tomogr. 2000;24:849-853. |
| 21. | Pisters PW, Lee JE, Vauthey JN, Charnsangavej C, Evans DB. Laparoscopy in the staging of pancreatic cancer. Br J Surg. 2001;88:325-337. |
| 22. | Bold RJ, Charnsangavej C, Cleary KR, Jennings M, Madray A, Leach SD, Abbruzzese JL, Pisters PW, Lee JE, Evans DB. Major vascular resection as part of pancreaticoduodenectomy for cancer: radiologic, intraoperative, and pathologic analysis. J Gastrointest Surg. 1999;3:233-243. |
| 23. | Beger HG, Rau B, Gansauge F, Poch B, Link KH. Treatment of pancreatic cancer: challenge of the facts. World J Surg. 2003;27:1075-1084. |
| 24. | Megibow AJ, Zhou XH, Rotterdam H, Francis IR, Zerhouni EA, Balfe DM, Weinreb JC, Aisen A, Kuhlman J, Heiken JP. Pancreatic adenocarcinoma: CT versus MR imaging in the evaluation of resectability--report of the Radiology Diagnostic Oncology Group. Radiology. 1995;195:327-332. |
| 25. | Horton KM, Fishman EK. Multidetector CT angiography of pancreatic carcinoma: part I, evaluation of arterial involvement. AJR Am J Roentgenol. 2002;178:827-831. |
| 26. | Valls C, Andía E, Sanchez A, Fabregat J, Pozuelo O, Quintero JC, Serrano T, Garcia-Borobia F, Jorba R. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol. 2002;178:821-826. |
| 27. | Roder JD, Stein HJ, Siewert JR. Carcinoma of the periampullary region: who benefits from portal vein resection? Am J Surg. 1996;171:170-174; discussion 174-175. |
| 28. | Horton KM, Fishman EK. Volume-rendered 3D CT of the mesenteric vasculature: normal anatomy, anatomic variants, and pathologic conditions. Radiographics. 2002;22:161-172. |
| 29. | Gritzmann N, Macheiner P, Hollerweger A, Hübner E. CT in the differentiation of pancreatic neoplasms--progress report. Dig Dis. 2004;22:6-17. |
| 30. | Horton KM, Fishman EK. Multidetector CT angiography of pancreatic carcinoma: part 2, evaluation of venous involvement. AJR Am J Roentgenol. 2002;178:833-836. |
| 31. | Poen JC, Ford JM, Niederhuber JE. Chemoradiotherapy in the management of localized tumors of the pancreas. Ann Surg Oncol. 1999;6:117-122. |
| 32. | Cunningham JD, Glajchen N, Brower ST. The use of spiral computed tomography in the evaluation of vessel encasement for pancreatic cancer. Int J Pancreatol. 1996;19:9-14. |
| 33. | Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Laméris JS, Stoker J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29:438-445. |
| 34. | Phoa SS, Reeders JW, Stoker J, Rauws EA, Gouma DJ, Laméris JS. CT criteria for venous invasion in patients with pancreatic head carcinoma. Br J Radiol. 2000;73:1159-1164. |
| 35. | Loyer EM, David CL, Dubrow RA, Evans DB, Charnsangavej C. Vascular involvement in pancreatic adenocarcinoma: reassessment by thin-section CT. Abdom Imaging. 1996;21:202-206. |
| 36. | Li H, Zeng MS, Zhou KR, Jin DY, Lou WH. Pancreatic adenocarcinoma: the different CT criteria for peripancreatic major arterial and venous invasion. J Comput Assist Tomogr. 2005;29:170-175. |
| 37. | Phoa SS, Tilleman EH, van Delden OM, Bossuyt PM, Gouma DJ, Laméris JS. Value of CT criteria in predicting survival in patients with potentially resectable pancreatic head carcinoma. J Surg Oncol. 2005;91:33-40. |
| 38. | Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439-1443. |
| 39. | Lepanto L, Arzoumanian Y, Gianfelice D, Perreault P, Dagenais M, Lapointe R, Létourneau R, Roy A. Helical CT with CT angiography in assessing periampullary neoplasms: identification of vascular invasion. Radiology. 2002;222:347-352. |
| 40. | Brügel M, Rummeny EJ, Dobritz M. Vascular invasion in pancreatic cancer: value of multislice helical CT. Abdom Imaging. 2004;29:239-245. |
| 41. | Hough TJ, Raptopoulos V, Siewert B, Matthews JB. Teardrop superior mesenteric vein: CT sign for unresectable carcinoma of the pancreas. AJR Am J Roentgenol. 1999;173:1509-1512. |
| 42. | Baek SY, Sheafor DH, Keogan MT, DeLong DM, Nelson RC. Two-dimensional multiplanar and three-dimensional volume-rendered vascular CT in pancreatic carcinoma: interobserver agreement and comparison with standard helical techniques. AJR Am J Roentgenol. 2001;176:1467-1473. |
| 43. | Kopka L, Rogalla P, Hamm B. [Multislice CT of the abdomen--current indications and future trends]. Rofo. 2002;174:273-282. |
| 44. | Nino-Murcia M, Tamm EP, Charnsangavej C, Jeffrey RB Jr. Multidetector-row helical CT and advanced postprocessing techniques for the evaluation of pancreatic neoplasms. Abdom Imaging. 2003;28:366-377. |
| 45. | Li H, Zeng MS, Zhou KR, Jin DY, Lou WH. Pancreatic adenocarcinoma: signs of vascular invasion determined by multi-detector row CT. Br J Radiol. 2006;79:880-887. |
| 46. | Yoshimi F, Hasegawa H, Koizumi S, Amemiya R, Ono H, Kobayashi H, Matsueda K, Itabashi M. Application of three-dimensional spiral computed tomographic angiography to pancreatoduodenectomy for cancer. Br J Surg. 1995;82:116-117. |
| 47. | Riker A, Libutti SK, Bartlett DL. Advances in the early detection, diagnosis, and staging of pancreatic cancer. Surg Oncol. 1997;6:157-169. |
| 48. | Fishman EK, Horton KM. Imaging pancreatic cancer: the role of multidetector CT with three-dimensional CT angiography. Pancreatology. 2001;1:610-624. |
| 49. | Chong M, Freeny PC, Schmiedl UP. Pancreatic arterial anatomy: depiction with dual-phase helical CT. Radiology. 1998;208:537-542. |
| 50. | Yamada Y, Mori H, Kiyosue H, Matsumoto S, Hori Y, Maeda T. CT assessment of the inferior peripancreatic veins: clinical significance. AJR Am J Roentgenol. 2000;174:677-684. |
| 51. | McNulty NJ, Francis IR, Platt JF, Cohan RH, Korobkin M, Gebremariam A. Multi--detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97-102. |
| 52. | Graf O, Boland GW, Warshaw AL, Fernandez-del-Castillo C, Hahn PF, Mueller PR. Arterial versus portal venous helical CT for revealing pancreatic adenocarcinoma: conspicuity of tumor and critical vascular anatomy. AJR Am J Roentgenol. 1997;169:119-123. |
| 53. | Lu DS, Vedantham S, Krasny RM, Kadell B, Berger WL, Reber HA. Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology. 1996;199:697-701. |
| 54. | Vedantham S, Lu DS, Reber HA, Kadell B. Small peripancreatic veins: improved assessment in pancreatic cancer patients using thin-section pancreatic phase helical CT. AJR Am J Roentgenol. 1998;170:377-383. |
| 55. | Imbriaco M, Megibow AJ, Camera L, Pace L, Mainenti PP, Romano M, Selva G, Salvatore M. Dual-phase versus single-phase helical CT to detect and assess resectability of pancreatic carcinoma. AJR Am J Roentgenol. 2002;178:1473-1479. |
| 56. | Schima W, Függer R, Schober E, Oettl C, Wamser P, Grabenwöger F, Ryan JM, Novacek G. Diagnosis and staging of pancreatic cancer: comparison of mangafodipir trisodium-enhanced MR imaging and contrast-enhanced helical hydro-CT. AJR Am J Roentgenol. 2002;179:717-724. |
| 57. | Adamek HE, Albert J, Breer H, Weitz M, Schilling D, Riemann JF. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: a prospective controlled study. Lancet. 2000;356:190-193. |
| 58. | Romijn MG, Stoker J, van Eijck CH, van Muiswinkel JM, Torres CG, Laméris JS. MRI with mangafodipir trisodium in the detection and staging of pancreatic cancer. J Magn Reson Imaging. 2000;12:261-268. |
| 59. | Trede M, Rumstadt B, Wendl K, Gaa J, Tesdal K, Lehmann KJ, Meier-Willersen HJ, Pescatore P, Schmoll J. Ultrafast magnetic resonance imaging improves the staging of pancreatic tumors. Ann Surg. 1997;226:393-405; discussion 405-407. |
| 60. | Lopez Hänninen E, Amthauer H, Hosten N, Ricke J, Böhmig M, Langrehr J, Hintze R, Neuhaus P, Wiedenmann B, Rosewicz S. Prospective evaluation of pancreatic tumors: accuracy of MR imaging with MR cholangiopancreatography and MR angiography. Radiology. 2002;224:34-41. |
| 61. | Catalano C, Pavone P, Laghi A, Panebianco V, Scipioni A, Fanelli F, Brillo R, Passariello R. Pancreatic adenocarcinoma: combination of MR imaging, MR angiography and MR cholangiopancreatography for the diagnosis and assessment of resectability. Eur Radiol. 1998;8:428-434. |
| 62. | Sironi S, De Cobelli F, Zerbi A, Angeli E, Balzano G, Taccagni G, Di Carlo V, Del Maschio A. Pancreatic adenocarcinoma: assessment of vascular invasion with high-field MR imaging and a phased-array coil. AJR Am J Roentgenol. 1996;167:997-1001. |
| 63. | Sarmiento JM, Sarr MG. Staging strategies for pancreatic adenocarcinoma: what the surgeon really wants to know. Curr Gastroenterol Rep. 2003;5:117-124. |
| 64. | Ito K, Blasbalg R, Hussain SM, Mitchell DG. Portal vein and its tributaries: evaluation with thin-section three-dimensional contrast-enhanced dynamic fat-suppressed MR imaging. Radiology. 2000;215:381-386. |
| 65. | Kanematsu M, Shiratori Y, Hoshi H, Kondo H, Matsuo M, Moriwaki H. Pancreas and peripancreatic vessels: effect of imaging delay on gadolinium enhancement at dynamic gradient-recalled-echo MR imaging. Radiology. 2000;215:95-102. |
| 66. | Gabata T, Matsui O, Kadoya M, Yoshikawa J, Miyayama S, Takashima T, Nagakawa T, Kayahara M, Nonomura A. Small pancreatic adenocarcinomas: efficacy of MR imaging with fat suppression and gadolinium enhancement. Radiology. 1994;193:683-688. |
| 67. | Ichikawa T, Haradome H, Hachiya J, Nitatori T, Ohtomo K, Kinoshita T, Araki T. Pancreatic ductal adenocarcinoma: preoperative assessment with helical CT versus dynamic MR imaging. Radiology. 1997;202:655-662. |
| 68. | Semelka RC, Kelekis NL, Molina PL, Sharp TJ, Calvo B. Pancreatic masses with inconclusive findings on spiral CT: is there a role for MRI? J Magn Reson Imaging. 1996;6:585-588. |
| 69. | Squillaci E, Fanucci E, Sciuto F, Masala S, Sodani G, Carlani M, Simonetti G. Vascular involvement in pancreatic neoplasm: a comparison between spiral CT and DSA. Dig Dis Sci. 2003;48:449-458. |
| 70. | Biehl TR, Traverso LW, Hauptmann E, Ryan JA Jr. Preoperative visceral angiography alters intraoperative strategy during the Whipple procedure. Am J Surg. 1993;165:607-612. |
| 71. | Kaneko T, Nakao A, Inoue S, Harada A, Nonami T, Itoh S, Endo T, Takagi H. Intraportal endovascular ultrasonography in the diagnosis of portal vein invasion by pancreatobiliary carcinoma. Ann Surg. 1995;222:711-718. |
| 72. | Hannesson PH, Lundstedt C, Dawiskiba S, Stridbeck H, Ihse I. Transhepatic intravascular ultrasound for evaluation of portal venous involvement in patients with cancer of the pancreatic head region. Eur Radiol. 2002;12:1150-1154. |
| 73. | Dooley WC, Cameron JL, Pitt HA, Lillemoe KD, Yue NC, Venbrux AC. Is preoperative angiography useful in patients with periampullary tumors? Ann Surg. 1990;211:649-654; discussion 654-655. |
| 74. | Ishikawa O, Ohigashi H, Imaoka S, Furukawa H, Sasaki Y, Fujita M, Kuroda C, Iwanaga T. Preoperative indications for extended pancreatectomy for locally advanced pancreas cancer involving the portal vein. Ann Surg. 1992;215:231-236. |
| 75. | Snady H, Cooperman A, Siegel J. Endoscopic ultrasonography compared with computed tomography with ERCP in patients with obstructive jaundice or small peri-pancreatic mass. Gastrointest Endosc. 1992;38:27-34. |
| 76. | Kaneko T, Nakao A, Inoue S, Endo T, Itoh S, Harada A, Nonami T, Takagi H. Portal venous invasion by pancreatobiliary carcinoma: diagnosis with intraportal endovascular US. Radiology. 1994;192:681-686. |
| 77. | Ueno N, Tomiyama T, Tano S, Wada S, Miyata T. Color Doppler ultrasonography in the diagnosis of portal vein invasion in patients with pancreatic cancer. J Ultrasound Med. 1997;16:825-830. |
| 78. | Clarke DL, Thomson SR, Madiba TE, Sanyika C. Preoperative imaging of pancreatic cancer: a management-oriented approach. J Am Coll Surg. 2003;196:119-129. |
| 79. | Tomiyama T, Ueno N, Tano S, Wada S, Kimura K. Assessment of arterial invasion in pancreatic cancer using color Doppler ultrasonography. Am J Gastroenterol. 1996;91:1410-1416. |
| 80. | Casadei R, Ghigi G, Gullo L, Moretti CC, Greco VM, Salizzoni E, Canini R, Marrano D. Role of color Doppler ultrasonography in the preoperative staging of pancreatic cancer. Pancreas. 1998;16:26-30. |
| 81. | Wren SM, Ralls PW, Stain SC, Kasiraman A, Carpenter CL, Parekh D. Assessment of resectability of pancreatic head and periampullary tumors by color flow Doppler sonography. Arch Surg. 1996;131:812-817; discussion 817-818. |
| 82. | Angeli E, Venturini M, Vanzulli A, Sironi S, Castrucci M, Salvioni M, Zerbi A, Di Carlo V, Del Maschio A. Color Doppler imaging in the assessment of vascular involvement by pancreatic carcinoma. AJR Am J Roentgenol. 1997;168:193-197. |
| 83. | Ishida H, Konno K, Hamashima Y, Naganuma H, Komatsuda T, Sato M, Ishida J, Masamune O. Assessment of resectability of pancreatic carcinoma by color Doppler sonography. Abdom Imaging. 1999;24:295-298. |
| 84. | Kobayashi A, Yamaguchi T, Ishihara T, Ohshima T, Ohno I, Seza K, Shirai Y, Sudo K, Nakagawa A, Tadenuma H. Assessment of portal vein invasion in pancreatic cancer by fusion 3-dimensional ultrasonography. J Ultrasound Med. 2005;24:363-369. |
| 85. | Morrin MM, Kruskal JB, Raptopoulos V, Weisinger K, Farrell RJ, Steer ML, Kane RA. State-of-the-art ultrasonography is as accurate as helical computed tomography and computed tomographic angiography for detecting unresectable periampullary cancer. J Ultrasound Med. 2001;20:481-490. |
| 86. | Ralls PW, Wren SM, Radin R, Stain SC, Yang J, Parekh D. Color flow sonography in evaluating the resectability of periampullary and pancreatic tumors. J Ultrasound Med. 1997;16:131-140. |
| 87. | Minniti S, Bruno C, Biasiutti C, Tonel D, Falzone A, Falconi M, Procacci C. Sonography versus helical CT in identification and staging of pancreatic ductal adenocarcinoma. J Clin Ultrasound. 2003;31:175-182. |
| 88. | Sugiyama M, Hagi H, Atomi Y. Reappraisal of intraoperative ultrasonography for pancreatobiliary carcinomas: assessment of malignant portal venous invasion. Surgery. 1999;125:160-165. |
| 89. | Gress FG, Hawes RH, Savides TJ, Ikenberry SO, Cummings O, Kopecky K, Sherman S, Wiersema M, Lehman GA. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc. 1999;50:786-791. |
| 90. | Krishna NB, LaBundy JL, Saripalli S, Safdar R, Agarwal B. Diagnostic value of EUS-FNA in patients suspected of having pancreatic cancer with a focal lesion on CT scan/MRI but without obstructive jaundice. Pancreas. 2009;38:625-630. |
| 91. | Sugiyama M, Hagi H, Atomi Y, Saito M. Diagnosis of portal venous invasion by pancreatobiliary carcinoma: value of endoscopic ultrasonography. Abdom Imaging. 1997;22:434-438. |
| 92. | Aslanian H, Salem R, Lee J, Andersen D, Robert M, Topazian M. EUS diagnosis of vascular invasion in pancreatic cancer: surgical and histologic correlates. Am J Gastroenterol. 2005;100:1381-1385. |
| 93. | Brugge WR, Lee MJ, Kelsey PB, Schapiro RH, Warshaw AL. The use of EUS to diagnose malignant portal venous system invasion by pancreatic cancer. Gastrointest Endosc. 1996;43:561-567. |
| 94. | Rösch T, Dittler HJ, Strobel K, Meining A, Schusdziarra V, Lorenz R, Allescher HD, Kassem AM, Gerhardt P, Siewert JR. Endoscopic ultrasound criteria for vascular invasion in the staging of cancer of the head of the pancreas: a blind reevaluation of videotapes. Gastrointest Endosc. 2000;52:469-477. |
| 95. | Tierney WM, Francis IR, Eckhauser F, Elta G, Nostrant TT, Scheiman JM. The accuracy of EUS and helical CT in the assessment of vascular invasion by peripapillary malignancy. Gastrointest Endosc. 2001;53:182-188. |
| 96. | Yusoff IF, Mendelson RM, Edmunds SE, Ramsay D, Cullingford GL, Fletcher DR, Zimmerman AM. Preoperative assessment of pancreatic malignancy using endoscopic ultrasound. Abdom Imaging. 2003;28:556-562. |
| 97. | Kahl S, Glasbrenner B, Zimmermann S, Malfertheiner P. Endoscopic ultrasound in pancreatic diseases. Dig Dis. 2002;20:120-126. |
| 98. | Buchs NC, Frossard JL, Rosset A, Chilcott M, Koutny-Fong P, Chassot G, Fasel JH, Poletti PA, Becker CD, Mentha G. Vascular invasion in pancreatic cancer: evaluation of endoscopic ultrasonography, computed tomography, ultrasonography, and angiography. Swiss Med Wkly. 2007;137:286-291. |
| 99. | Rösch T, Braig C, Gain T, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188-199. |
| 100. | Yasuda K, Mukai H, Nakajima M, Kawai K. Staging of pancreatic carcinoma by endoscopic ultrasonography. Endoscopy. 1993;25:151-155. |
| 101. | Rivadeneira DE, Pochapin M, Grobmyer SR, Lieberman MD, Christos PJ, Jacobson I, Daly JM. Comparison of linear array endoscopic ultrasound and helical computed tomography for the staging of periampullary malignancies. Ann Surg Oncol. 2003;10:890-897. |
| 102. | Schwarz M, Pauls S, Sokiranski R, Brambs HJ, Glasbrenner B, Adler G, Diederichs CG, Reske SN, Möller P, Beger HG. Is a preoperative multidiagnostic approach to predict surgical resectability of periampullary tumors still effective? Am J Surg. 2001;182:243-249. |
| 103. | Fritscher-Ravens A, Knoefel WT, Krause C, Swain CP, Brandt L, Patel K. Three-dimensional linear endoscopic ultrasound-feasibility of a novel technique applied for the detection of vessel involvement of pancreatic masses. Am J Gastroenterol. 2005;100:1296-1302. |
| 104. | Kaneko T, Nakao A, Takagi H. Intraportal endovascular ultrasonography for pancreatic cancer. Semin Surg Oncol. 1998;15:47-51. |
| 105. | Nakao A, Kaneko T. Intravascular ultrasonography for assessment of portal vein invasion by pancreatic carcinoma. World J Surg. 1999;23:892-895. |
| 106. | Hannesson PH, Stridbeck H, Lundstedt C, Dawiskiba S, Andrén-Sandberg A, Ihse I. Intravascular ultrasound for evaluation of portal venous involvement in pancreatic cancer. Eur Radiol. 1997;7:21-25. |
| 107. | Kaneko T, Nakao A, Harada A, Nomami T, Takagi H. Intraportal endovascular ultrasonography in pancreatic cancer--a new technique for the diagnosis of portal vein invasion: a preliminary report. Surgery. 1994;115:438-444. |
| 108. | Stein M, Schneider PD, Ho HS, Eckert R, Urayama S, Bold RJ. Percutaneous transhepatic portography with intravascular ultrasonography for evaluation of venous involvement of hepatobiliary and pancreatic tumors. J Vasc Interv Radiol. 2002;13:805-814. |
| 109. | Kaneko T, Inoue S, Sugimoto H, Takeda S, Harada A, Nakao A. Intraoperative diagnosis of pancreatic cancer extension using IVUS. Hepatogastroenterology. 2001;48:944-948. |
| 110. | Tezel E, Kaneko T, Takeda S, Inoue S, Nagasaka T, Nakao A. Intraportal endovascular ultrasound for portal vein resection in pancreatic carcinoma. Hepatogastroenterology. 2005;52:237-242. |
| 111. | Manninen HI, Räsänen H. Intravascular ultrasound in interventional radiology. Eur Radiol. 2000;10:1754-1762. |
| 112. | Cuschieri A, Hall AW, Clark J. Value of laparoscopy in the diagnosis and management of pancreatic carcinoma. Gut. 1978;19:672-677. |
| 113. | Cuschieri A. Laparoscopy for pancreatic cancer: does it benefit the patient? Eur J Surg Oncol. 1988;14:41-44. |
| 115. | Conlon KC, Dougherty E, Klimstra DS, Coit DG, Turnbull AD, Brennan MF. The value of minimal access surgery in the staging of patients with potentially resectable peripancreatic malignancy. Ann Surg. 1996;223:134-140. |
| 116. | Barreiro CJ, Lillemoe KD, Koniaris LG, Sohn TA, Yeo CJ, Coleman J, Fishman EK, Cameron JL. Diagnostic laparoscopy for periampullary and pancreatic cancer: what is the true benefit? J Gastrointest Surg. 2002;6:75-81. |
| 117. | Potter MW, Shah SA, McEnaney P, Chari RS, Callery MP. A critical appraisal of laparoscopic staging in hepatobiliary and pancreatic malignancy. Surg Oncol. 2000;9:103-110. |
| 118. | Edwin B, Mala T, Mathisen Ø, Gladhaug I, Buanes T, Lunde OC, Søreide O, Bergan A, Fosse E. Laparoscopic resection of the pancreas: a feasibility study of the short-term outcome. Surg Endosc. 2004;18:407-411. |
| 119. | Menack MJ, Spitz JD, Arregui ME. Staging of pancreatic and ampullary cancers for resectability using laparoscopy with laparoscopic ultrasound. Surg Endosc. 2001;15:1129-1134. |
| 120. | Merchant NB, Conlon KC. Laparoscopic evaluation in pancreatic cancer. Semin Surg Oncol. 1998;15:155-165. |
| 121. | Nieveen van Dijkum EJ, Romijn MG, Terwee CB, de Wit LT, van der Meulen JH, Lameris HS, Rauws EA, Obertop H, van Eyck CH, Bossuyt PM. Laparoscopic staging and subsequent palliation in patients with peripancreatic carcinoma. Ann Surg. 2003;237:66-73. |
| 122. | Doran HE, Bosonnet L, Connor S, Jones L, Garvey C, Hughes M, Campbell F, Hartley M, Ghaneh P, Neoptolemos JP. Laparoscopy and laparoscopic ultrasound in the evaluation of pancreatic and periampullary tumours. Dig Surg. 2004;21:305-313. |
| 123. | John TG, Greig JD, Carter DC, Garden OJ. Carcinoma of the pancreatic head and periampullary region. Tumor staging with laparoscopy and laparoscopic ultrasonography. Ann Surg. 1995;221:156-164. |
| 124. | Minnard EA, Conlon KC, Hoos A, Dougherty EC, Hann LE, Brennan MF. Laparoscopic ultrasound enhances standard laparoscopy in the staging of pancreatic cancer. Ann Surg. 1998;228:182-187. |
| 125. | Murugiah M, Paterson-Brown S, Windsor JA, Miles WF, Garden OJ. Early experience of laparoscopic ultrasonography in the management of pancreatic carcinoma. Surg Endosc. 1993;7:177-181. |
| 126. | Rau B, Hünerbein M, Schlag PM. Is there additional information from laparoscopic ultrasound in tumor staging? Dig Surg. 2002;19:479-483. |
| 127. | Bemelman WA, de Wit LT, van Delden OM, Smits NJ, Obertop H, Rauws EJ, Gouma DJ. Diagnostic laparoscopy combined with laparoscopic ultrasonography in staging of cancer of the pancreatic head region. Br J Surg. 1995;82:820-824. |
| 128. | John TG, Wright A, Allan PL, Redhead DN, Paterson-Brown S, Carter DC, Garden OJ. Laparoscopy with laparoscopic ultrasonography in the TNM staging of pancreatic carcinoma. World J Surg. 1999;23:870-881. |
| 129. | Vollmer CM, Drebin JA, Middleton WD, Teefey SA, Linehan DC, Soper NJ, Eagon CJ, Strasberg SM. Utility of staging laparoscopy in subsets of peripancreatic and biliary malignancies. Ann Surg. 2002;235:1-7. |
| 130. | Pietrabissa A, Caramella D, Di Candio G, Carobbi A, Boggi U, Rossi G, Mosca F. Laparoscopy and laparoscopic ultrasonography for staging pancreatic cancer: critical appraisal. World J Surg. 1999;23:998-1002; discussion 1003. |
| 131. | Holzman MD, Reintgen KL, Tyler DS, Pappas TN. The role of laparoscopy in the management of suspected pancreatic and periampullary malignancies. J Gastrointest Surg. 1997;1:236-243; discussion 243-244. |
| 132. | Maire F, Sauvanet A, Trivin F, Hammel P, O’Toole D, Palazzo L, Vilgrain V, Belghiti J, Ruszniewski P, Levy P. Staging of pancreatic head adenocarcinoma with spiral CT and endoscopic ultrasonography: an indirect evaluation of the usefulness of laparoscopy. Pancreatology. 2004;4:436-440. |
| 133. | Rumstadt B, Schwab M, Schuster K, Hagmüller E, Trede M. The role of laparoscopy in the preoperative staging of pancreatic carcinoma. J Gastrointest Surg. 1997;1:245-250. |
| 134. | Annovazzi A, Peeters M, Maenhout A, Signore A, Dierckx R, Van De Wiele C. 18-fluorodeoxyglucose positron emission tomography in nonendocrine neoplastic disorders of the gastrointestinal tract. Gastroenterology. 2003;125:1235-1245. |
| 135. | Delbeke D, Pinson CW. Pancreatic tumors: role of imaging in the diagnosis, staging, and treatment. J Hepatobiliary Pancreat Surg. 2004;11:4-10. |
| 136. | Kalra MK, Maher MM, Boland GW, Saini S, Fischman AJ. Correlation of positron emission tomography and CT in evaluating pancreatic tumors: technical and clinical implications. AJR Am J Roentgenol. 2003;181:387-393. |
| 137. | Diederichs CG, Staib L, Vogel J, Glasbrenner B, Glatting G, Brambs HJ, Beger HG, Reske SN. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000;20:109-116. |
| 138. | Goh BK, Tan YM, Chung YF. Utility of fusion CT-PET in the diagnosis of small pancreatic carcinoma. World J Gastroenterol. 2005;11:3800-3802. |
| 139. | Heinrich S, Goerres GW, Schäfer M, Sagmeister M, Bauerfeind P, Pestalozzi BC, Hany TF, von Schulthess GK, Clavien PA. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg. 2005;242:235-243. |
| 140. | Jadvar H, Fischman AJ. Evaluation of pancreatic carcinoma with FDG PET. Abdom Imaging. 2001;26:254-259. |
| 141. | Rose DM, Delbeke D, Beauchamp RD, Chapman WC, Sandler MP, Sharp KW, Richards WO, Wright JK, Frexes ME, Pinson CW. 18Fluorodeoxyglucose-positron emission tomography in the management of patients with suspected pancreatic cancer. Ann Surg. 1999;229:729-737; discussion 737-738. |
| 142. | Nakamoto Y, Higashi T, Sakahara H, Tamaki N, Kogire M, Doi R, Hosotani R, Imamura M, Konishi J. Delayed (18)F-fluoro-2-deoxy-D-glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer. 2000;89:2547-2554. |
| 143. | Rajput A, Stellato TA, Faulhaber PF, Vesselle HJ, Miraldi F. The role of fluorodeoxyglucose and positron emission tomography in the evaluation of pancreatic disease. Surgery. 1998;124:793-797; discussion 797-798. |
| 144. | Syrota A, Duquesnoy N, Paraf A, Kellershohn C. The role of positron emission tomography in the detection of pancreatic disease. Radiology. 1982;143:249-253. |
| 145. | Delbeke D, Rose DM, Chapman WC, Pinson CW, Wright JK, Beauchamp RD, Shyr Y, Leach SD. Optimal interpretation of FDG PET in the diagnosis, staging and management of pancreatic carcinoma. J Nucl Med. 1999;40:1784-1791. |
| 146. | Zimny M, Bares R, Fass J, Adam G, Cremerius U, Dohmen B, Klever P, Sabri O, Schumpelick V, Buell U. Fluorine-18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: a report of 106 cases. Eur J Nucl Med. 1997;24:678-682. |
| 147. | Sendler A, Avril N, Helmberger H, Stollfuss J, Weber W, Bengel F, Schwaiger M, Roder JD, Siewert JR. Preoperative evaluation of pancreatic masses with positron emission tomography using 18F-fluorodeoxyglucose: diagnostic limitations. World J Surg. 2000;24:1121-1129. |
| 148. | Sperti C, Pasquali C, Decet G, Chierichetti F, Liessi G, Pedrazzoli S. F-18-fluorodeoxyglucose positron emission tomography in differentiating malignant from benign pancreatic cysts: a prospective study. J Gastrointest Surg. 2005;9:22-82; discussion 28-29. |
| 149. | Diederichs CG, Staib L, Glatting G, Beger HG, Reske SN. FDG PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med. 1998;39:1030-1033. |
| 150. | Lemke AJ, Niehues SM, Hosten N, Amthauer H, Boehmig M, Stroszczynski C, Rohlfing T, Rosewicz S, Felix R. Retrospective digital image fusion of multidetector CT and 18F-FDG PET: clinical value in pancreatic lesions--a prospective study with 104 patients. J Nucl Med. 2004;45:1279-1286. |
| 151. | Mertz HR, Sechopoulos P, Delbeke D, Leach SD. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc. 2000;52:367-371. |
| 152. | Hosten N, Lemke AJ, Wiedenmann B, Böhmig M, Rosewicz S. Combined imaging techniques for pancreatic cancer. Lancet. 2000;356:909-910. |
| 153. | Cusack JC Jr, Fuhrman GM, Lee JE, Evans DB. Managing unsuspected tumor invasion of the superior mesenteric-portal venous confluence during pancreaticoduodenectomy. Am J Surg. 1994;168:352-354. |
| 154. | Fuhrman GM, Leach SD, Staley CA, Cusack JC, Charnsangavej C, Cleary KR, El-Naggar AK, Fenoglio CJ, Lee JE, Evans DB. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg. 1996;223:154-162. |
| 155. | Sakorafas GH, Farnell MB, Nagorney DM, Farley DR, Que FG, Donohue JH, Thompson GG, Sarr MG. Management of peri-pancreatic vasculature during pancreatoduodenectomy: tips to avoid severe haemorrhage. Eur J Surg Oncol. 1999;25:524-528. |
| 156. | Takahashi S, Ogata Y, Tsuzuki T. Combined resection of the pancreas and portal vein for pancreatic cancer. Br J Surg. 1994;81:1190-1193. |
| 157. | Harrison LE, Klimstra DS, Brennan MF. Isolated portal vein involvement in pancreatic adenocarcinoma. A contraindication for resection? Ann Surg. 1996;224:342-347; discussion 347-349. |
| 158. | Imamura M, Doi R. Treatment of locally advanced pancreatic cancer: should we resect when resectable? Pancreas. 2004;28:293-295. |
| 159. | Sindelar WF. Clinical experience with regional pancreatectomy for adenocarcinoma of the pancreas. Arch Surg. 1989;124:127-132. |
| 160. | Nagakawa T, Mori K, Nakano T, Kadoya M, Kobayashi H, Akiyama T, Kayahara M, Ohta T, Ueno K, Higashino Y. Perineural invasion of carcinoma of the pancreas and biliary tract. Br J Surg. 1993;80:619-621. |
| 161. | Fortner JG. Technique of regional subtotal and total pancreatectomy. Am J Surg. 1985;150:593-600. |
| 162. | Fortner JG. Regional resection of cancer of the pancreas: a new surgical approach. Surgery. 1973;73:307-320. |
| 163. | Konishi M, Kinoshita T, Nakagori T, Inoue K, Oda T, Kimata T, Kikuchi H, Ryu M. Distal pancreatectomy with resection of the celiac axis and reconstruction of the hepatic artery for carcinoma of the body and tail of the pancreas. J Hepatobiliary Pancreat Surg. 2000;7:183-187. |
| 164. | Li B, Chen FZ, Ge XH, Cai MZ, Jiang JS, Li JP, Lu SH. Pancreatoduodenectomy with vascular reconstruction in treating carcinoma of the pancreatic head. Hepatobiliary Pancreat Dis Int. 2004;3:612-615. |
| 165. | Hirai I, Kimura W, Kamiga M, Mizutani M, Takeshita A, Watanabe T, Fuse A. The significance of intraoperative Doppler ultrasonography in evaluating hepatic arterial flow when assessing the indications for the Appleby procedure for pancreatic body cancer. J Hepatobiliary Pancreat Surg. 2005;12:55-60. |
| 166. | Horiguchi A, Miyakawa S, Mizuno K, Ishihara S, Miura K. Portal vein resection without reconstruction during Appleby operation in a patient with pancreatic body carcinoma with cavernous transformation. Hepatogastroenterology. 1999;46:2628-2630. |
| 167. | Kimura W, Han I, Furukawa Y, Sunami E, Futakawa N, Inoue T, Shinkai H, Zhao B, Muto T, Makuuchi M. Appleby operation for carcinoma of the body and tail of the pancreas. Hepatogastroenterology. 1997;44:387-393. |
| 168. | Yamaguchi K, Nakano K, Kobayashi K, Ogura Y, Konomi H, Sugitani A, Tanaka M. Appleby operation for pancreatic body-tail carcinoma: report of three cases. Surg Today. 2003;33:873-878. |
| 169. | Gagandeep S, Artinyan A, Jabbour N, Mateo R, Matsuoka L, Sher L, Genyk Y, Selby R. Extended pancreatectomy with resection of the celiac axis: the modified Appleby operation. Am J Surg. 2006;192:330-335. |
| 170. | Hirano S, Kondo S, Hara T, Ambo Y, Tanaka E, Shichinohe T, Suzuki O, Hazama K. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg. 2007;246:46-51. |
| 171. | Kusano T, Tamai O, Miyazato H, Isa T, Shiraishi M, Muto Y. Vascular reconstruction of the hepatic artery using the gastroepiploic artery: a case report. Hepatogastroenterology. 1999;46:2278-2280. |
| 172. | Ohwada S, Ogawa T, Ohya T, Kawashima Y, Nakamura S, Satoh Y, Saitoh A, Takeyoshi I, Yokoe T, Morishita Y. Gonadal vein graft for hepatic artery reconstruction. Hepatogastroenterology. 1999;46:1823-1826. |
| 173. | Sarmiento JM, Panneton JM, Nagorney DM. Reconstruction of the hepatic artery using the gastroduodenal artery. Am J Surg. 2003;185:386-387. |
| 174. | Kondo S, Katoh H, Shimizu T, Omi M, Hirano S, Ambo Y, Okushiba S, Morikawa T. Preoperative embolization of the common hepatic artery in preparation for radical pancreatectomy for pancreas body cancer. Hepatogastroenterology. 2000;47:1447-1449. |
| 175. | Makary MA, Fishman EK, Cameron JL. Resection of the celiac axis for invasive pancreatic cancer. J Gastrointest Surg. 2005;9:503-507. |
| 176. | Mayumi T, Nimura Y, Kamiya J, Kondo S, Nagino M, Kanai M, Miyachi M, Hamaguchi K, Hayakawa N. Distal pancreatectomy with en bloc resection of the celiac artery for carcinoma of the body and tail of the pancreas. Int J Pancreatol. 1997;22:15-21. |
| 177. | Shimada K, Sano T, Sakamoto Y, Kosuge T. Clinical implications of combined portal vein resection as a palliative procedure in patients undergoing pancreaticoduodenectomy for pancreatic head carcinoma. Ann Surg Oncol. 2006;13:1569-1578. |
| 178. | Schäfer M, Müllhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg. 2002;236:137-148. |
| 179. | Strasberg SM, Drebin JA, Soper NJ. Evolution and current status of the Whipple procedure: an update for gastroenterologists. Gastroenterology. 1997;113:983-994. |
| 180. | Takahashi S, Ogata Y, Aiura K, Kitajima M, Hiramatsu K. Combined resection of the portal vein for pancreatic cancer: preoperative diagnosis of invasion by portography and prognosis. Hepatogastroenterology. 2000;47:545-549. |
| 181. | Riediger H, Makowiec F, Fischer E, Adam U, Hopt UT. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg. 2006;10:1106-1115. |
| 182. | Carrère N, Sauvanet A, Goere D, Kianmanesh R, Vullierme MP, Couvelard A, Ruszniewski P, Belghiti J. Pancreaticoduodenectomy with mesentericoportal vein resection for adenocarcinoma of the pancreatic head. World J Surg. 2006;30:1526-1535. |
| 183. | Fukuda S, Oussoultzoglou E, Bachellier P, Rosso E, Nakano H, Audet M, Jaeck D. Significance of the depth of portal vein wall invasion after curative resection for pancreatic adenocarcinoma. Arch Surg. 2007;142:172-179; discussion 180. |
| 184. | Clavien PA, Rüdiger HA. A simple technique of portal vein resection and reconstruction during pancreaticoduodenectomy. J Am Coll Surg. 1999;189:629-634. |
| 185. | van Geenen RC, ten Kate FJ, de Wit LT, van Gulik TM, Obertop H, Gouma DJ. Segmental resection and wedge excision of the portal or superior mesenteric vein during pancreatoduodenectomy. Surgery. 2001;129:158-163. |
| 186. | Chae YS, Choi JS, Kim KS, Seong JS, Lee WJ, Kim BR. Preoperative chemoradiation and pancreaticoduodenectomy with portal vein resection for localized advanced pancreatic cancer. Yonsei Med J. 2003;44:551-556. |
| 187. | Harrison LE, Brennan MF. Portal vein resection for pancreatic adenocarcinoma. Surg Oncol Clin N Am. 1998;7:165-181. |
| 188. | Launois B, Stasik C, Bardaxoglou E, Meunier B, Campion JP, Greco L, Sutherland F. Who benefits from portal vein resection during pancreaticoduodenectomy for pancreatic cancer? World J Surg. 1999;23:926-929. |
| 189. | Tashiro S, Uchino R, Hiraoka T, Tsuji T, Kawamoto S, Saitoh N, Yamasaki K, Miyauchi Y. Surgical indication and significance of portal vein resection in biliary and pancreatic cancer. Surgery. 1991;109:481-487. |
| 190. | Nakao A, Harada A, Nonami T, Kaneko T, Takagi H. Regional vascular resection using catheter bypass procedure for pancreatic cancer. Hepatogastroenterology. 1995;42:734-739. |
| 191. | Howard TJ, Villanustre N, Moore SA, DeWitt J, LeBlanc J, Maglinte D, McHenry L. Efficacy of venous reconstruction in patients with adenocarcinoma of the pancreatic head. J Gastrointest Surg. 2003;7:1089-1095. |
| 192. | Allema JH, Reinders ME, van Gulik TM, van Leeuwen DJ, de Wit LT, Verbeek PC, Gouma DJ. Portal vein resection in patients undergoing pancreatoduodenectomy for carcinoma of the pancreatic head. Br J Surg. 1994;81:1642-1646. |
| 193. | Launois B, Franci J, Bardaxoglou E, Ramee MP, Paul JL, Malledant Y, Campion JP. Total pancreatectomy for ductal adenocarcinoma of the pancreas with special reference to resection of the portal vein and multicentric cancer. World J Surg. 1993;17:122-126; discussion 126-127. |
| 194. | Aramaki M, Matsumoto T, Etoh T, Ishio T, Himeno Y, Sasaki A, Yada K, Kawano K, Kitano S. Clinical significance of combined pancreas and portal vein resection in surgery for pancreatic adenocarcinoma. Hepatogastroenterology. 2003;50:263-266. |
| 195. | Bachellier P, Nakano H, Oussoultzoglou PD, Weber JC, Boudjema K, Wolf PD, Jaeck D. Is pancreaticoduodenectomy with mesentericoportal venous resection safe and worthwhile? Am J Surg. 2001;182:120-129. |
| 196. | Leach SD, Lee JE, Charnsangavej C, Cleary KR, Lowy AM, Fenoglio CJ, Pisters PW, Evans DB. Survival following pancreaticoduodenectomy with resection of the superior mesenteric-portal vein confluence for adenocarcinoma of the pancreatic head. Br J Surg. 1998;85:611-617. |
| 197. | Shibata C, Kobari M, Tsuchiya T, Arai K, Anzai R, Takahashi M, Uzuki M, Sawai T, Yamazaki T. Pancreatectomy combined with superior mesenteric-portal vein resection for adenocarcinoma in pancreas. World J Surg. 2001;25:1002-1005. |
| 198. | Pedrazzoli S, Pasquali C, Sperti C. General aspects of surgical treatment of pancreatic cancer. Dig Surg. 1999;16:265-275. |
| 199. | Machado MC, Penteado S, Cunha JE, Jukemura J, Herman P, Bacchella T, Machado MA, Montagnini AL. Pancreatic head tumors with portal vein involvement: an alternative surgical approach. Hepatogastroenterology. 2001;48:1486-1487. |
| 200. | Zhou GW, Wu WD, Xiao WD, Li HW, Peng CH. Pancreatectomy combined with superior mesenteric-portal vein resection: report of 32 cases. Hepatobiliary Pancreat Dis Int. 2005;4:130-134. |
| 201. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Pancreaticoduodenectomy with en bloc portal vein resection for pancreatic carcinoma with suspected portal vein involvement. World J Surg. 2004;28:602-608. |
| 202. | Siriwardana HP, Siriwardena AK. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg. 2006;93:662-673. |
| 203. | Koniaris LG, Staveley-O’Carroll KF, Zeh HJ, Perez E, Jin XL, Maley WR, Zabari G, Bartlett DL, Khanna A, Franceschi D. Pancreaticoduodenectomy in the presence of superior mesenteric venous obstruction. J Gastrointest Surg. 2005;9:915-921. |
| 204. | Moore GE, Sako Y, Thomas LB. Radical pancreatoduodenectomy with resection and reanastomosis of the superior mesenteric vein. Surgery. 1951;30:550-553. |
| 205. | Park DI, Lee JK, Kim JE, Hyun JG, Shim SG, Lee KT, Palk SW, Rhee JC, Choi KW, Lim JH. The analysis of resectability and survival in pancreatic cancer patients with vascular invasion. J Clin Gastroenterol. 2001;32:231-234. |
| 206. | Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, Gomez HF, Sun CC, Crane CH, Wolff RA. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935-949; discussion 949-950. |
| 207. | Turrini O, Viret F, Moureau-Zabotto L, Guiramand J, Moutardier V, Lelong B, de Chaisemartin C, Giovannini M, Delpero JR. Neoadjuvant 5 fluorouracil-cisplatin chemoradiation effect on survival in patients with resectable pancreatic head adenocarcinoma: a ten-year single institution experience. Oncology. 2009;76:413-419. |
| 208. | Raptopoulos V, Steer ML, Sheiman RG, Vrachliotis TG, Gougoutas CA, Movson JS. The use of helical CT and CT angiography to predict vascular involvement from pancreatic cancer: correlation with findings at surgery. AJR Am J Roentgenol. 1997;168:971-977. |
| 209. | Diehl SJ, Lehmann KJ, Sadick M, Lachmann R, Georgi M. Pancreatic cancer: value of dual-phase helical CT in assessing resectability. Radiology. 1998;206:373-378. |
| 210. | House MG, Yeo CJ, Cameron JL, Campbell KA, Schulick RD, Leach SD, Hruban RH, Horton KM, Fishman EK, Lillemoe KD. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg. 2004;8:280-288. |
| 211. | Nakao A, Harada A, Nonami T, Kaneko T, Inoue S, Takagi H. Clinical significance of portal invasion by pancreatic head carcinoma. Surgery. 1995;117:50-55. |
| 212. | Hartel M, Niedergethmann M, Farag-Soliman M, Sturm JW, Richter A, Trede M, Post S. Benefit of venous resection for ductal adenocarcinoma of the pancreatic head. Eur J Surg. 2002;168:707-712. |