Published online Dec 28, 2010. doi: 10.3748/wjg.v16.i48.6128
Revised: August 30, 2010
Accepted: September 7, 2010
Published online: December 28, 2010
AIM: To study tissue factor (TF) in acute pancreatitis and evaluate the role of TF as a predictive marker of severity.
METHODS: Forty-nine consecutive patients admitted to Lund University Hospital, fulfilling the criteria of predicted severe acute pancreatitis (AP), were recruited prospectively between 2002 and 2004. Blood samples for TF analyses were drawn at inclusion in the study and 12 h, 1 d and 3 d later.
RESULTS: Twenty-seven patients developed mild AP, and 22 patients severe AP. At inclusion in the study, the groups were comparable with respect to gender, aetiology, Acute Physiology and Chronic Health Evaluation II score, and duration of pain. At inclusion in the study and at 12 h, TF was higher in the severe AP group (P = 0.035 and P = 0.049, respectively). After 1 and 3 d, no differences in TF levels were noted. Interleukin (IL)-6 was significantly higher in the severe AP group at all of the studied time points. C-reactive protein (CRP) was significantly higher in the AP group at 1 and 3 d. In receiver operating characteristic-curves, the area under the curve (AUC) for TF was 0.679 (P = 0.035) at inclusion in the study, and a cut off level for TF of 40 pg/mL showed a sensitivity of 71% and a specificity of 67%, whereas corresponding AUC for IL-6 was 0.775, P = 0.001, and for CRP was 0.653. IL-6 showed better AUC-values than TF at all time points studied.
CONCLUSION: TF-levels are raised early in severe AP. TF as an early predictive marker of severe AP is superior to CRP, but inferior to IL-6.
- Citation: Andersson E, Axelsson J, Eckerwall G, Ansari D, Andersson R. Tissue factor in predicted severe acute pancreatitis. World J Gastroenterol 2010; 16(48): 6128-6134
- URL: https://www.wjgnet.com/1007-9327/full/v16/i48/6128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i48.6128
Severe acute pancreatitis (AP) is one example of critical illness where both the inflammatory system and the coagulation system are to be considered as ticking bombs, where the most extreme scenarios result in multiple organ dysfunction and disseminated intravascular coagulation. Microcirculatory disturbances with micro vascular thromboses appear to play an important role both in the inflamed pancreas itself and in remote organ failure[1,2]. Clinical evidence is still sparse[3-5], but several experimental studies have suggested an important role of the coagulation system in the pathophysiology of AP[6-8]. One key to the cross-talk between inflammation and coagulation are proteases, with enzymatic capacity to activate both inflammation and coagulation. Coagulation factors, such as factor VII (FVII) and tissue factor (TF), as well as thrombin, can bind to protease activated receptors (PARs) on various cells and elicit intracellular signalling, resulting in modulation of inflammatory response[9]. The PAR family has at least four members (PAR 1-4) where TF-FVII has been shown to be able to act through PAR-2, while TF-FVII-FX also activates PAR-1. PAR-2 is the only PAR not activated by thrombin[10].
Tissue factor is a trans-membrane glycoprotein, initiating the most important pathway of coagulation[11,12]. Tissue factor is expressed in the vascular adventitia, but may also be expressed in micro-particles which can be shed from leukocytes, endothelial cells, vascular smooth muscle cells and platelets[13]. In the normal setting TF is not in contact with circulating blood. When vessels are injured or when TF-expressing cells are stimulated by circulating pro-inflammatory cytokines or lipopolysaccharide (LPS), TF is exposed to the bloodstream. TF then binds and activates factor VII. Factor VII is a vitamin K-dependent trypsin-like serine protease, produced in the liver. It circulates in an inactive form, and requires the action of its allosteric regulator, TF, to convert it to the active enzyme (FVIIa). The TF-factor VII complex initiates coagulation by activating FX, eventually resulting in conversion of pro-thrombin to thrombin. Thrombin cleaves fibrinogen, resulting in abundant fibrin production and the formation of a clot. The activity of TF is counterbalanced by circulating tissue factor pathway inhibitor (TFPI). In addition to its well-established role in coagulation, TF, and to a lesser extent FVII, have also been associated with various other physiological processes of gene transcription, apoptosis and cytoskeleton reformation, such as in inflammation, sepsis, metastasis, angiogenesis and atherosclerosis, where the TF-FVIIa complex acts as a signalling receptor[14-17]. The role of TF/FVIIa signalling in inflammatory conditions is confirmed by TF/FVIIa regulated expression of the pro-inflammatory cytokine interleukin (IL)-8 in keratinocytes[18], and a role in the regulation of both IL-6 and IL-8 expression in monocytes/macrophages[19]. Confirming the effect of FVIIa on expression of interleukins, recombinant FVIIa administered to healthy humans caused a three- to four-fold increase in plasma levels of IL-6 and IL-8[20]. A role of TF/FVIIa signalling in the regulation of inflammatory genes has been demonstrated in LPS-stimulated macrophages, where TF-FVIIa signalling activated genes coding for tumor necrosis factor-α, IL-6, and IL-8[21].
Recent clinical studies have suggested a potential role of coagulation variables, such as TF, TFPI and D-dimer, in predicting risk of developing organ failure and severe AP[22-24]. However, the evidence supporting their use as predictors of severity of AP is still weak, compared to C-reactive protein (CRP) and IL-6, which to date are the most well-documented laboratory parameters to predict severe AP[25-28].
The present study aimed to investigate plasma levels of TF in the initial phase of predicted severe AP, and to assess the ability of this biochemical marker to predict severe AP.
Consecutive patients admitted to Lund University Hospital with the clinical diagnosis of acute pancreatitis, were recruited prospectively between June 2002 and December 2004. Inclusion and exclusion criteria are listed in Table 1.
| Inclusion criteria | Exclusion criteria |
| > 18 yr | Acute pancreatitis due to surgery |
| Abdominal pain | Trauma |
| Amylase > 3 times upper normal limit | Cancer |
| Onset of abdominal pain within 48 h | Inflammatory bowel disease |
| APACHE II score > 8 and/or | Stoma |
| CRP > 150 and/or | Short bowel |
| Peripancreatic fluid collection on CT | Chronic pancreatitis |
Written informed consent was obtained and the study was approved by the local research ethics committee. This study was part of a prospective single-centre study on early enteral nutrition vs total parenteral nutrition in AP, where parts of the data on IL-6 and CRP have been published[29]. Venous blood was taken for measurement of plasma levels of TF, FVII, fibrinogen, IL-6 and CRP. Not all markers were measured at all time points in the study. TF and IL-6 were measured at inclusion, after 12 h, and after 1 and 3 d. CRP was measured at inclusion, and after 1 and 3 d. Fibrinogen and FVII were only measured at inclusion in the study.
Descriptive data were recorded including age, gender, aetiology, time from onset of pain to inclusion in the study, Acute Physiology and Chronic Health Evaluation (APACHE) II score on day 1 and 3, organ failure, and mortality. The severity of pancreatitis was assessed according to the Atlanta classification[30].
Peripheral blood samples were taken from each patient on study inclusion, at 12 h, and after 1 and 3 d. Admission plasma levels of FVII were analysed, and to detect the prevalence of fibrinolysis and fibrinogen consumption at admission, plasma fibrinogen was analysed. Fibrinogen is an acute phase protein, affected by pathologic proteolysis such as in disseminated intravascular coagulation, where low levels of fibrinogen are to be expected. TF, IL-6 and CRP were analysed at repeated time points during three days after inclusion in the study.
Tissue factor and fibrinogen were collected using citrate tubes, and ethylenediaminetetraacetic acid tubes were used for IL-6 and CRP. All samples were centrifuged at 2200 g for 10 min (3200 r/min, rotor diameter 19.1 cm). The plasma was decanted and stored at -70°C until further analysis.
TF and FVII were assessed by enzyme-linked immunosorbent assay (ELISA)-kits according to the manufacturer’s instructions (Assaypro St. Charles, MO, USA). The TF-ELISA recognizes TF-apo, TF and TF-VII complexes. The FVII-ELISA detects free FVII and FVIIa, as well as complexes with TF, TF/factor VII and TF/FVIIa.
Fibrinogen was analysed by Sysmex CA-7000 (Sysmex Corporation, Kobe, Japan) according to the operator’s manual. The procedure involves mixing citrate plasma with buffer. After incubation, coagulation was initiated by adding an excess of thrombin. The time between addition of thrombin and coagulation was registered photo-optically and is inversely proportional to the concentration of fibrinogen.
IL-6 was measured by an ELISA-kit according to the manufacturer’s instructions (Quantikine, R6D systems Europe, Abingdon, UK). CRP was measured by Cobas 6000 (Roche Corporation, Basel, Switzerland) according to the operator’s manual. The complex binding between CRP and CRP monoclonal antibodies attached to latex particles was registered as an increase in absorbance, measured photo-optically, and the increase in absorbance was related to the concentration of CRP.
Data are presented as median and interquartile range, when applicable. Outliers are not shown in the box-plots, but are included in all calculations. Comparisons between groups were performed with the χ2 test for binary data or Fisher’s exact test for small samples. Continuous variables were compared with the Mann-Whitney U-test. To evaluate TF as a predictor of severe AP, receiver operating characteristics (ROC) curves were plotted and positive likelihood ratios (PLR) and negative likelihood ratios (NLR) were calculated to detect optimal cut-off levels. As a comparison, figures calculated from levels of CRP and IL-6 were used, as they are known to be good predictors of severity, IL-6 already at admission[27] while CRP peaks about 48 h later.
In a ROC curve, the true positive rate (sensitivity) is plotted in function of the false positive rate (100 - specificity) for different cut-off points. Each point on the ROC plot represents a sensitivity/specificity pair corresponding to a particular decision threshold. A test with perfect discrimination (no overlap in the two distributions) has a ROC plot that passes through the upper left corner (100% sensitivity, 100% specificity). The closer the ROC plot is to the upper left corner, and the greater the area under the curve, the higher the overall accuracy of the test is[31]. The Likelihood Ratio (LR) is the likelihood that a given test result would be expected in a patient with the target disorder, compared to the likelihood that the same result would be expected in a patient without the target disorder.
Statistical analyses were performed with SPSS version PASW Statistics 18 (SPSS Inc, Chicago, IL, USA).
According to the Atlanta classification, 22 patients (45%) fulfilled the criteria of severe AP, and 27 patients (55%) were classified as having mild AP. One patient in the severe AP group died, rendering an overall mortality rate of 2.0%. At inclusion in the study, the groups with mild and severe pancreatitis were comparable with respect to gender, aetiology, APACHE II score, and duration of pain prior to inclusion. Age was lower in the severe AP group, compared to the mild AP group. Patient characteristics and laboratory variables at time of inclusion are presented in Table 2.
| MAP (n = 27) | SAP (n = 22) | P | |
| Age (yr)1 | 76 (63-85) | 63 (56-77) | 0.042 |
| Sex (M:F) | 14:13 | 10:12 | 0.664 |
| APACHE II1 | 9 (8-11) | 9 (7-13) | 0.860 |
| Aetiology | |||
| Biliary | 15 | 16 | 0.248 |
| Alcohol | 4 | 3 | 1.000 |
| ERCP | 3 | 1 | 0.617 |
| Unknown | 5 | 2 | 0.436 |
| Duration of pain (h)1 | 34 (21-43) | 25 (22-29) | 0.160 |
| Amylase1 | 8.2 (2.7-13.7) | 9.8 (4.3-15.3) | 0.690 |
| IL-6 (pg/mL)1 | 100 (55-210) | 275 (158-315) | 0.001 |
| CRP (mg/mL)1 | 106 (69-167) | 173 (104-209) | 0.071 |
| Tissue factor (pg/mL)1 | 35 (23-50) | 49 (36-101) | 0.035 |
| Fibrinogen (g/L)1 | 4.8 (4.4-6.2) | 4.0 (3.8-7.2) | 0.047 |
| FVII (ng/mL)1 | 155 (46-294) | 136 (88-296) | 0.608 |
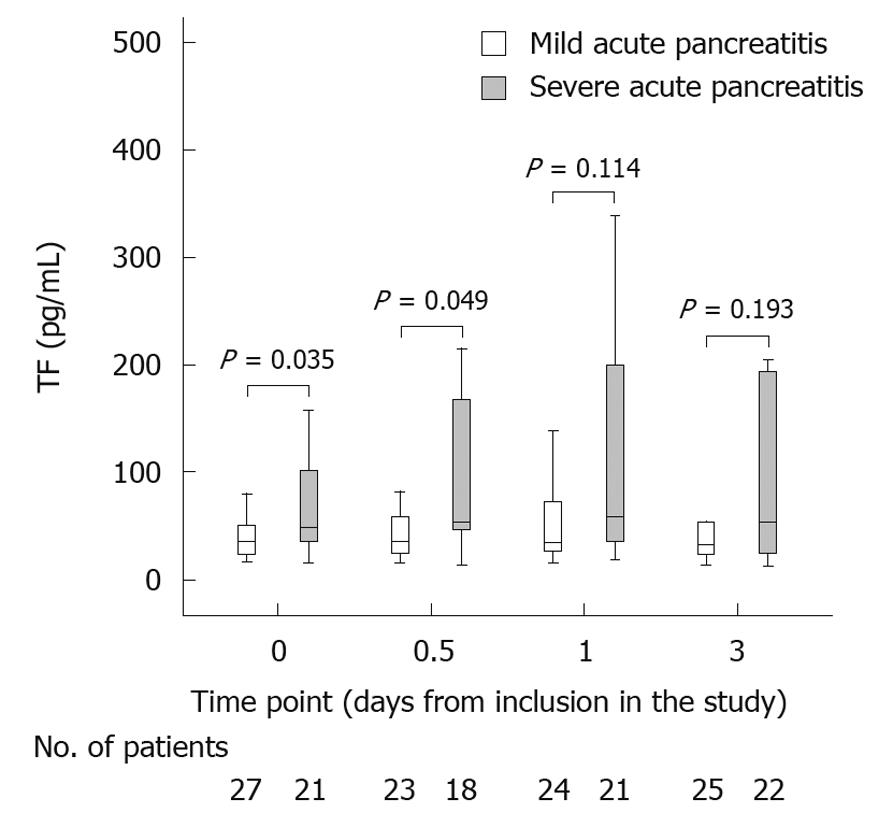
Because some blood samples were not taken properly, there are different numbers of patients at the different time points. At inclusion in the study, TF was higher in the severe AP group, whereas fibrinogen was lower in the severe AP group compared to the group with mild AP [Figure 1, tissue factor (pg/mL)].
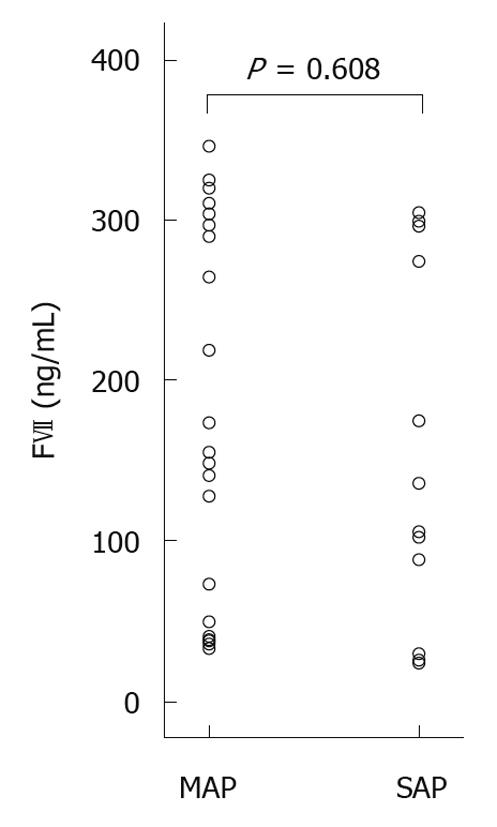
There was no difference in FVII-levels between the groups (P = 0.608). A large variation in inter-individual levels of FVII was noted [Figure 2, scattergram of FVII plasma levels at admission (ng/mL)]. IL-6 was higher in the severe AP group (P = 0.001), and CRP showed a tendency towards higher levels in the severe AP group (P = 0.071) at time of inclusion in the study (Table 2).
When looking at changes over time, TF was slightly higher in the severe AP group at 12 h (P = 0.049). After 1 and 3 d no differences in TF levels were noted between the mild and the severe AP group [Figure 1, tissue factor (pg/mL)].
IL-6 peaked at 12 h and was significantly higher in the severe AP group at all of the studied time points (at inclusion P = 0.001, 12 h P < 0.001, 1 d P < 0.001 and 3 d P = 0.000, respectively). CRP peaked at day 3, and was significantly higher in the AP group at 1 and 3 d (P = 0.001 and P < 0.001, respectively).
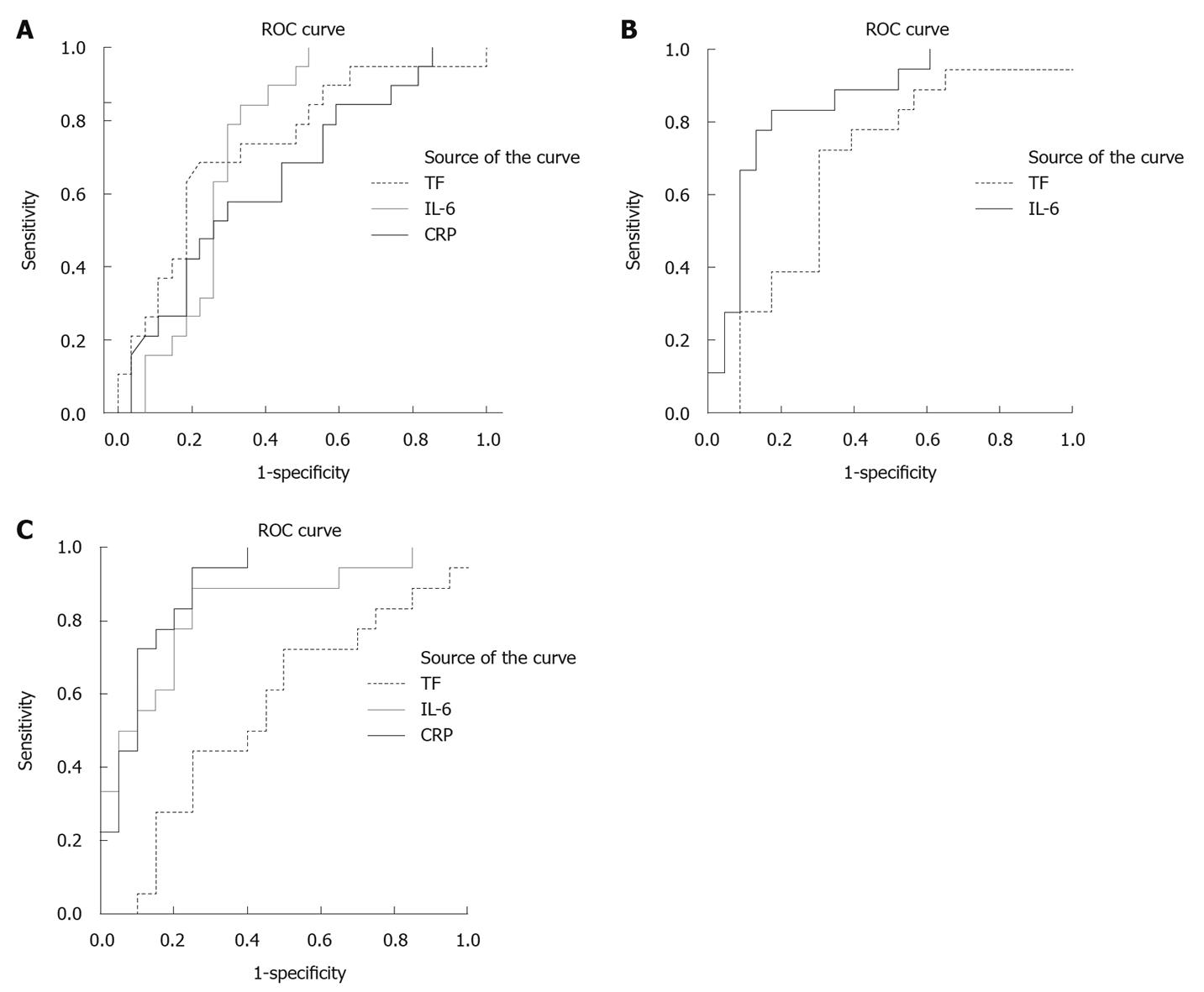
To evaluate the utility of TF as an early predictor of severe AP, ROC-curves were plotted for the time of inclusion (Figure 3A, ROC curves of TF, IL-6 and CRP at time of inclusion in the study), and for 12 h (Figure 3B, ROC curve of TF and IL-6 at 12 h after inclusion in the study), 1 d (results not shown) and 3 d after inclusion in the study (Figure 3C, ROC curves of TF, CRP and IL-6 at 3 d after inclusion). As a comparison, ROC-curves were plotted for CRP and IL-6. Area under the curve (AUC) values at the different time points were studied. Based on these results, possible cut-off levels for TF are suggested at inclusion and after 12 h, based on sensitivity, specificity, PLR and NLR. Table 3 shows AUC-values, P-values, possible cut-off levels, sensitivity, specificity, PLR and NLR for TF (pg/mL).
| Time point1 | AUC | P | Cut-off TF (pg/mL) | Sensitivity | Specificity | PLR | NLR |
| 0 | 0.679 | 0.035 | 32 | 86 | 48 | 1.65 | 0.30 |
| 40 | 71 | 67 | 2.14 | 0.43 | |||
| 46 | 62 | 74 | 2.39 | 0.51 | |||
| 0.5 | 0.681 | 0.049 | 33 | 90 | 43 | 1.57 | 0.26 |
| 41 | 78 | 56 | 1.79 | 0.39 | |||
| 47 | 72 | 70 | 2.37 | 0.40 | |||
| 1 | 0.652 | 0.078 | |||||
| 3 | 0.621 | 0.151 |
Several previous studies on coagulation factors in AP have been published. In a study on 36 patients with AP, elevated levels of TF were detected at admission. In that study only 5 patients were classified as having moderate AP, while 31 had severe AP according to the Japanese Severity Score[22]. A correlation between higher levels of TF and development of organ failure was demonstrated, but in contrast to the results from the present study no correlation with overall severity was detected. In the present study, TF was higher in severe AP compared to mild AP at inclusion in the study, i.e. close to admission, and after 12 h.
The levels of fibrinogen in both mild and severe AP were in the higher span or above the reference for normal human plasma levels, consistent with fibrinogen being an acute phase protein. A slightly lower level of fibrinogen was noted in the group with severe AP at inclusion in the study. The results are, however, hard to interpret as fibrinogen is an acute phase protein and the level of fibrinogen in the severe AP group was just above normal level. It should be stressed that the result for fibrinogen was of weak significance, and further studies of other parameters of fibrinolysis, such as D-dimer and fibrin degradation products should be conducted in order to tell whether early fibrinolysis is the explanation for the lower levels of fibrinogen in the severe AP group.
In a study on 91 patients with AP, D-dimer, pro-thrombin time and fibrinogen were different when comparing patients developing organ failure and patients not developing organ failure, both at admission and 24 h later. D-dimer was the best predictive marker of organ failure (sensitivity 90%, specificity 89%)[23]. In a study on 139 patients with AP, the levels of antithrombin III (AT-III), fibrin/fibrinogen degradation products, platelet count, D-dimer, and antithrombin-AT-III complex at admission were associated with severity and prognosis of AP. AT-III, fibrin/fibrinogen degradation products, platelet count, D-dimer, and thrombin-AT-III complex at admission showed better area under the ROC curve values compared to CRP. AT-III was the best predictor of fatal outcome (sensitivity 81%, specificity 86%)[32].
In experimental studies, deficiency of FVII has been shown to reduce inflammation[33,34] and high levels of FVII have been suggested to be associated with ischemic heart disease and inflammation[35,36]. In the present study, concentrations of FVII did not differ between the mild AP group and the severe AP group, and hence levels of FVII do not seem to be affected in the early course of the disease. The large variation in levels of FVII is consistent with reported findings on a strong contribution of the FVII genotype to levels of FVII. Different FVII genotypes can result in up to several-fold differences in mean FVII levels[37].
Early recognition of patients at risk of developing severe AP with multiple organ failure and high risk of mortality remains a challenge, despite the use of multifactor scoring systems such as APACHE-II and Ranson’s score[38]. Obesity, age, alcohol consumption and use of tobacco are known to predispose to a severe disease course[39,40]. The most widely used laboratory parameter to predict severity of AP and development of complications is CRP. A meta-analysis on the ability of IL-6 to predict severe AP concludes that these cytokines perform at an acceptable level in predicting severe AP[26]. The pooled IL-6 sensitivities ranged between 81.0% and 83.6% and specificities between 75.6% and 85.3% with PLRs of 3.43, 4.90 and 4.40 for days 1, 2 and 3, respectively. The IL-6 AUCs were 0.75, 0.88 and 0.85 for days 1, 2 and 3, which are in accordance with the AUCs for IL-6 in the present study.
Data concerning the role of coagulation variables as predictors of severe AP are scarce. In a study of 44 patients with AP, TFPI measured at admission was shown to be related to severity[24]. Among the three variables in the present study, fibrinogen, FVII and TF, TF was significantly raised at admission, when comparing the severe and the mild AP group. With this result in mind, TF was explored as a marker of severity at four different time points, by area under ROC-curves. At admission and after 12 h, the AUC for TF was 0.68, and when evaluating different cut-off points the best PLR was 2.4, with a sensitivity of 62% and a specificity of 72%, which implies a quite low impact on the likelihood of severe disease, much less impact than IL-6 at corresponding time points.
We conclude that levels of TF, but not FVII, are higher in “true severe” AP than in those patients with predicted severe AP who turn out to develop mild AP. Our results stress the need of more reliable predictors of severity, as only 45% of the patients in our study with predicted severe disease actually developed severe AP. The value of TF as a predictive marker of severe AP early in the course of the disease is not as good as IL-6, but superior to CRP. The results do not indicate a role for TF as a valuable predictive marker of severity on its own. The higher levels of TF in the early course of severe AP suggest, however, a potential role of TF in the development of severe disease, and may reflect a window for therapeutic inhibition of TF in AP.
Acute pancreatitis affects about 20-40/100 000 inhabitants each year. One fifth of these patients will develop a severe form of AP with multiple organ failure and a high risk of death. There is no reliable marker to early predict which patients will develop the severe form. In severe disease, such as AP, a close interplay between coagulation and inflammation is known to exist, and take part in the development of the disease. In this paper, tissue factor, which is a key player in the crosstalk between inflammation and coagulation, is measured in the plasma of patients with predicted severe pancreatitis.
Data concerning the role of coagulation variables as predictors of severe acute pancreatitis (AP) are still sparse. The results from one study on patients with AP, showed that levels of tissue factor (TF) were related to the development of pancreatic necrosis in alcoholic severe AP, but no association with overall severity was demonstrated (Sawa et al 2006). In another study of AP, the coagulation parameters D-dimer, pro-thrombin time and fibrinogen were different in the group of AP patients developing organ failure compared to the patients who did not develop organ failure, both at admission and 24 h later. D-dimer was the best predictive marker of organ failure (Radenkovic et al 2009). In yet another study on AP, the levels of the coagulation parameters antithrombin III (AT-III), fibrin/fibrinogen degradation products, platelet count, D-dimer, and thrombin-AT-III complex at admission were associated with severity and prognosis of AP. AT-III was the best predictor of fatal outcome (Maeda et al 2006).
The authors show that levels of TF measured early in the course of the disease are higher in patients who develop severe AP. These results are consistent with the possible role of coagulation variables in the development of AP.
The role of TF as an early predictor of severe AP is inferior to interleukin-6, which has been shown to be of value in various previous studies, however, TF is superior to the most frequently used laboratory parameter, C-reactive protein. The results indicate a role for TF in the development of severe AP, and the effect of tissue factor pathway-inhibitors in AP should be studied.
Acute pancreatitis is an acute inflammation of the pancreatic gland, most often elicited by alcohol ingestion or gall stone disease. Tissue factor is located in the membrane of various cells surrounding the blood vessels throughout the body, and is exposed to circulating blood when vessels are ruptured or may be expressed by white blood cells or cells on the inside of blood vessels in inflammatory conditions, such as acute pancreatitis. When tissue factor binds to factor VII, circulating in the blood, the coagulation cascade is initiated, but tissue factor - factor VII may also modulate the inflammatory response.
This clinically relevant study of the predictors of pancreatitis severity looks fine.
Peer reviewer: Natalia A Osna, MD, PhD, Liver Study Unit, Research Service (151), VA Medical Center, 4101 Woolworth Avenue, Omaha, NE 68105, United States
S- Editor Sun H L- Editor Webster JR E- Editor Lin YP
| 1. | Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518-530. |
| 2. | Foitzik T, Eibl G, Hotz B, Hotz H, Kahrau S, Kasten C, Schneider P, Buhr HJ. Persistent multiple organ microcirculatory disorders in severe acute pancreatitis: experimental findings and clinical implications. Dig Dis Sci. 2002;47:130-138. |
| 3. | Ranson JH, Lackner H, Berman IR, Schinella R. The relationship of coagulation factors to clinical complications of acute pancreatitis. Surgery. 1977;81:502-511. |
| 4. | Lasson A, Ohlsson K. Consumptive coagulopathy, fibrinolysis and protease-antiprotease interactions during acute human pancreatitis. Thromb Res. 1986;41:167-183. |
| 5. | Salomone T, Tosi P, Palareti G, Tomassetti P, Migliori M, Guariento A, Saieva C, Raiti C, Romboli M, Gullo L. Coagulative disorders in human acute pancreatitis: role for the D-dimer. Pancreas. 2003;26:111-116. |
| 6. | Ottesen LH, Bladbjerg EM, Osman M, Lausten SB, Jacobsen NO, Gram J, Jensen SL. Protein C activation during the initial phase of experimental acute pancreatitis in the rabbit. Dig Surg. 1999;16:486-495. |
| 7. | Yamaguchi H, Weidenbach H, Lührs H, Lerch MM, Dickneite G, Adler G. Combined treatment with C1 esterase inhibitor and antithrombin III improves survival in severe acute experimental pancreatitis. Gut. 1997;40:531-535. |
| 8. | Alsfasser G, Warshaw AL, Thayer SP, Antoniu B, Laposata M, Lewandrowski KB, Fernández-del Castillo C. Decreased inflammation and improved survival with recombinant human activated protein C treatment in experimental acute pancreatitis. Arch Surg. 2006;141:670-676; discussion 676-677. |
| 9. | Poulsen LK, Jacobsen N, Sørensen BB, Bergenhem NC, Kelly JD, Foster DC, Thastrup O, Ezban M, Petersen LC. Signal transduction via the mitogen-activated protein kinase pathway induced by binding of coagulation factor VIIa to tissue factor. J Biol Chem. 1998;273:6228-6232. |
| 11. | Ruf W, Riewald M. Tissue factor-dependent coagulation protease signaling in acute lung injury. Crit Care Med. 2003;31:S231-S237. |
| 12. | Monroe DM, Key NS. The tissue factor-factor VIIa complex: procoagulant activity, regulation, and multitasking. J Thromb Haemost. 2007;5:1097-1105. |
| 13. | Mackman N. The many faces of tissue factor. J Thromb Haemost. 2009;7 Suppl 1:136-139. |
| 14. | Watanabe T, Yasuda M, Yamamoto T. Angiogenesis induced by tissue factor in vitro and in vivo. Thromb Res. 1999;96:183-189. |
| 15. | Rao LV, Pendurthi UR. Tissue factor-factor VIIa signaling. Arterioscler Thromb Vasc Biol. 2005;25:47-56. |
| 16. | Abe K, Shoji M, Chen J, Bierhaus A, Danave I, Micko C, Casper K, Dillehay DL, Nawroth PP, Rickles FR. Regulation of vascular endothelial growth factor production and angiogenesis by the cytoplasmic tail of tissue factor. Proc Natl Acad Sci USA. 1999;96:8663-8668. |
| 17. | Falciani M, Gori AM, Fedi S, Chiarugi L, Simonetti I, Dabizzi RP, Prisco D, Pepe G, Abbate R, Gensini GF. Elevated tissue factor and tissue factor pathway inhibitor circulating levels in ischaemic heart disease patients. Thromb Haemost. 1998;79:495-499. |
| 18. | Wang X, Gjernes E, Prydz H. Factor VIIa induces tissue factor-dependent up-regulation of interleukin-8 in a human keratinocyte line. J Biol Chem. 2002;277:23620-23626. |
| 19. | Muth H, Kreis I, Zimmermann R, Tillmanns H, Hölschermann H. Differential gene expression in activated monocyte-derived macrophages following binding of factor VIIa to tissue factor. Thromb Haemost. 2005;94:1028-1034. |
| 20. | de Jonge E, Friederich PW, Vlasuk GP, Rote WE, Vroom MB, Levi M, van der Poll T. Activation of coagulation by administration of recombinant factor VIIa elicits interleukin 6 (IL-6) and IL-8 release in healthy human subjects. Clin Diagn Lab Immunol. 2003;10:495-497. |
| 21. | Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci USA. 2001;98:7742-7747. |
| 22. | Sawa H, Ueda T, Takeyama Y, Yasuda T, Matsumura N, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Elevation of plasma tissue factor levels in patients with severe acute pancreatitis. J Gastroenterol. 2006;41:575-581. |
| 23. | Radenkovic D, Bajec D, Ivancevic N, Milic N, Bumbasirevic V, Jeremic V, Djukic V, Stefanovic B, Stefanovic B, Milosevic-Zbutega G. D-dimer in acute pancreatitis: a new approach for an early assessment of organ failure. Pancreas. 2009;38:655-660. |
| 24. | Yasuda T, Ueda T, Kamei K, Shinzaki W, Sawa H, Shinzeki M, Ku Y, Takeyama Y. Plasma tissue factor pathway inhibitor levels in patients with acute pancreatitis. J Gastroenterol. 2009;44:1071-1079. |
| 25. | Puolakkainen P, Valtonen V, Paananen A, Schröder T. C-reactive protein (CRP) and serum phospholipase A2 in the assessment of the severity of acute pancreatitis. Gut. 1987;28:764-771. |
| 26. | Aoun E, Chen J, Reighard D, Gleeson FC, Whitcomb DC, Papachristou GI. Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: a meta-analysis. Pancreatology. 2009;9:777-785. |
| 27. | Heath DI, Cruickshank A, Gudgeon M, Jehanli A, Shenkin A, Imrie CW. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993;34:41-45. |
| 28. | Papachristou GI, Whitcomb DC. Predictors of severity and necrosis in acute pancreatitis. Gastroenterol Clin North Am. 2004;33:871-890. |
| 29. | Eckerwall GE, Axelsson JB, Andersson RG. Early nasogastric feeding in predicted severe acute pancreatitis: A clinical, randomized study. Ann Surg. 2006;244:959-965; discussion 965-967. |
| 30. | Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. |
| 31. | Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561-577. |
| 32. | Maeda K, Hirota M, Ichihara A, Ohmuraya M, Hashimoto D, Sugita H, Takamori H, Kanemitsu K, Baba H. Applicability of disseminated intravascular coagulation parameters in the assessment of the severity of acute pancreatitis. Pancreas. 2006;32:87-92. |
| 33. | Shinagawa K, Ploplis VA, Castellino FJ. A severe deficiency of coagulation factor VIIa results in attenuation of the asthmatic response in mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L763-L770. |
| 34. | Xu H, Ploplis VA, Castellino FJ. A coagulation factor VII deficiency protects against acute inflammatory responses in mice. J Pathol. 2006;210:488-496. |
| 35. | Meade TW, Mellows S, Brozovic M, Miller GJ, Chakrabarti RR, North WR, Haines AP, Stirling Y, Imeson JD, Thompson SG. Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet. 1986;2:533-537. |
| 36. | Heinrich J, Balleisen L, Schulte H, Assmann G, van de Loo J. Fibrinogen and factor VII in the prediction of coronary risk. Results from the PROCAM study in healthy men. Arterioscler Thromb. 1994;14:54-59. |
| 37. | Roberts HR, Monroe DM, Hoffman M. Molecular biology and biochemistry of the coagulation factors and pathways of hemostasis. William’s Hematology. 6th ed. New York: McGraw-Hill 2001; 1409-1434. |
| 38. | Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150. |
| 39. | Compañy L, Sáez J, Martínez J, Aparicio JR, Laveda R, Griñó P, Pérez-Mateo M. Factors predicting mortality in severe acute pancreatitis. Pancreatology. 2003;3:144-148. |
| 40. | Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279-285. |