Published online Feb 14, 2010. doi: 10.3748/wjg.v16.i6.749
Revised: December 16, 2009
Accepted: December 23, 2009
Published online: February 14, 2010
AIM: To explore the interventional effects and mechanism of in vitro cultivated Calculus Bovis compound preparation (ICCBco) on pulmonary lesions in portal hypertensive rabbits with schistosomiasis.
METHODS: The experimental group included 20 portal hypertensive rabbits with schistosomiasis treated by ICCBco. The control group included 20 portal hypertensive rabbits with schistosomiasis treated by praziquantel. The morphological changes of the pulmonary tissues were observed under light and electron microscopy. The expression of fibronectin (FN) and laminin (LN) in the lung tissues was analyzed by immunohistochemistry.
RESULTS: Under light microscope, the alveolar exudation in the lung tissue was more frequently observed in the control group, while the alveolar space was fairly dry in the lung tissue of ICCBco group. Under electron microscope, more alveolar exudation in the lung tissue, and more macrophages, alveolar angiotelectasis and the blurred three-tier structure of alveolar-capillary barrier could be seen in the control group. In ICCBco group, fibers within the alveolar interspace slightly increased in some lung regions, and the structure of type I epithelium, basement membrane and endodermis was complete, and no obvious exudation from the alveolar space, and novascular congestion could be observed. There was a positive or strong positive expression of FN and LN in the lung tissue of the control group, while there was a negative or weak positive expression of FN and LN in ICCBco group.
CONCLUSION: ICCBco can effectively prevent pulmonary complications in portal hypertensive rabbits with schistosomiasis by means of improving lung microcirculation and lowering the content of extracellular matrix.
-
Citation: Li T, Yang Z, Cai HJ, Song LW, Lu KY, Zhou Z, Wu ZD. Effects of
in vitro cultivatedCalculus Bovis compound on pulmonary lesions in rabbits with schistosomiasis. World J Gastroenterol 2010; 16(6): 749-754 - URL: https://www.wjgnet.com/1007-9327/full/v16/i6/749.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i6.749
Portal hypertension is a vascular lesion that initially arises in liver, but is also accompanied with structural and functional changes of blood vessels in extrahepatic portal system, systemic circulation and pulmonary circulation, which now collectively called portal hypertensive vascular lesions[1]. In clinical practice, much attention has been paid to the prevention and treatment of complications such as ascites, esophagogastric variceal bleeding; however the management of pulmonary complications is ignored which affects the prognosis of patients. Hence, drugs used for prevention and treatment of pulmonary complications seems to be very important. In vitro cultivated Calculus Bovis (ICCB)[2] developed by Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, with independent intellectual property rights, is one of the first-class national Chinese herbal medicine certificate of new drugs. ICCB is bilirubin calcium stones from in vitro cultivated bovine bile by simulating the formation principle and biochemical processes of gallstone in vivo using the modern bio-medical technology. The pharmacy, pharmacology and toxicology of the drug and phase I-IV clinical trials show that ICCB is consistent with natural bezoar in property, structure, composition, content and clinical efficacy, and no obvious toxicity and adverse effects could been observed (SFDA approval number Z20010075). Calculus Bovis has effects of clearing heat and toxic materials, promoting blood circulation and reducing swelling, eliminating stasis and facilitating tissue recovery, lowering vascular permeability, clearing softened blood vessels, scavenging free radicals and anti-anoxia in the principle of traditional Chinese medicine. In vitro cultivated Calculus Bovis compound preparation (ICCBco)[3-5] is mainly composed of ICCB, Chinese Paris Rhizome, polygonum cuspidatum, appendiculate cremastra pseudobulb, frankincense, and myrrh, with functions of clearing away heat and toxic materials, removing blood stasis, reducing swelling, eliminating blood stasis and promoting tissue regeneration. To evaluate the efficacy of ICCBco in the treatment of lung lesions in portal hypertensive rabbits with schistosomiasis as the experimental animal model, we performed a randomized, double-blind, controlled trial to observe the pathological changes and pathological mechanism of fibronectin (FN) and laminin (LN) expressions in the lung tissue of portal hypertensive rabbits with schistosomiasis.
Experimental animal: Forty healthy adult rabbits (male, 2.5 kg in weight) provided by the Medical Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology.
Medicine: ICCBco (0.25 g/granule, 60 granules/bottle), Levo-praziquantel (0.1 g/tablet, 10/box), both offered by Tongji Medical College, Huazhong University of Science and Technology.
Reagents: Sheep serum, rabbit anti-human FN and LN primary antibody serum (1:100) were purchased from Boster Company, Wuhan. SABC kit was obtained from Zhongshan Company, Beijing.
Instruments: OPTON transmission electron microscopy (TEM, Carl Zeiss EM 10 C, Oberkochen, Germany) and optical microscopy (Olympus, Japan) were used.
Animal model establishment: The hair over the abdomen was shaved off, and the rabbits were infected by cercarie of oncomelania hupensis by the sticking and pasting method[6]. The solution containing 200 ± 5 cercarie was dripped on the shaved area of each rabbit and covered by a slide for 15 min, which led to acute infection. After 40 d, ICCBco was administered to the rabbits (6 granules/d). After 60 d, levo-praziquantel was perfused to kill parasites with a dosage of 500 mg/d for two consecutive days. The pulmonary fibrosis model was established in 120 d or so, and the experimental animals were killed by necropsy procedure in 4 mo. Pulmonary samples were obtained by autopsy. The experimental animals were divided into two groups: group A (control group), treated with praziquantel (n = 20); group B, treated with praziquantel plus ICCBco (n = 20).
Sample collection: Batches of rabbits were sacrificed by injecting an overdose of anesthesia with 1% Thiopental Sodium (50 mg/kg) via the ear vein. A small sample of the left lung tissue about the size of 2 cm × l cm × l cm was harvested after laparotomy, routinely fixed with 10% formaldehyde solution, and embedded in paraffin wax within 12 h for pathological examination. Another sample of the left lung tissue with a size of l mm × l mm × 0.5 mm was immediately put in a vial containing 20 g/L glutaraldehyde, and then sent to the department of ultrastructural pathology for TEM examination in 1 h.
Staining method: Samples of lung tissue were fixed in 40 g/L neutral buffered formaldehyde solution, routinely embedded in paraffin wax, and serial sections were made at a thickness of 5 μm for hematoxylin and eosin (HE) staining and observed under optical microscopy.
Samples were fixed in 10% formaldehyde solution, and then treated with 2.5% potassium dichromate mordant prepared by 5% acetic acid for 12-18 h, and immersed in water washing for 10 min. Sections were treated with sodium thiosulfate in order to remove mercury deposition, and then fully washed with water, and stained for 2-5 min in Ehrlich’s hematoxylin followed by wash in water. Differentiation was done in acid alcohol and thoroughly washed in running water until the sections turned blue, and then stained in 1% aqueous acid fuchsin solution for 5 min followed by rinsing sections in running water for 30 s or longer until color of collagen disappeared, and rinsed in distilled water. Sections were stained in Aniline Blue/Orange G for 20 min, washed in running water for 2-5 min, dehydrated, differentiated through 95% alcohols, washed in absolute alcohol and then passed into xylene for tissue transparent. At last, sections were mounted with gelatin and observed under optical microscopy.
Flesh sample of the lung tissue were cut into slices about a size of 1 cm × l cm × l cm, and placed in 25 mg/L glutaraldehyde for 2 h as pre-fixation, then washed in pH 7.4 phosphate buffer solution followed by post- fixation in 10 g/L osmium acid under 4°C for 2 h. The slices were dehydrated with progressively increased concentration of alcohol and acetone, embedded in epoxy resin EPON 812. Ultrathin sections were cut and stained with double staining of uranium and lead (acetic acid-uranium-lead citrate) so as to be observed under TEM.
Sections were routinely deparaffinized, treated with 30 mL/L hydrogen peroxide, which was freshly prepared by distilled water, for 10 min at room temperature to inactive endogenous peroxidases and rinsed three times in distilled water. Normal goat serum blocking solution was added and incubated at room temperature for 30 min in order to reduce non-specific background staining. Excessive liquid was removed without rinse. Sections were incubated in primary antibody (1:100) at 4°C overnight, then washed thrice in PBS (0.1 mol/L), and incubated in biotinylated secondary antibody for 30 min at room temperature, then rinsed thrice in PBS for 5 min. And they were incubated with SABC for 30 min at 37°C followed by rinse four times in PBS for 5 min. Sections were incubated in DAB with DAB reagent kit: One drop of reagent A and reagent B were added in 1 mL distilled water, and placed the well-mixed reagents onto the slices. The reaction was allowed to develop for 3-10 min under the control of microscopy at room temperature, and rinsed in distilled water. Sections were lightly counterstained with hematoxylin. After dehydration, transparent, and mounting process, slides were observed under microscopy. PBS was used as negative control instead of primary antibody and a known positive slice was taken as positive control. Brownish yellow granules in the cytoplasm were considered as positive FN and LN. Selected regions containing endothelial cells under optical microscopy were input into HPIAS-l000 automatic color image analysis system and the results were shown as the average absorbance.
All data were presented as the mean ± SD. Statistical analysis was done using SPSS software version 11.0. Statistical differences between the two groups were analyzed using ANOVA and t tests. P values less than 0.05 were considered significant.
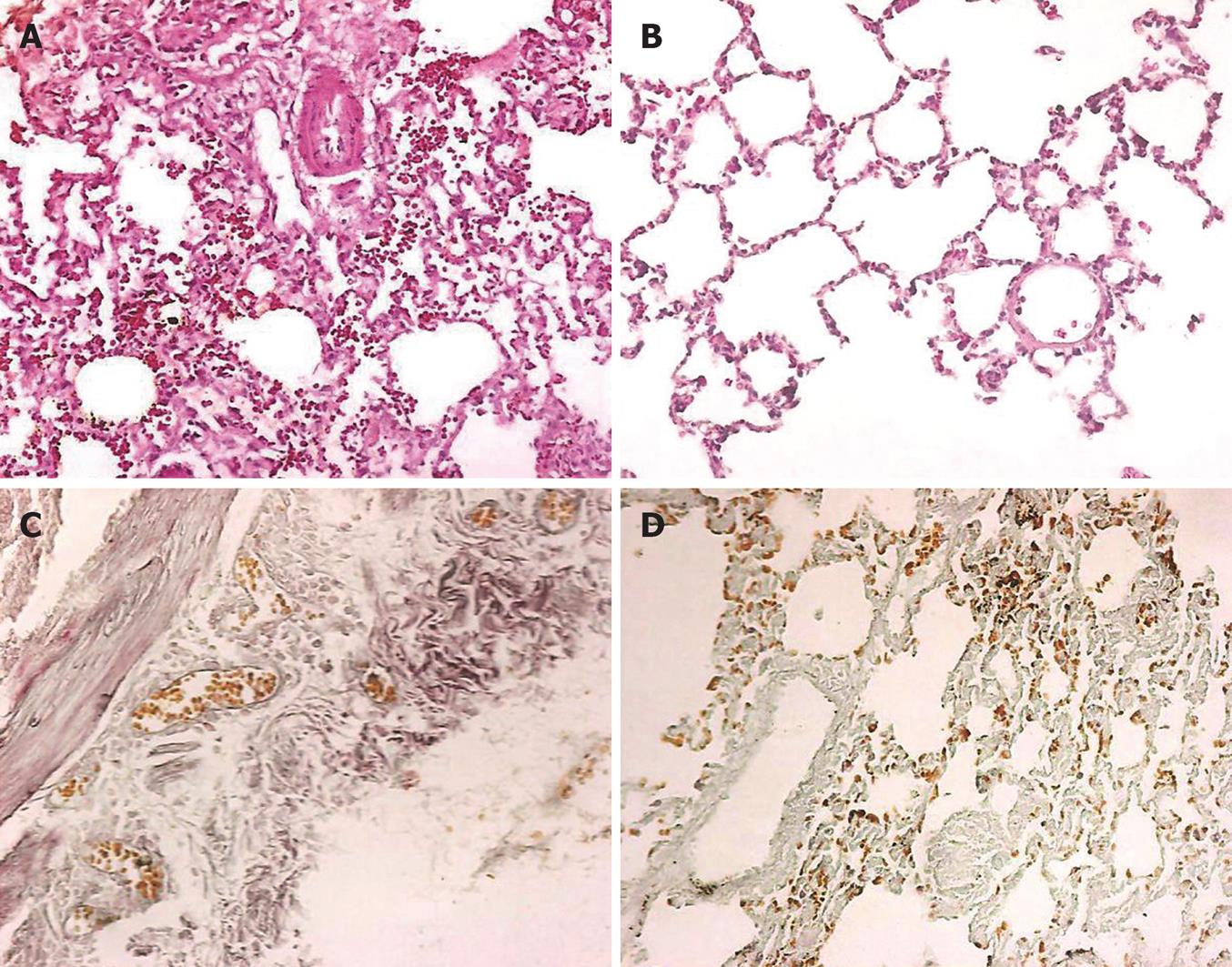
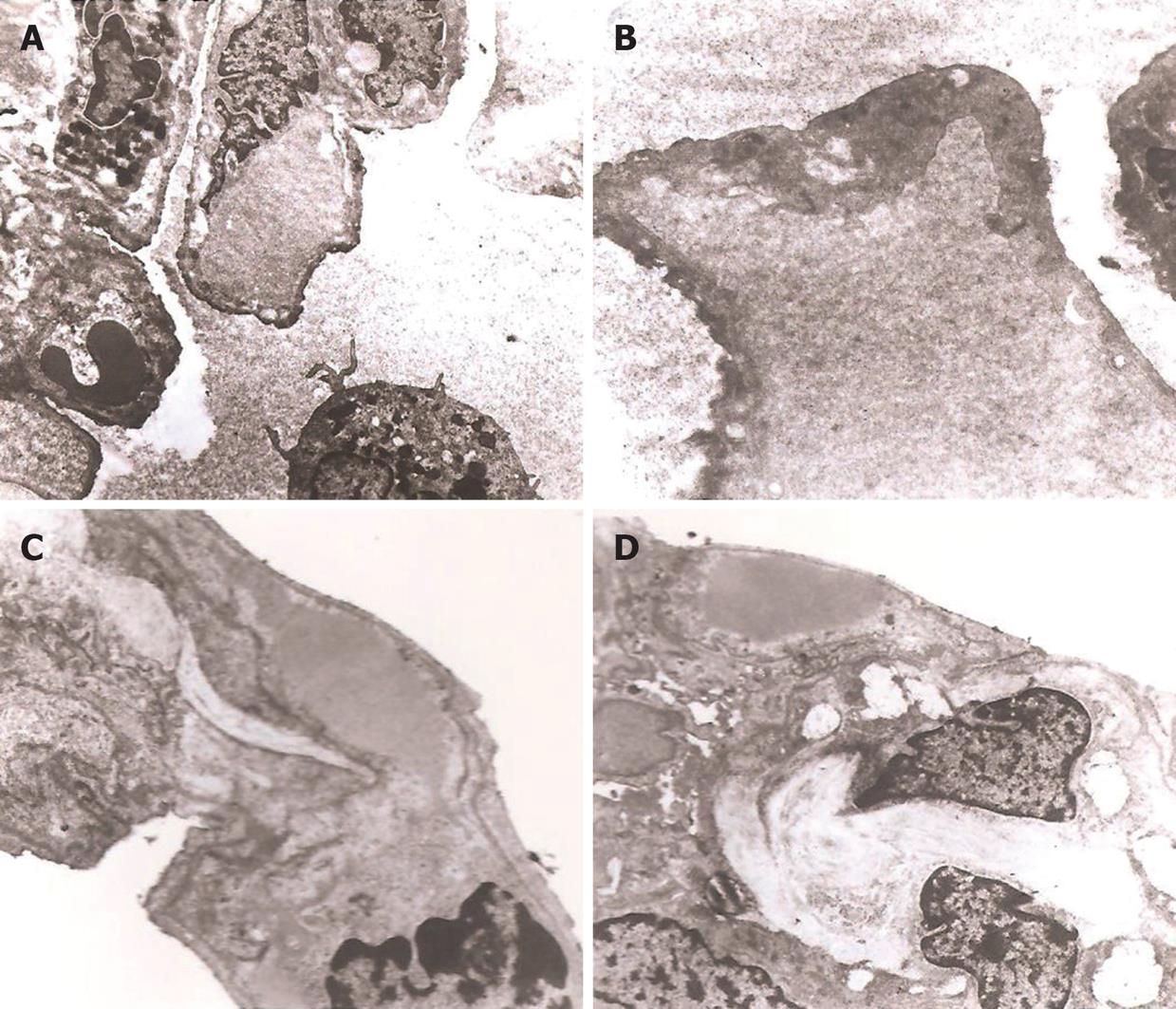
HE staining results showed that the alveolar exudation in the lung tissue was seen more frequently in group A, while the alveolar space was fairly dry in the lung tissue of group B (Figure 1A and B). Mallory trichrome staining results showed more alveolar exudation and collagen fibers in the lung tissue of group A, while fairly dry alveolar space and less collagen fibers were seen in group B (Figure 1C and D). More alveolar exudation was found in group A, and more macrophages, alveolar angiotelectasis and the blurred three-tier structure of alveolar-capillary barrier could also be seen under TEM observation (Figure 2A and B). In group B, fibers within the alveolar interspace slightly increased in some lung regions, and the structure of type I epithelium, basement membrane and endodermis was complete, and no obvious exudation in alveolar space and no vascular congestion were observed (Figure 2C and D).
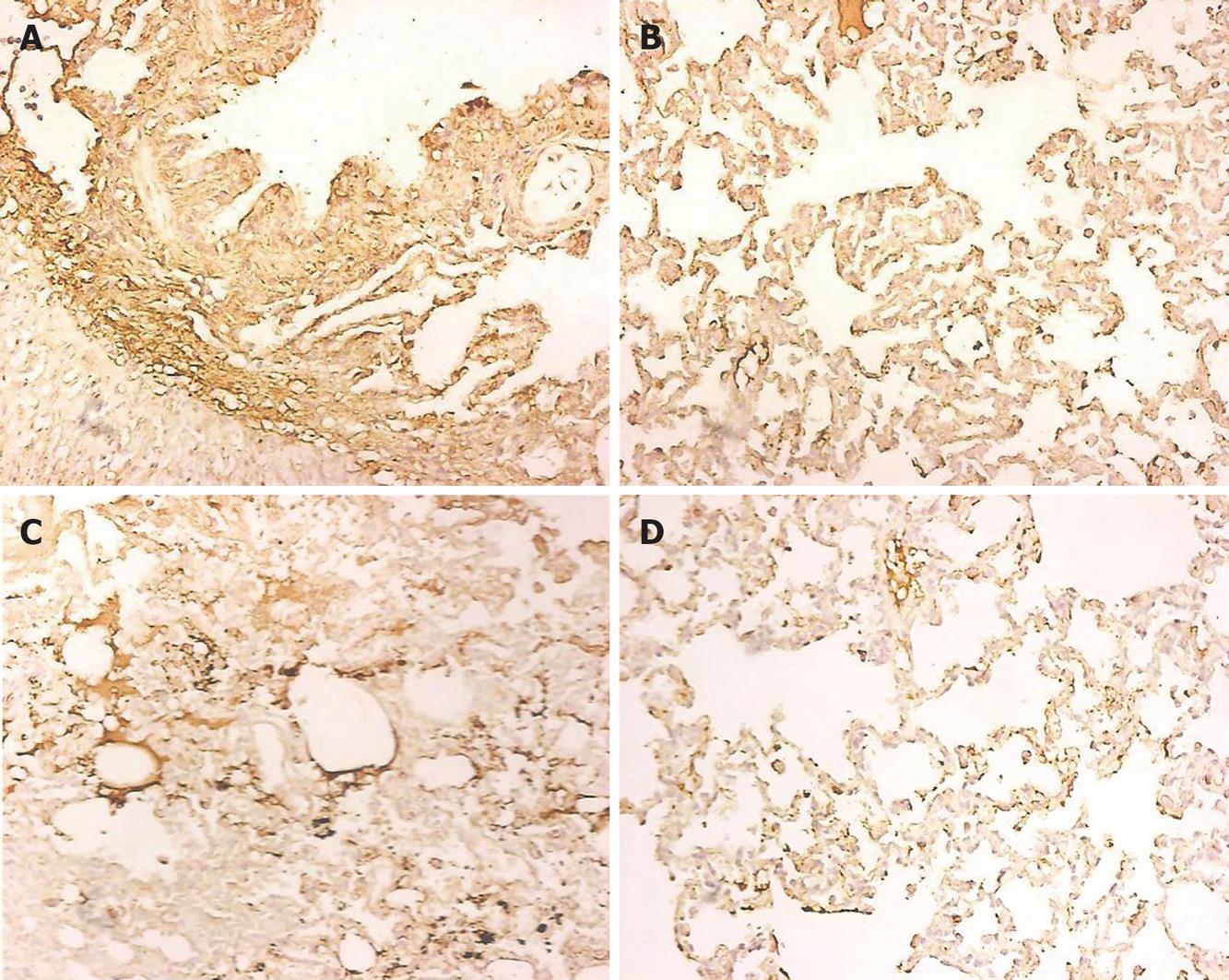
There was a positive or strong positive expression of FN in the lung tissue of group A, while a negative or weak positive expression of FN in group B (Figure 3A and B). Group A showed a positive or strong positive expression of LN in the lung tissue, while group B showed a negative or weak positive expression of LN (Figure 3C and D). The integrated optical density (IOD) values are shown in Table 1.
| Group | n | IOD value of FN | IOD value of LN |
| Control group | 20 | 19.47 ± 1.21 | 20.23 ± 0.87 |
| ICCBco group | 20 | 13.15 ± 0.94 | 12.08 ± 1.26 |
Rabbit model of hepatic fibrosis of schistosomiasis was consistent with the natural course and pathological development process of human liver fibrosis; previous studies have confirmed that 4 mo after rabbits were infected with schistosomiasis, the hepatic fibrosis formed, indicating it is a mature model of liver fibrosis.
Schistosomiasis can not only cause liver fibrosis and portal hypertension, but also cause tissue lesions. The most dangerous lesion is pulmonary vascular lesion characterized by hypoxemia and decreased oxygen saturation. However, the exact mechanisms of hypoxemia are still controversial, and it is currently believed to be caused by the three factors together - intrapulmonary shunt, ventilation - perfusion imbalance and pulmonary diffusion dysfunction. The important fundamental cause of these pathologic changes is pulmonary vasodilation[7]. Our results showed that there was a slight increase in fibers between alveolar gap in some parts of the lung tissue, and the structure of type I epithelium, basement membrane and endodermis was complete, and there was no obvious exudation in alveolar space, and no vascular congestion in the ICCBco group, while more alveolar exudation, more macrophages, alveolar angiotelectasis and the blurred three-tier structure of alveolar-capillary barrier were seen in praziquantel control group. This indicates that ICCBco could make a marked improvement in pulmonary ischemia, hypoxia, pulmonary function, blood flow blockade, damage, and connective tissue hyperplasia, which would shed light on pathological basis of clinical manifestations.
Extracellular matrix (ECM) proteins can be categorized into two kinds: collagen and non-collagen protein. ECM not only provides supporting structure and attachment for tissues but also regulates cell adhesion, migration, proliferation, differentiation, and tissue trauma repair and fibrosis. FN is a glycoprotein of large molecular weight with highly active adhesion, mainly derived from macrophage. FN induces chemotactic migration of interstitial cells[8], and promotes fibroblast division and proliferation[9]. Studies have found that FN mRNA and protein expression of alveolar macrophages and interstitial fibroblasts were markedly elevated in patients with pulmonary fibrosis[10], FN can transmit messages into cells through adhesion molecules on the surface of fibroblast cells and plays an initial role in the lung fibrosis process. It is currently believed that FN first appears in the early pulmonary fibrosis, and after then other interstitial elements occurs. LN is a large-molecular-weight non-collagen glycoprotein existing in the transparent layer of the basement membrane, which plays an important role in the maintenance of structure and function of alveolar and capillary basement membrane. Basement membrane provides a support for the regeneration of injured epithelial cells, and is the barrier for the entry of molecules and cells into the alveolar cavity[11,12]. The normal lung tissue contains very little LN. There is a significant increase of LN in pulmonary fibrosis or liver cirrhosis, which is about 10 times the level of LN in the normal lung and is consistent with collagen content in fibrosis[13]. Some scholars found that LN fluorescence in alveolar septa was enhanced in rats with early stage pulmonary fibrosis, later became thicker and deranged, presuming that LN participated in the whole process of experimental pulmonary fibrosis, and might play an important role in the development of fibrosis[14]. Previous experiments showed that abnormal accumulation of type I and Ш collagen, FN and LN in extracellular matrix at the early stage of schistosomiasis-induced liver fibrosis[15]. FN and LN are, therefore, better indicators for pulmonary fibrosis. In this study, we found that there was a significant decrease in the expression of FN and LN in ICCBco group compared with praziquantel control group, which suggests ICCBco could effectively inhibit the formation of pulmonary fibrosis. With functions of clearing away heat and toxic materials, removing blood stasis, reducing swelling, eliminating blood stasis and promoting tissue regeneration, it could improve pulmonary microcirculation, inhibit connective tissue proliferation, degrade extracellular matrix, reduce pulmonary damage, hence inhibiting the formation of pulmonary fibrosis.
In summary, ICCBco can effectively prevent pulmonary complications of portal hypertensive rabbits with schistosomiasis. Its function in suppressing pulmonary lesions is achieved by removing heat, toxic materials and blood stasis, reducing swelling, eliminating blood stasis and promoting tissue regeneration to block the activation of alveolar macrophages, improve pulmonary microcirculation, and reduce ECM. The successful development of ICCB and the preliminary study on portal hypertensive pulmonary lesions caused by schistosomiasis suggest that it is of great significance and prospects for further basic and clinical research, development and clinical application of new drugs and preparations to treat portal hypertensive pulmonary lesions induced by schistosomiasis.
Portal hypertension is a vascular lesion that initially arises in liver, but structural and functional changes of blood vessels in extrahepatic portal system, systemic circulation and pulmonary circulation also accompany, which now collectively called portal hypertensive vascular lesions. In clinical practice, much attention has been paid to the prevention and treatment of complications such as ascites, esophagogastric variceal bleeding; however the management of pulmonary complications is ignored which affects the prognosis of patients. Hence, drugs used for prevention and treatment of pulmonary complications seem to be very important.
In vitro cultivated Calculus Bovis compound preparation (ICCBco) is composed of ICCB, Chinese Paris Rhizome, polygonum cuspidatum, appendiculate cremastra pseudobulb, frankincense, and myrrh, and has the functions of clearing away heat and toxic materials, removing blood stasis, reducing swelling, eliminating blood stasis and promoting tissue regeneration, according to the principle of traditional Chinese medicine. However, the topic has not been unequivocally addressed. This study evaluated the efficacy of ICCBco in the treatment of lung lesions in portal hypertensive rabbits with schistosomiasis as the experimental animal model.
The present study explored the pathogenesis of portal hypertension and the prevention and treatment of its pulmonary complications (hepatopulmonary syndrome, pulmonary fibrosis, pulmonary hypertension, pulmonary venous hypertension) from a new perspective of portal hypertensive vascular disease. ICCB is a Class 1 new Chinese medicine developed by Wuhan Tongji Hospital with independent intellectual property rights, is a treasure of traditional Chinese medicine. To investigate its role in the treatment of schistosomiasis-induced pulmonary complications of portal hypertension has far-reaching significance.
The successful development of ICCB and the preliminary study on portal hypertensive pulmonary lesions caused by schistosomiasis suggest that it is of great significance and prospects for further basic and clinical research, development and clinical application of new drugs and preparations to treat portal hypertensive pulmonary lesions induced by schistosomiasis.
Composed of ICCB, Chinese Paris Rhizome, polygonum cuspidatum, appendiculate cremastra pseudobulb, frankincense, and myrrh, ICCBco can effectively prevent pulmonary complications of portal hypertensive rabbits with schistosomiasis. ICCBco could improve pulmonary microcirculation, inhibit connective tissue proliferation, degrade extracellular matrix, reduce pulmonary damage, hence inhibiting the formation of pulmonary fibrosis.
This is a very interesting research but not well planned.
Peer reviewer: Heitor Rosa, Professor, Department of Gastroenterology and Hepatology, Federal University School of Medicine, Rua 126 n.21, Goiania - GO 74093-080, Brazil
S- Editor Wang YR L- Editor Ma JY E- Editor Lin YP
| 1. | Yang Z, Tian L, Mba M, Qiu F. Portal Hypertensive Vasculopathy. J Tongji Med Univ. 1996;25:309-310. |
| 2. | Cai HJ, Qiu FZ, Liu RZ. Studies on the pharmacy of in-vitro Cultivated Calculus bovis (ICCB). Zhongguo Tianran Yaowu. 2004;2:335-338. |
| 3. | Du ZH, Cai HJ, Yang RG, Liu RZ, Cao FD, Qiu FZ. Experimental studies on anti-inflammatory effect of in-vitro Cultivated Calculus bovis (ICCB). Zhongyao Xinyao Yu Linchuang Yaoli. 1996;7:27-29. |
| 4. | Cai HJ, Wang SY, Zhang YH, Li QM, Xia JY, Wang Q, Xie H, Lai SL. In vitro Cultured Calculus Bovis in the Treatment of Epidemic Encephalitis B. Huazhong Keji Daxue Xuebao (Health Sci). 2003;32:604-606. |
| 5. | Cai HJ, Wang SY, Liu LG, Yao P, Guan Y. Effect of anti-hypoxia and elimination of free radicals of in vitro Cultured Calculus. Zhongyao Yaoli Yu Linchuang. 2003;19:20-22. |
| 6. | Yang Z, Qiu FZ, Wang ZH. Establishment and characteristic rabbit model of schistosomiasis. Zhonghua Shiyan Waike Zazhi. 1993;10:145-146. |
| 7. | Fallon MB, Abrams GA. Pulmonary dysfunction in chronic liver disease. Hepatology. 2000;32:859-865. |
| 8. | Shoji S, Rickard KA, Ertl RF, Robbins RA, Linder J, Rennard SI. Bronchial epithelial cells produce lung fibroblast chemotactic factor: fibronectin. Am J Respir Cell Mol Biol. 1989;1:13-20. |
| 9. | Bitterman PB, Rennard SI, Adelberg S, Crystal RG. Role of fibronectin as a growth factor for fibroblasts. J Cell Biol. 1983;97:1925-1932. |
| 10. | Limper AR, Broekelmann TJ, Colby YV, McDonald JA. Analysis of local Mrna expression for extracellular matrix proteins and growth factors using insitu hybridization in fibroproliferative lung disorders. Chest. 1991;99 Supple 3:55S-56S. |
| 11. | Vracko R. Significance of basal lamina for regeneration of injured lung. Virchows Arch A Pathol Pathol Anat. 1972;355:264-274. |
| 12. | Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974;77:314-346. |
| 14. | Jiang L, Chen BY, Hou XM. Changes of extracellular matrix proteins in rats with pulmonary fibrosis. Zhongguo Yike Daxue Xuebao. 1998;27:26-29. |
| 15. | Yang Z, Liu RZ, Yang RG, Cai HJ. Changes of hepatic extracellular matrix in schistosomiasis rats at early stage of liver fibrosis. Zhonghua Shiyan Waike Zazhi. 1995;12:157-158. |