Published online Feb 14, 2010. doi: 10.3748/wjg.v16.i6.728
Revised: November 4, 2009
Accepted: November 11, 2009
Published online: February 14, 2010
AIM: To assess the feasibility of endoscopic ultrasound (EUS)-guided celiac plexus neurolysis (CPN) using a poloxamer.
METHODS: In this prospective evaluation, six Yorkshire pigs underwent EUS-guided CPN. Three received an injection of 10 mL of 0.25% Lidocaine plus methylene blue (group 1) and three received an injection of 10 mL of 0.25% Lidocaine plus blue colored poloxamer (PS137-25) (group 2). Necropsy was performed immediately after the animals were sacrificed. The abdominal and pelvic cavities were examined for the presence of methylene blue and the blue colored poloxamer.
RESULTS: EUS-guided CPN was successfully performed in all 6 pigs without immediate complication. Methylene blue was identified throughout the peritoneal and retroperitoneal cavity in group 1. The blue colored poloxamer was found in the retroperitoneal cavity immediately adjacent to the aorta, in the exact location of the celiac plexus in group 2.
CONCLUSION: EUS-guided CPN using a reverse phase polymer in a non-survival porcine model was technically feasible. The presence of a poloxamer gel at the site of the celiac plexus at necropsy indicates a precise delivery of the neurolytic agent.
- Citation: Obstein KL, Martins FP, Fernández-Esparrach G, Thompson CC. Endoscopic ultrasound-guided celiac plexus neurolysis using a reverse phase polymer. World J Gastroenterol 2010; 16(6): 728-731
- URL: https://www.wjgnet.com/1007-9327/full/v16/i6/728.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i6.728
Pancreatic cancer and chronic pancreatitis commonly cause pain that is difficult to control[1-3]. Opioids are frequently used in an attempt to mitigate pain, however, tolerance, nausea, constipation and other side effects develop[4,5]. Non-pharmacologic therapies are often employed to improve pain control and quality of life while reducing drug-related side effects. Celiac plexus blockade (CPB) using steroids or celiac plexus neurolysis (CPN) using alcohol has been utilized and considered safe. Endoscopic ultrasound (EUS)-guided CPB and CPN have demonstrated safety and efficacy through real-time imaging and anterior access to the celiac plexus from the posterior gastric wall, thereby avoiding complications related to the puncture of spinal nerves, arteries and the diaphragm.
Unfortunately, EUS-guided CPN and CPB provide limited benefit in terms of degree and duration of pain relief[3]. While benefit duration of EUS CPN diminishes after 8-12 wk, the etiology remains unknown[6,7]. One theory is that the neurolytic or blockade agent washes away from the celiac plexus injection site due to its liquid free-flowing form and does not remain in the ideal anatomical location. Thus, if a neurolytic or blockade agent could be delivered in an alternate phase (solid or gel), it could offer the potential for enhanced efficacy and safety[8].
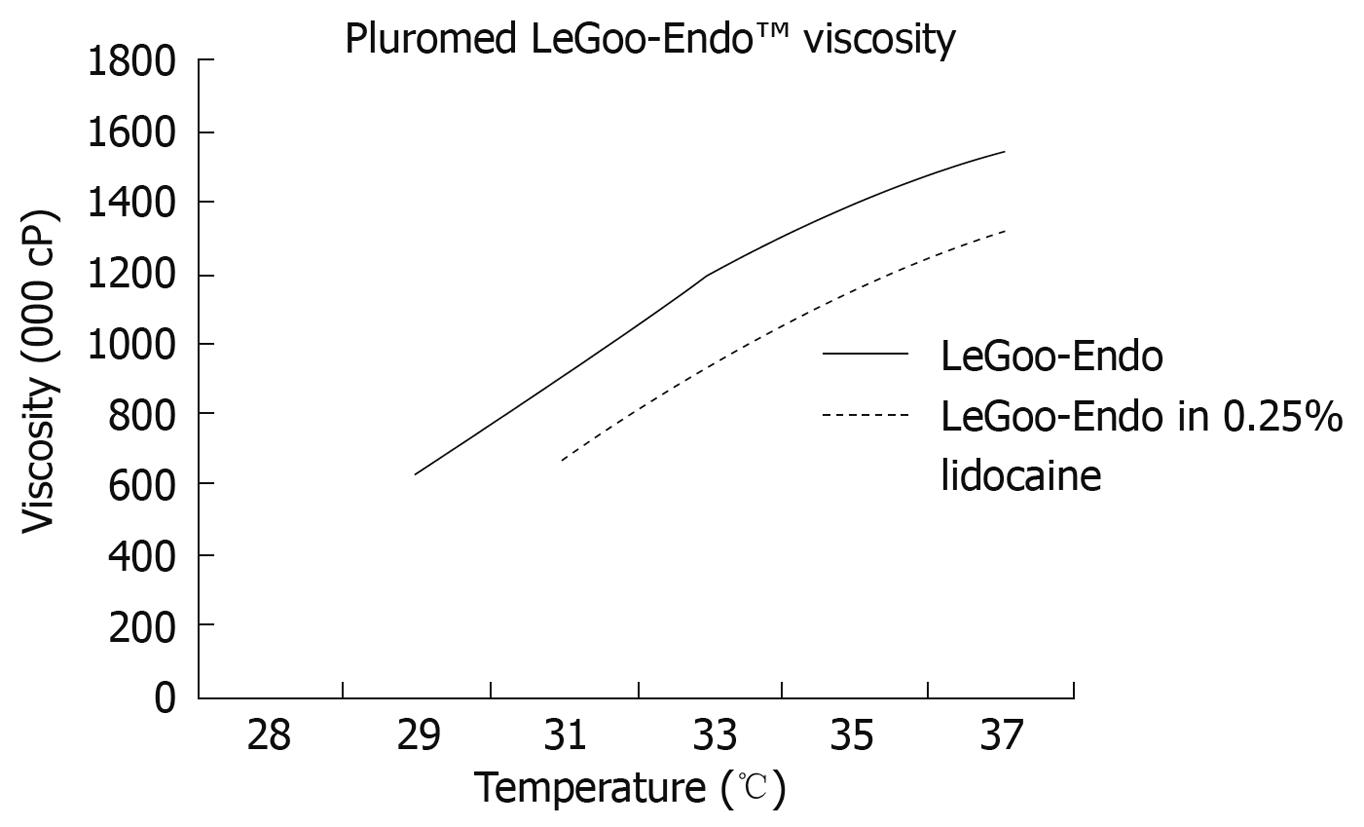
Recently, non-ionic surfactant triblock (ABA) copolymers of polyethylene oxidea-polypropylene oxideb-polyethylene oxidea (PEOa-PPOb-PEOa), also termed as poloxamers, have been widely used in industrial and medical applications[9-14]. Certain poloxamers have demonstrated rapid reverse phase thermosensitive properties at certain concentrations. Purified poloxamers PS138-25, PS107-20 and PS137-25 (Pluromed Inc., Woburn, MA, USA) are thin liquids at room temperature while at body temperature they are solid gel plugs (Figure 1). Therefore using a neurolytic or blockade agent as an additive in a purified poloxamer will potentially form a solid gel plug at the exact location of injection at the celiac plexus with enhancement of efficacy and safety. This study will assess the feasibility of EUS-guided CPN/CPB using a poloxamer in a non-survival porcine model.
Six Yorkshire pigs (25-30 kg) were food restricted for 24 h prior to the procedure. Intravenous (iv) Telazol (4.4 mg/kg), Atropine sulfate (0.04 mg/kg) and Xylazine (2.2 mg/kg) were used for anesthesia induction followed by inhaled Isoflurane (1% to 3%) on a semi-closed circuit for anesthesia maintenance after endotracheal intubation.
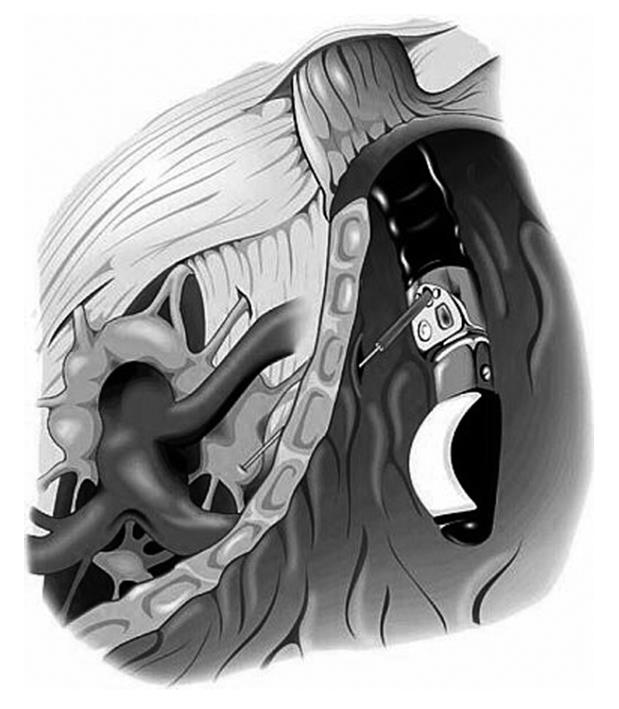
EUS-guided CPN was then performed using a linear echoendoscope (GF-UC140P, Olympus, Tokyo, Japan). Once the location of the celiac plexus was identified by its position relative to the celiac artery, a 19-guage needle (Wilson-Cook Medical, Inc., Winston-Salem, NC, USA) was introduced under direct EUS visualization (Figure 2). The needle was flushed with 2 mL normal saline and aspiration was performed to evaluate for vessel penetration prior to additional injections.
Three pigs were randomly assigned to receive a single injection of 10 mL of Lidocaine (0.25%) plus methylene blue (group 1) and three pigs randomly received a single injection of 10 mL of Lidocaine (0.25%) plus blue colored poloxamer PS137-25 (LeGoo-endo™, Pluromed, Inc., Woburn, MA, USA) (group 2). Due to the increased viscosity of the poloxamer, a greater amount of force was required for injection. This was easily overcome with the use of a Controlled Radial Expansion (CRE) balloon dilator inflation hand pump system (Boston Scientific, Natick, MA, USA). During the procedure, the blood pressure, heart rate, temperature, ventilation, and oxygenation status of the pigs were continuously monitored by the professional veterinary team of the Animal Research at Children’s Hospital (ARCH) (Boston, MA, USA). The investigators were not blinded to the injection group.
Using Fatal Plus (86 mg/kg), the group 1 pigs were immediately sacrificed after the procedure and the group 2 pigs were sacrificed at 60 min after the procedure. Necropsy was performed immediately upon death of the pigs and close examinations of the peritoneal and retroperitoneal cavities were made. Photographic and video records were obtained. This study was approved by the Animal Research Committee of the ARCH and complied with the National Academy of Sciences Guide for the Care and Use of Laboratory animals.
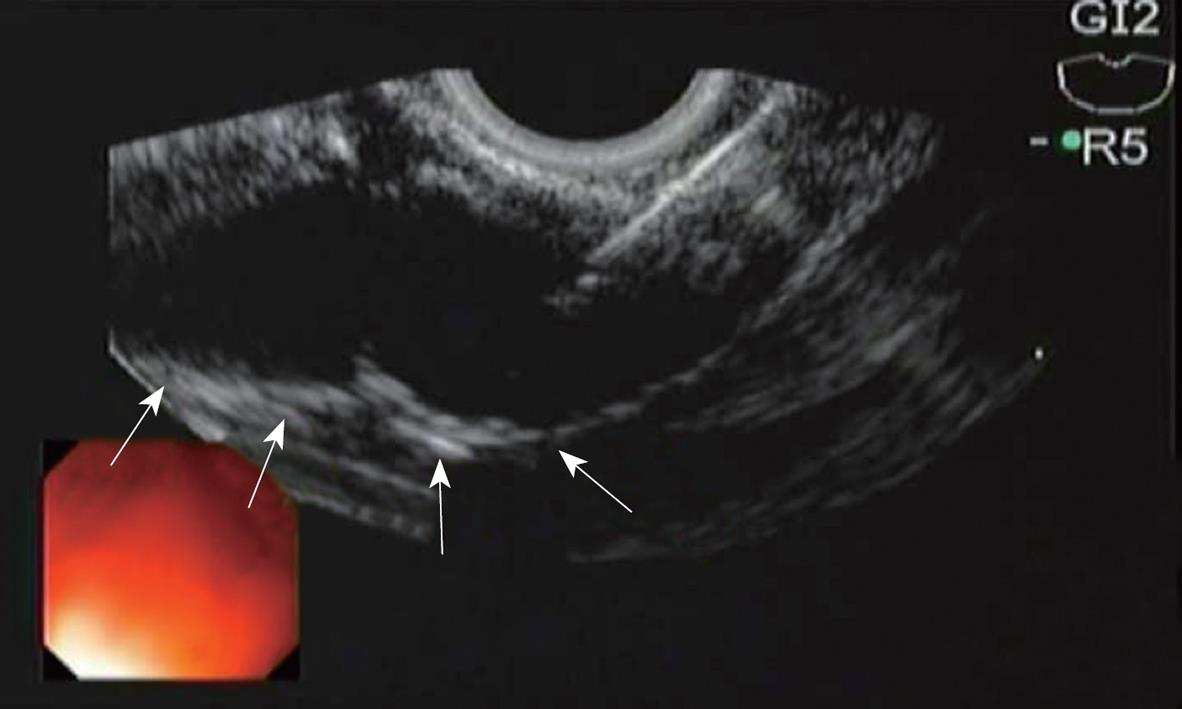
All the six pigs tolerated the procedure well with no immediate complications. EUS-guided CPN was successfully performed in both groups. An echodense smudge was endosonographically visualized after injection of Lidocaine plus methylene blue and Lidocaine plus blue colored poloxamer PS 137-25 (Figure 3).
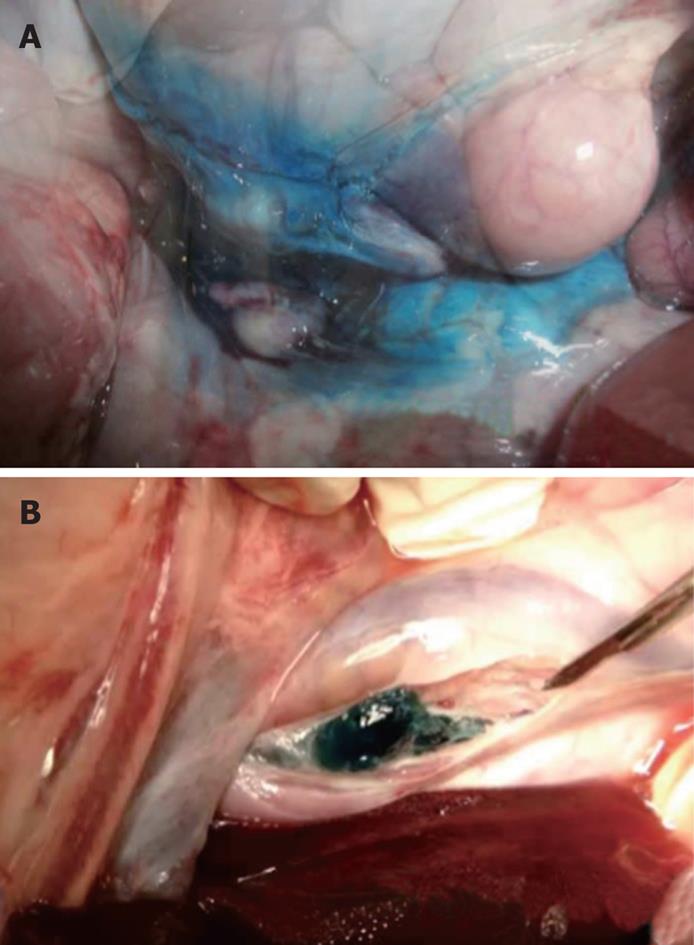
At necropsy, methylene blue was identified spread throughout the peritoneal and retroperitoneal cavities in group 1. In group 2, a blue gel plug was identified in the retroperitoneal cavity, adjacent to the aorta, in the exact location of the celiac plexus without evidence of any blue gel elsewhere in the peritoneal or retroperitoneal cavity (Figure 4).
EUS-guided CPN and CPB are safe and effective methods for pain control in patients with pancreatic neoplasms and chronic pancreatitis[3-6,15-18]. Unfortunately, the degree and duration of therapeutic effect vary. Gress et al[18] performed EUS-guided CPB in 90 patients with chronic pancreatitis and found that 55% of patients had decreased pain scores at a mean follow-up of 8 wk. This included a reduction in pain medication requirements as reported by the patients in the study. Persistent benefit was found in 26% of patients at week 12 and in 10% at week 24. Gunaratnam et al[6] performed EUS-guided CPN in 58 patients with pancreatic cancer, 78% of whom experienced a decline on a continuous 11-point visual analog pain scale 2 wk after the procedure. Only 54% experienced a decline of greater than 2 points after EUS-guided CPN and the efficacy diminished 8-12 wk after the procedure in those not receiving adjuvant therapy. Those authors also found that opioid administration increased throughout the study, however this increase was not statistically significant. Levy et al[3] administered EUS-guided direct ganglia injection in 33 patients with pancreatic cancer and chronic pancreatitis, 94% and 80% of the patients reported pain relief with alcohol injection at 2-4 wk after the procedure, however long-term follow-up data was not recorded.
In a report in 1996, fluoroscopic evaluation of the abdomen was conducted in patients who underwent EUS-guided CPN. Of those examined, all were noted to have injected material spread in a periaortic distribution with dye spread anterior and lateral to the aorta with extension in both the cranial and caudal direction[16]. This study supports our findings in group 1 pigs where the injected methylene blue was identified spread throughout the peritoneal and retroperitoneal cavities.
While short-term reduction in pain has been indicated, long-term benefit with this technique is limited. This may be explained by the fact that until recently, the celiac ganglia was unable to be directly visualized, leading to a less precise delivery of therapy[3,19,20]. Other possibilities include interference with direct visualization due to the echodense smudge and potential alterations in anatomy after injecting one side of the aorta with the therapeutic agent using the double injection technique. Additionally, dispersion of the therapeutic agent away from the desired location may act to influence the efficacy and duration.
The goal of this study was to evaluate the feasibility of EUS-guided CPN using a poloxamer that would potentially remain in the desired target location. This may yield a safer and more durable therapeutic result. In group 2, where the PS137-25 was injected, a gel plug was successfully created at the intended location of the celiac ganglia. The gel maintained the therapeutic agent in the celiac plexus and potentially released the drug slowly over time, thereby optimizing long-term therapeutic results.
A limitation of this study is that it is an acute, non-survival evaluation without long-term follow-up. It is therefore unclear how the injected gel plug interacts with the porcine model over time. Additionally, the study was performed using Lidocaine instead of a steroid or alcohol as it was simpler to integrate into the PS137-25. Steroid or alcohol integration may influence the temperature phase transition point of the poloxamer, however, the formation of a gel at body temperature and liquid at room temperature would remain intact. Lastly, the quantity and rate of drug release from the gel plug was unable to be determined in this current study.
In conclusion, EUS-guided CPN using a reverse phase polymer is feasible in a non-survival porcine model. The formation of a gel plug at the exact location of the celiac ganglia may avoid dispersion of the injected therapeutic agent and increase the duration of analgesic effect. A survival study is now necessary to determine the duration and breakdown of the gel plug within the body. This would provide useful information on potential complications related to the plugs presence and allow for assessment of the therapy from the gel plug over time. Future studies will also integrate the use of steroids and alcohols into the poloxamer.
Pancreatic cancer and chronic pancreatitis commonly cause pain that is difficult to control. Nonpharmacologic therapies are often employed to improve pain control and quality of life while reducing drug-related side effects. Endoscopic ultrasound (EUS)-guided celiac plexus blockade (CPB) using steroids or celiac plexus neurolysis (CPN) have demonstrated safety and efficacy. Unfortunately, the neurolytic or blockade agent may wash away from the celiac plexus injection site due to its liquid free-flowing form and does not remain in the ideal anatomical location. Therefore, using a neurolytic or blockade agent as an additive in a reverse thermodynamic phase poloxamer will potentially form a solid gel plug at the exact location of injection at the celiac plexus with enhancement of efficacy and safety.
Expanded research utilizing reverse phase poloxamers for additional applications in medical practice. Survival studies utilizing reverse phase poloxamers to determine the duration and breakdown of the gel plug within the body. Enhancement of the efficacy and safety of CPB and CPN.
Recently, non-ionic surfactant triblock (ABA) copolymers of polyethylene oxidea-polypropylene oxideb-polyethylene oxidea (PEOa-PPOb-PEOa), also termed as poloxamers, have been widely used in industrial and medical applications. Certain poloxamers have demonstrated rapid reverse phase thermosensitive properties at certain concentrations. Purified poloxamers PS138-25, PS107-20 and PS137-25 (Pluromed Inc., Woburn, MA, USA) are thin liquids at room temperature while at body temperature they are solid gel plugs. Therefore, the novel use of a neurolytic or blockade agent as an additive in a purified poloxamer will potentially form a solid gel plug at the exact location of injection at the celiac plexus.
EUS-guided CPN using a reverse phase polymer is feasible in a non-survival porcine model. The formation of a gel plug at the exact location of the celiac ganglia may avoid dispersion of the injected therapeutic agent and increase the duration of analgesic effect. A survival study is now necessary to determine the duration and breakdown of the gel plug within the body. Future studies will also integrate the use of steroids and alcohols into the poloxamer.
Poloxamer: A non-ionic surfactant triblock (ABA) copolymer of polyethylene oxidea-polypropylene oxideb-polyethylene oxidea (PEOa-PPOb-PEOa). Reverse phase thermosensitivity: When a material or substance is in its liquid phase at cold temperature and in its solid phase at hot temperature.
Dr. Thompson et al present an animal experience on the use of reverse phase polymer to increase the efficacy of EUS-guided celiac plexus injection for pancreatic pain control. This represent an interesting experiment outlining the possible improvement of the procedure by using a compound which facilitate the retention of the injectate at the local level (celiac plexus).
Peer reviewer: Dr. Massimo Raimondo, Division of Gastroenterology and Hepatology, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, United States
S- Editor Tian L L- Editor Ma JY E- Editor Lin YP
| 1. | Ventafridda GV, Caraceni AT, Sbanotto AM, Barletta L, De Conno F. Pain treatment in cancer of the pancreas. Eur J Surg Oncol. 1990;16:1-6. |
| 2. | Lankisch PG. Natural course of chronic pancreatitis. Pancreatology. 2001;1:3-14. |
| 3. | Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, de la Mora JG, Gleeson FC, Pearson RK, Pelaez MC. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct Ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98-103. |
| 4. | Tran QN, Urayama S, Meyers FJ. Endoscopic ultrasound-guided celiac plexus neurolysis for pancreatic cancer pain: a single-institution experience and review of the literature. J Support Oncol. 2006;4:460-462, 464; discussion 463-464. |
| 5. | Michaels AJ, Draganov PV. Endoscopic ultrasonography guided celiac plexus neurolysis and celiac plexus block in the management of pain due to pancreatic cancer and chronic pancreatitis. World J Gastroenterol. 2007;13:3575-3580. |
| 6. | Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316-324. |
| 7. | Polati E, Finco G, Gottin L, Bassi C, Pederzoli P, Ischia S. Prospective randomized double-blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer. Br J Surg. 1998;85:199-201. |
| 8. | Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321-339. |
| 9. | Nace VM. Nonionic surfactants: polyoxyalkylene block copolymers. Surfactant Science Series, vol. 60. New York: Marcel Dekker 1996; 280. |
| 10. | Maynard C, Swenson R, Paris JA, Martin JS, Hallstrom AP, Cerqueira MD, Weaver WD. Randomized, controlled trial of RheothRx (poloxamer 188) in patients with suspected acute myocardial infarction. RheothRx in Myocardial Infarction Study Group. Am Heart J. 1998;135:797-804. |
| 11. | O'Keefe JH, Grines CL, DeWood MA, Schaer GL, Browne K, Magorien RD, Kalbfleisch JM, Fletcher WO Jr, Bateman TM, Gibbons RJ. Poloxamer-188 as an adjunct to primary percutaneous transluminal coronary angioplasty for acute myocardial infarction. Am J Cardiol. 1996;78:747-750. |
| 12. | Orringer EP, Casella JF, Ataga KI, Koshy M, Adams-Graves P, Luchtman-Jones L, Wun T, Watanabe M, Shafer F, Kutlar A. Purified poloxamer 188 for treatment of acute vaso-occlusive crisis of sickle cell disease: A randomized controlled trial. JAMA. 2001;286:2099-2106. |
| 13. | Raymond J, Metcalfe A, Salazkin I, Schwarz A. Temporary vascular occlusion with poloxamer 407. Biomaterials. 2004;25:3983-3989. |
| 14. | Boodhwani M, Cohn WE, Feng J, Ramlawi B, Mieno S, Schwarz A, Sellke FW. Safety and efficacy of a novel gel for vascular occlusion in off-pump surgery. Ann Thorac Surg. 2005;80:2333-2337. |
| 15. | Levy MJ, Wiersema MJ. EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc. 2003;57:923-930. |
| 16. | Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656-662. |
| 17. | Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol. 1999;94:900-905. |
| 18. | Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol. 2001;96:409-416. |
| 19. | Gleeson FC, Levy MJ, Papachristou GI, Pelaez-Luna M, Rajan E, Clain JE, Topazian MD. Frequency of visualization of presumed celiac ganglia by endoscopic ultrasound. Endoscopy. 2007;39:620-624. |
| 20. | Levy M, Rajan E, Keeney G, Fletcher JG, Topazian M. Neural ganglia visualized by endoscopic ultrasound. Am J Gastroenterol. 2006;101:1787-1791. |