Published online Dec 28, 2010. doi: 10.3748/wjg.v16.i48.6111
Revised: September 13, 2010
Accepted: September 20, 2010
Published online: December 28, 2010
AIM: To determine whether mitochondrial dysfunction resulting from high-fat diet is related to impairment of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt, also known as PKB) pathway.
METHODS: Rat models of nonalcoholic fatty liver were established by high-fat diet feeding. The expression of total and phosphorylated P13K and Akt proteins in hepatocytes was determined by Western blotting. Degree of fat accumulation in liver was measured by hepatic triglyceride. Mitochondrial number and size were determined using quantitative morphometric analysis under transmission electron microscope. The permeability of the outer mitochondrial membrane was assessed by determining the potential gradient across this membrane.
RESULTS: After Wistar rats were fed with high-fat diet for 16 wk, their hepatocytes displayed an accumulation of fat (103.1 ± 12.6 vs 421.5 ± 19.7, P < 0.01), deformed mitochondria (9.0% ± 4.3% vs 83.0% ± 10.9%, P < 0.05), and a reduction in the mitochondrial membrane potential (389.385% ± 18.612% vs 249.121% ± 13.526%, P < 0.05). In addition, the expression of the phosphorylated P13K and Akt proteins in hepatocytes was reduced, as was the expression of the anti-apoptotic protein Bcl-2, while expression of the pro-apoptotic protein caspase-3 was increased. When animals were treated with pharmacological inhibitors of P13K or Akt, instead of high-fat diet, a similar pattern of hepatocellular fat accumulation, mitochondrial impairment, and change in the levels of PI3K, Akt, Bcl-2 was observed.
CONCLUSION: High-fat diet appears to inhibit the PI3K/Akt signaling pathway, which may lead to hepatocellular injury through activation of the mitochondrial membrane pathway of apoptosis.
- Citation: Han JW, Zhan XR, Li XY, Xia B, Wang YY, Zhang J, Li BX. Impaired PI3K/Akt signal pathway and hepatocellular injury in high-fat fed rats. World J Gastroenterol 2010; 16(48): 6111-6118
- URL: https://www.wjgnet.com/1007-9327/full/v16/i48/6111.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i48.6111
Nonalcoholic fatty liver disease (NAFLD) is caused by triglyceride (TG) accumulation within the liver and can either be a benign self-limiting state or a condition associated with steatohepatitis (NASH), which may develop to fibrosis, cirrhosis and liver failure[1,2]. Triacylglycerol formation in the hepatocytes may also be cytotoxic to hepatocytes[3,4]. Multiple lines of evidence support the role of intrahepatic fat in causing hepatic insulin resistance. Hepatic insulin resistance is considered to be the fundamental mechanism in the prevalence and progression of the disease. It is also a critical component in the development of NAFLD, which is characterized by a marked reduction in the activity of the insulin signaling pathway[5].
In the presence of insulin, the insulin receptor normally phosphorylates insulin receptor substrate (IRS) proteins, which are linked to the activation of several signaling pathways, including the metabolic phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt, also known as PKB) pathway. Phosphatidylinositol 3-kinase (PI3K) and its downstream effector Akt regulate a diverse array of cellular events, including survival and apoptosis of a number of cell types[6].
Hepatocyte apoptosis is a key histologic feature of NAFLD, and correlates with progressive inflammation and fibrosis[7]. The molecular pathways leading to hepatocyte apoptosis are not fully defined; however, mitochondrial dysfunction is an important element in the pathogenesis of NAFLD[8]. Recent evidence showed that mitochondria participate in the regulation of both cell proliferation and death, including apoptosis[9], and are thus potential mediators of the PI3K/Akt signaling pathway. Although multiple lines of evidence have suggested a close linkage between insulin-induced signaling and mitochondrial functions[10], the potential relationship between the PI3K/Akt signaling pathway and the mitochondrial abnormalities that underlie NAFLD remain unclear.
The present study was undertaken to investigate the relationship between mitochondria impairment and the activity of the PI3K/Akt signaling pathway during the development of NAFLD.
Male Wistar rats, 12 wk old were obtained from Harbin Medical University.
Laboratories (stock No. 002207). All experiments and animal care complied with the guidelines for the humane treatment of animals set by the Association of Laboratory Animal Sciences and the Center for Laboratory Animal Sciences, Harbin Medical University. At 6 wk of age, the 60 mice were randomly divided into four groups: (1) Normal control (NC) group; (2) NC plus the PI3K inhibitor LY294002 (NC + LY, 15 μg/kg daily injected via the tail CA 440206, Calbiochem); (3) NC plus the AKT inhibitor 1-L-6-hydroxymethyl-chiro-inositol2-(R)-2-O-methyl-3-O-octadecylcarbonate (NC + AI, 20 μg/kg daily via tail injection CA124005, Calbiochem); and (4) High-fat diet (HFD). The normal control rats were fed a commercial rat diet (7%-10% fat, 68%-70% carbohydrates, 18%-20% protein, 1%-2% vitamins and minerals; 210 kcal/100 g per day) for 16 wk, while rats in the treatment group (HFD group) were fed a high-fat diet (40% fat, 38%-40% carbohydrates, 18%-20% protein, 1%-2% vitamins and minerals; 210 kcal/100 g per day) for the same period of time.
Blood samples from the retro-orbital sinus were collected before and after the treatment. Rats were fasted overnight before the collection of the blood samples. Plasma insulin was determined using ELISA. Insulin resistance was evaluated using a homeostasis model assessment of insulin resistance (HOMA). Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyltransferase (GGT) levels were measured using spectrophotometric assay kits (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. Insulin resistance was assessed by computing insulin resistant index (HOMA-IR). The formula used was as follows: HOMA-IR = Insulin (μg/L) × glucose (mmol/L)/22.5.
The liver (100 mg wet tissue) was homogenized in an ice-cold 0.05% butylhydroxytoluene solution. After lipids were extracted from the liver according to the method of Folch et al[11], TG content in each sample was measured with a commercial assay kit (Wako Pure Chemical Industries, Osaka, Japan CA 290-63701).
Hepatocytes were isolated from the liver (20-25 mg) of each mouse by the collagenase perfusion method. Each liver was pre-perfused at 37°C with buffer containing 100 mmol/L HEPES (pH 7.4), 143 mmol/L NaCl, and 7 mmol/L KCl, and then perfused with buffer containing 0.05% collagenase and 5 mmol/L CaCl2. Following digestion, the liver was dispersed in the perfusion solution and incubated in the perfusion buffer at 37°C for an additional 5 min. The dispersed cell suspension was then filtered through a nylon mesh and centrifuged at 100 ×g for 3 min at 25°C. The resulting cell pellets were resuspended in the hepatocyte medium, and cell viability was then determined using a trypan-blue-exclusion test.
The integrity of the inner mitochondrial membrane was assessed by determining the potential gradient across this membrane. Rhodamine 123 (Rh123) powder was dissolved in methanol and stored at -20°C as a 1 g/L solution, which was diluted to 5 mg/L with phosphate buffered solution (PBS) before each experiment. Hepatocytes (1 × 106) were washed three times with PBS that had been preheated to 4°C. They were then resuspended in 300 mL PBS, incubating with Rh123 (final concentration 2.5 mg/L) for 1 h at 37°C, and then filtered through a 200-mesh screen. Approximately 10 000 cells were measured using a FACS Calibur flow cytometer (BD Biosciences, San Diego, CA, USA) using Cell Quest software (a maximum absorbing wave length 590 nm, an excitation wave length 488 nm) (BD Biosciences). Rh123 and tetramethylrhodamineethylester (TMRE) were purchased from Invitrogen (Karlsruhe, Germany).
For transmission electron microscopy, small liver fragments were fixed in 4% glutaraldehyde and then processed using standard methods. Sections were viewed under microscope by a pathologist (Dr. Chang H, Department of Pathology, Harbin Medical University). Mitochondrial number and size were determined using quantitative morphometric analysis under transmission electron microscope (Model HB601UX, Vacuum Generators, Hastings, United Kingdom).
Ten μg protein was subjected to SDS-PAGE (10% acrylamide gel) and then transferred to a PVDF membrane for 2 h (120 V) using a Bio-Rad Mini Trans Blot electrophoretic transfer unit (Bio-Rad, Marnes-la-Coquette, France). The membranes were blocked for nonspecific binding with 5% nonfat dry milk and then probed with the specific primary antibodies (Abcam, CA ab74136, ab63566, ab79360, ab8805, 1:1000 dilution) at 4°C overnight. After 3 washes with TBS-T, membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz, CA SC2030). Separated proteins were visualized by an ECL kit (GE Healthcare Life Sciences, CA RPN2135) and light emission was captured on X-ray film (GE Healthcare). Intensities of the respective bands were examined by densitometric analysis (Scion Image Analyst program).
Results were presented as mean ± SE and were analyzed using one-way analysis of variance followed by the Bonferroni multiple comparisons test. All tests were 2-sided, and P < 0.05 was considered to be statistically significant. All statistical analyses were performed using INSTAT version 3 (Graph Pad Software, San Diego, CA, USA).
Compared with normal control groups, the mean serum transaminase, hepatic TG content, body and liver weight, insulin resistant index were all increased in PI3K inhibitor, Akt inhibitor and high-fat groups (P < 0.05, Table 1). Hepatocytes could be damaged after PI3K/Akt pathway signal transduction was blocked, but blocked PI3K/Akt pathway signal transduction could lead to TG accumulation in liver to liver weight gain and insulin resistance.
| NC | NC + LY | NC + AI | HFD | |
| Tissue weight | ||||
| Liver (g) | 1.55 ± 0.14 | 1.99 ± 0.32a | 1.89 ± 0.43a | 3.98 ± 0.64a |
| Serum | ||||
| TG (mg/dL) | 183.8 ± 70.4 | 396.7 ± 72.3 | 376.7 ± 68.4 | 589 ± 98.4b |
| ALT (U/L) | 34 ± 3.1 | 86 ± 5.58a | 89 ± 5.2a | 207 ± 35.5b |
| AST (U/L) | 36 ± 3.4 | 88 ± 6.1 | 99 ± 5.2a | 187 ± 35.5b |
| GGT (U/L) | 52 ± 6.4 | 48 ± 3.9 | 62 ± 4.4 | 232 ± 67.8b |
| Glucose (mmol/L) | 5.1 ± 0.4 | 6.1 ± 0.5 | 5.7 ± 0.4 | 7.1 ± 71.3a |
| Insulin (mIu) | 4.7 ± 0.7 | 14.9 ± 2.0b | 15.0 ± 2.9b | 21.0 ± 3.8b |
| Liver | ||||
| IRI (HOMA-IR) | 1.06 | 4.03a | 3.8a | 6.6b |
| TG (mg/g) | 103. 1 ± 12.6 | 324.6 ± 13.4 | 2336.8 ± 11.6 | 421.5 ± 19.7b |
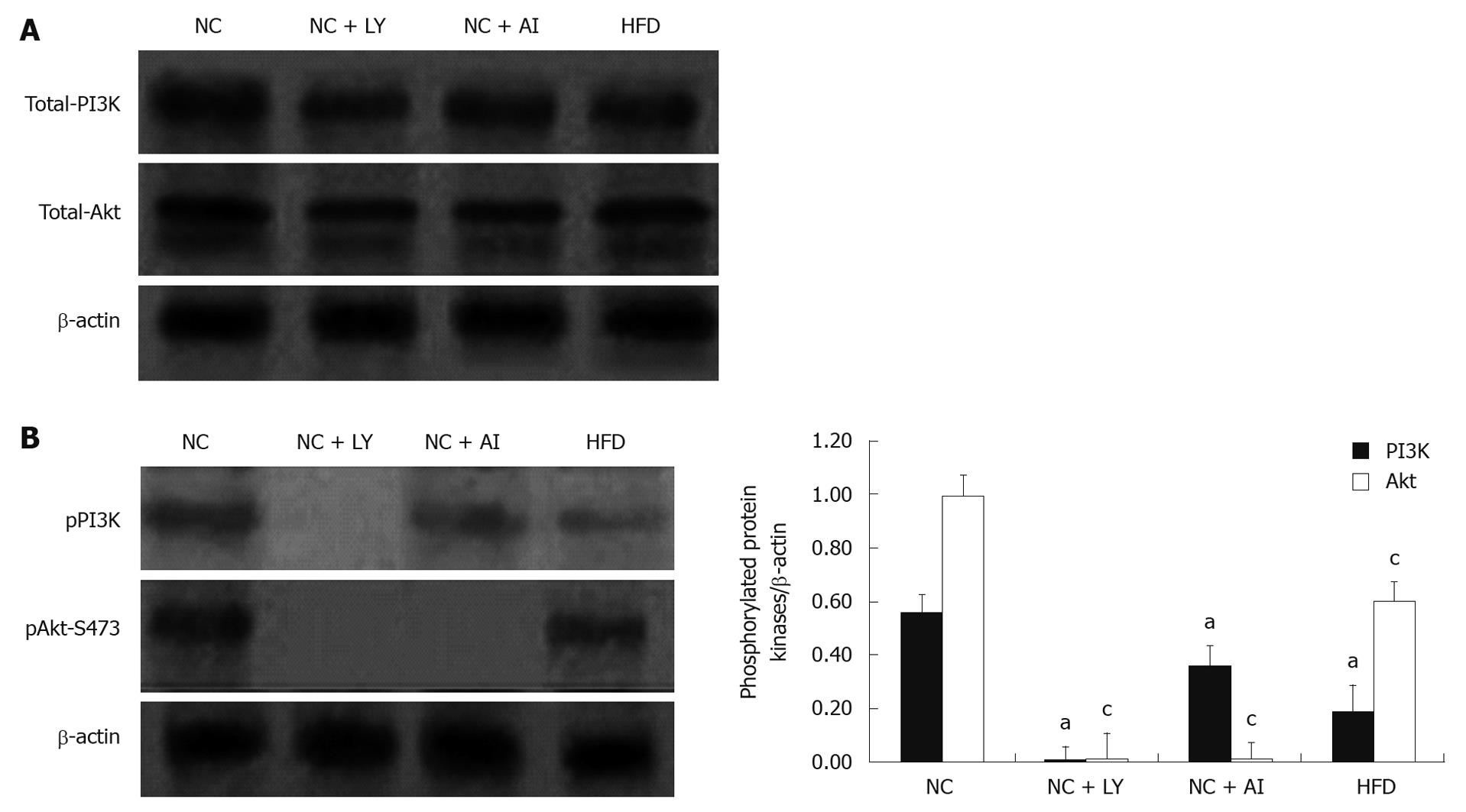
To further investigate whether the PI3K/Akt pathway mediates injury of hepatocytes, we measured the protein expression levels of total and phosphorylated PI3K and Akt in the four groups. The protein expression levels of total PI3K and Akt showed no significant difference among the four groups (Figure 1). But compared with the normal control groups, the expression levels of pPI3K and pAkt markedly decreased in the high-fat group. Moreover, in PI3K inhibitor groups, neither pPI3K nor pAkt was expressed. In Akt inhibitor groups, pAkt showed no expression, and pPI3K had no significant difference compared with the normal control group (Figure 1). These results suggested that fat mass accumulation in the liver may lead to decreased expression of pPI3K and pAkt in fatty liver induced by high fat.
To investigate further whether the fat accumulation in the liver was involved in PI3K/Akt pathway, TG content in each sample was measured in the four groups. The results showed that TG content of liver was elevated in high-fat diet, PI3K and Akt inhibitor groups.
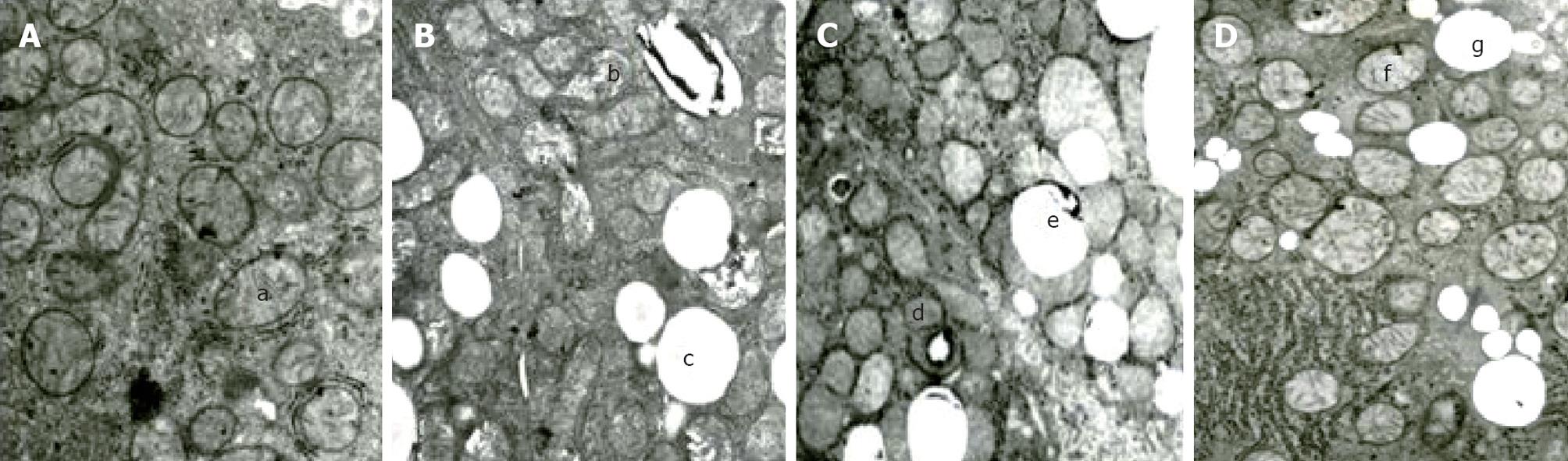
To determine whether the PI3K pathway has an anti-apoptotic effect in liver cells, we compared the mitochondrial morphology in the four groups (Figure 2A). Electron microscopy revealed that the hepatocytes of the control group rats were rich in mitochondria, the shape and size of mitochondria were normal and had few lipid droplets. In contrast, many mitochondria from rats in the high-fat group were enlarged and showed morphological changes, including a rarefied matrix and large lipid droplet. In the high-fat group, 83.0% ± 10.9% of mitochondria had abnormal morphology, compared to 9.0% ± 4.3% in the control group (P < 0.05) (Figure 2B). The changes of mitochondria in the PI3K-inhibitor and Akt- inhibitor groups were similar to those of the high- fat group, although they differed significantly from the high-fat group in the PI3K and Akt inhibitor groups (Figure 2C and D). This indicated that PI3K/Akt pathway blocking could affect ultrastructural changes of hepatocellular mitochondria. The change was similar to that resulting from a high-fat diet.
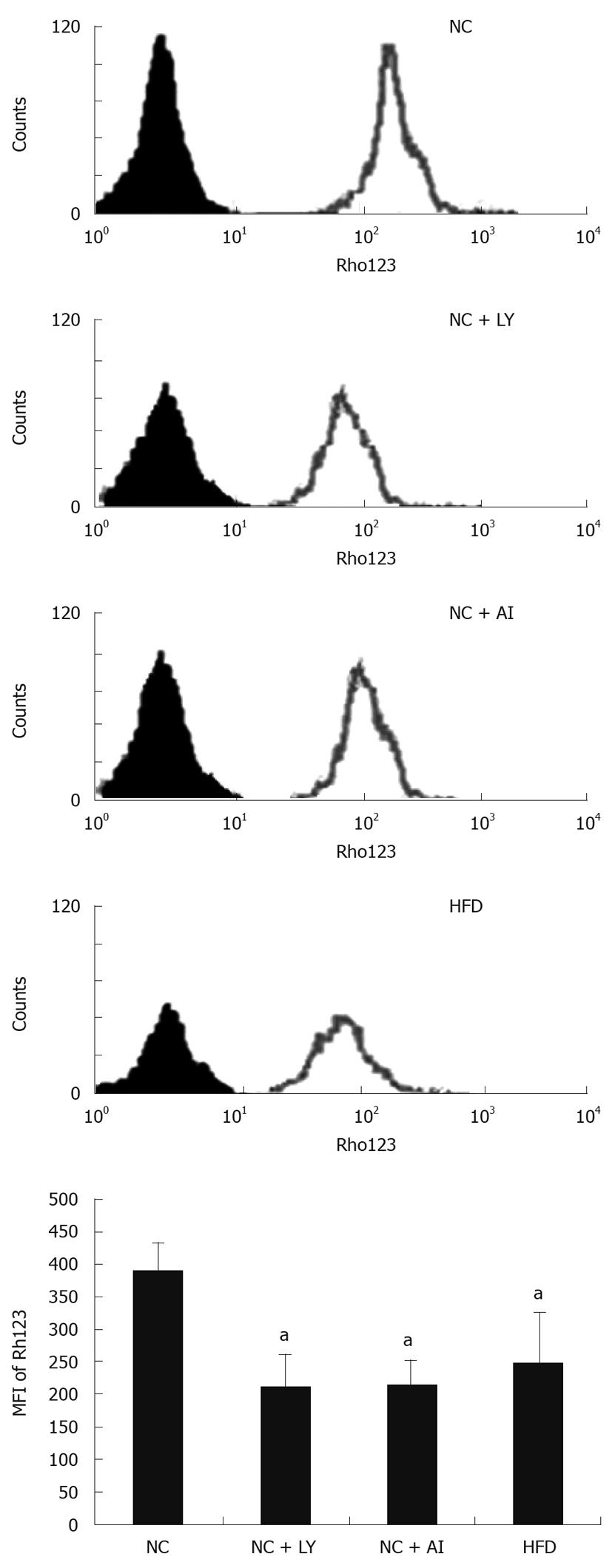
To investigate whether PI3K/Akt signaling is associated with an increase in the permeability of the outer mitochondrial membrane, we measured the mean fluorescence intensity (MFI) of Rh123 on the mitochondrial membrane in animals of the four groups. The MFI was 249.121% ± 13.526% in the high-fat group, and 389.385% ± 18.612% in the control group, indicating that there was a significant decrease in the mitochondrial membrane potential in the high-fat group. The PI3K and Akt inhibitor groups showed similar decreases in the mitochondrial membrane potential (211.326% ± 12.114% and 214.326% ± 13.321%, respectively) compared with the control group (Figure 3). These results suggested that mitochondrial function was impaired to the same degree in the high-fat and both inhibitor groups, strongly suggesting that the PI3K/Akt signal transduction pathway is associated with permeabilization of the outer mitochondrial membrane.
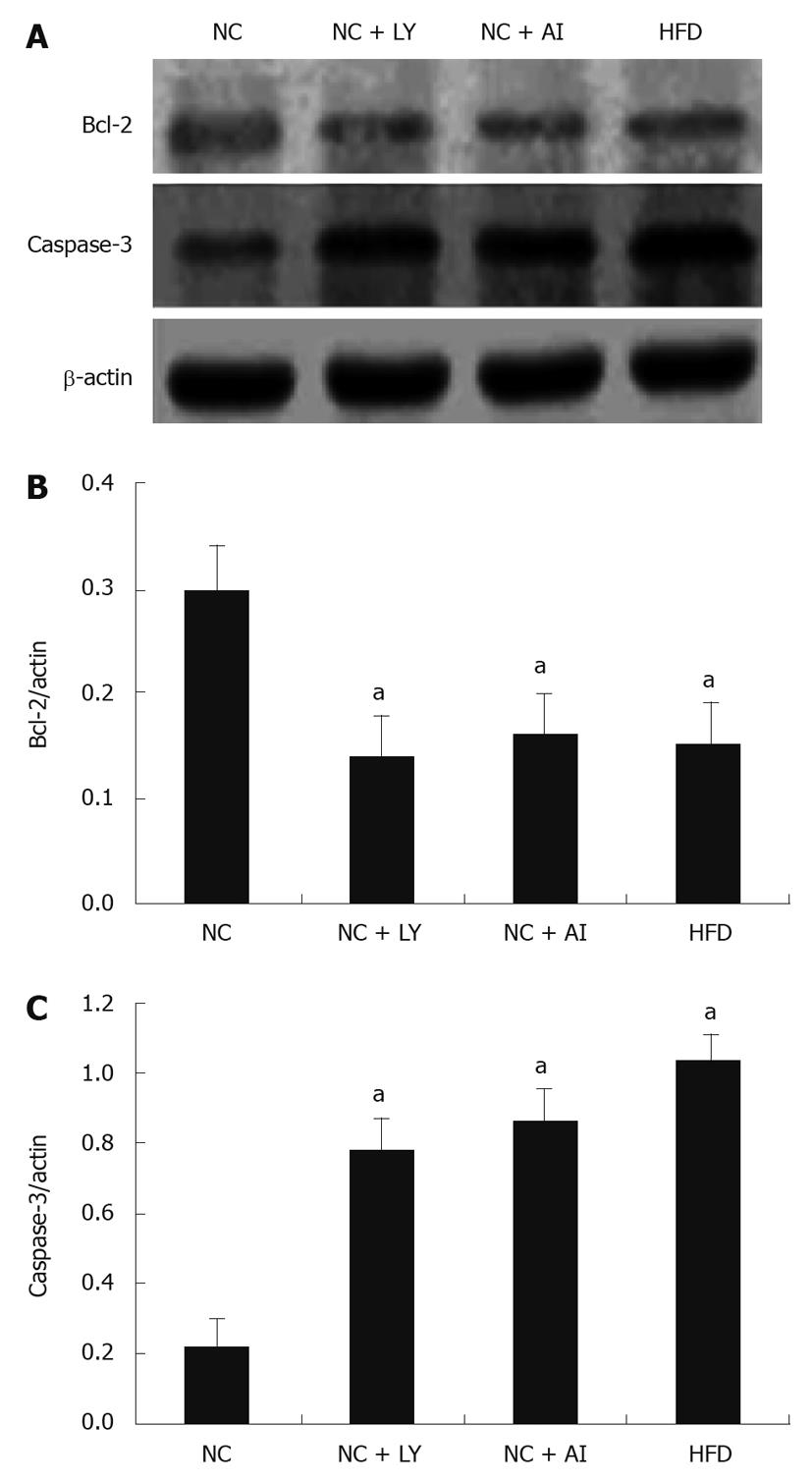
One way in which changes in the PI3K/Akt signaling pathway might have induced mitochondrial apoptosis was through the effects of the apoptosis-related proteins Bcl-2 and caspase-3. To test this theory, we measured the expression level of these two proteins using Western blottings (Figure 4). We found that Bcl-2 expression showed a similar decrease in the high-fat, P13K-inhibitor, and Akt-inhibitor groups (by 64%, 61% and 62%, respectively) compared to the control group, Conversely, the level of caspase-3 increased by a similar margin in the 3 experimental groups (42%, 31% and 29.5%, respectively) compared to the control group. This result demonstrated that there may be a link between the PI3K/Akt signaling pathway and the mitochondrial pathway of apoptosis in rats treated with high-fat, P13K inhibitor, or Akt inhibitor.
In this study, we attempted to investigate whether the PI3K/Akt pathway could mediate mitochondrial impairment during the development of NAFLD, and whether this might explain why hepatic insulin resistance is critical to the development of this disease. We found that hepatocytes of rats fed a high-fat diet accumulated fat and developed deformed mitochondria, a decreased mitochondrial membrane potential, and decreased expression of PI3K and Akt proteins. Unexpectedly, we found that blocking the PI3K/Akt pathway with either a PI3K or Akt inhibitor led to hepatocellular fat accumulation and mitochondrial impairment indistinguishable from that of high-fat fed rats. These findings suggest that signals transduced by the PI3K/Akt pathway are involved in the pathogenesis of NAFLD.
The phosphatidylinositol 3-kinase (PI3K)/Akt signaling cascade is an important component of insulin signaling in normal tissues, where it mediates glucose uptake and homeostasis, as well as being an important regulator of cell survival in numerous cell types[12]. In this study, we found that both the insulin resistance index (HOMA-IR) and serum levels of hepatocellular enzymes were significantly increased in response to a high-fat diet, suggesting that insulin resistance and hepatocyte damage may coexist in our high-fat experimental model.
Mitochondrial dysfunction is known to play a central role in the hepatocyte damage of NAFLD[13]. Although our current experiment confirmed that mitochondrial dysfunction is associated with progression of liver pathological changes, the mechanisms initiating mitochondrial dysfunction in this disease are unknown. Specifically, it is not clear whether mitochondrial damage and changes in insulin signal transduction are directly related to the pathophysiology of nonalcoholic fatty liver induced by high fat. A number of studies have indicated that insulin can stimulate mitochondrial biogenesis[14] and alter mitochondrial morphology in obese, insulin-resistant and type 2 diabetic individuals[15]. In the current study, we found that a high-fat diet led to an increase in the proportion of morphologically abnormal mitochondria as well as an increase in the insulin resistance index. Similarly, less marked results were seen with treatment of the P13K- or Akt inhibitors, suggesting that changes in the PI3K/Akt signal transduction pathway may mediate these changes in mitochondria morphology.
The transmembrane potential (Dym) of mitochondria is known to play a crucial role in their normal function[16]. We found that the mitochondrial transmembrane potential in the high-fat group, as well as those treated with either the PI3K- or AKT inhibitor, was significantly lower than control values, further implicating the P13K/Akt pathway in mitochondrial dysfunction.
Our results have confirmed some previous findings by Mehta et al[17], who reported that hepatic steatosis is frequently associated with obesity, type 2 diabetes, and hyperlipidemia, with insulin resistance being a key pathogenic factor in NAFLD and mitochondrial damage being characteristic of the disease. However, the current study is the first to implicate the PI3K/Akt signal transduction pathway in the morphological and functional changes in hepatocyte mitochondria, as well as insulin resistance induced by high fat.
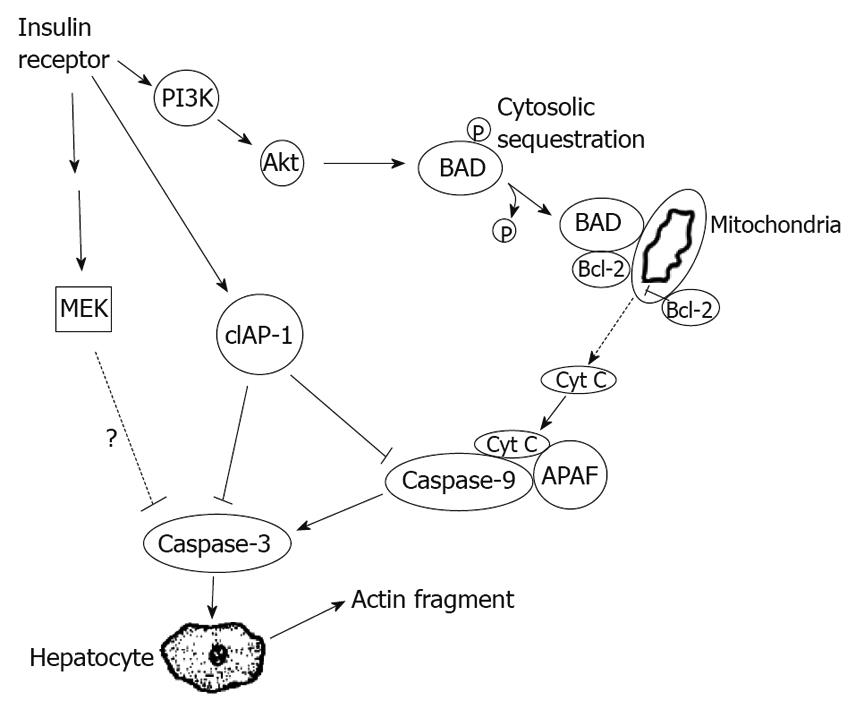
Recent evidence suggests that hepatocellular injury in a number of liver diseases is accompanied by activation of the apoptotic pathways[18]. We found morphological (deformed mitochondria), and functional abnormalities (a reduction in the mitochondrial membrane potential) in hepatocyte mitochondria consistent with apoptosis in high-fat, PI3K and Akt inhibitor groups. We also examined the expression of two proteins in the Bcl-2 family of anti-apoptotic proteins. Proteins in this family are known to regulate apoptosis at peri-mitochondrial sites. The PI3K/Akt signaling pathway has been shown to have an anti-apoptotic effect by activating Bcl-2 to inhibit the apoptotic mediator caspase-3[19]. We found that, in rats fed a high-fat diet, expression of PI3K, phosphorylated Akt, and Bcl-2 decreased, but the expression of caspase-3 increased, suggesting a mechanism by which apoptosis may be triggered in NAFLD. To further support a role for the P13K pathway in mediating apoptosis in NAFLD, we found that the P13K- and Akt inhibitors led to a similar decrease in Bcl-2 expression and a significant increase in caspase-3 expression (Figure 5).
Apoptosis is a process of active cellular self-destruction that requires the expression of specific genes including those of the Bcl-2 gene family[20]. Of these, Bax, Bad and Bak promote cell death, whereas Bcl-2 and Bcl-xL inhibit apoptosis and promote cell survival[21]. The results of a recent study suggest that caspase-3 can cause permeabilization of cells, with the help of pro-apoptotic Bcl-2 proteins. Until recently, the prevailing view has been that caspase-3 activation represents the apex of the caspase cascade within the mitochondrial apoptotic pathway.
Some Bcl-2 family members located on the mitochondrial membrane have been shown to be able to alter the permeability of the mitochondrial membrane and trigger the activation of caspases[22]. Programmed cell death might thus be activated via a membrane bound pathway, in which the signal is initiated at the mitochondrion[23]. Pro-apoptotic compounds are normally sequestered in the intermembrane space[24]. When the permeability of the outer mitochondrial membrane increases, these proteins are released into the cytosol, forming the apoptosome and subsequently activating caspase-3[25]. The results of the current study indicate that not only high fat, but also blockage of the PI3K/Akt pathway signal, lead to an increase in the permeability of the hepatic mitochondrial membrane, implicating this pathway in the apoptotic mechanisms triggered by a high-fat diet. All these results lead us to propose a disease model in which the PI3K/Akt signal transduction pathway induces apoptosis via a Bcl-2/caspase-3 mitochondrial-dependent pathway, in which phosphorylation of Bad results in targeting of Bcl-xL to the mitochondrial membrane, where Bcl-2 interacts with and inactivates anti-apoptotic Bcl-2 proteins, thereby inducing apoptosis[26].
In conclusion, the present study suggests that fat accumulation in the liver may impair PI3K/Akt pathway signal transduction and thereby activate the mitochondrial membrane pathway of apoptosis, leading to hepatocyte damage.
Nonalcoholic fatty liver disease (NAFLD) is caused by triglyceride (TG) accumulation within the liver, which may progress to fibrosis, cirrhosis and liver failure. Mitochondrial dysfunction which caused hepatocyte apoptosis is an important element in the pathogenesis of NAFLD. Hepatic insulin resistance is also a critical component in the development of NAFLD, which is characterized by a marked reduction in the activity of the insulin signaling pathway, including the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt, also known as PKB) pathway. But the potential relationship between the PI3K/Akt signaling pathway and the mitochondrial abnormalities that underlie NAFLD remain unclear.
Recent reports have highlighted the insulin-induced signaling pathways in various cells. In the presence of insulin, the insulin receptor normally phosphorylates insulin receptor substrate (IRS) proteins, which are linked to the activation of several signaling pathways, including the PI3K/Akt pathway. PI3K and its downstream effector Akt regulate a diverse array of cellular events, including survival and apoptosis of a number of cell types. The mitochondrial impairment during the development of NAFLD is also an area of intense research. Mitochondrial dysfunction is an important element in the pathogenesis of NAFLD, mainly hepatocyte apoptosis. Recent evidence showed that mitochondria participates in the regulation of both cell proliferation and death, including apoptosis, and are thus potential mediators of the PI3K/Akt signaling pathway.
This is the first study to report the relationship between mitochondria impairment and the activity of the PI3K/Akt signaling pathway during the development of NAFLD, which suggests that fat accumulation in the liver may impair PI3K/Akt pathway signal transduction and thereby activate the mitochondrial membrane pathway of apoptosis, leading to hepatocyte damage.
The results of this study indicated that the PI3K/Akt pathway may mediate mitochondrial impairment during the development of NAFLD, and this might explain why hepatic insulin resistance is critical to the development of this disease.
Insulin signaling pathways, including the metabolic PI3K/Akt pathway. PI3K and its downstream effector Akt regulate a diverse array of cellular events, including survival and apoptosis of a number of cell types. Mitochondrial impairment, an important element in the pathogenesis of NAFLD. Mitochondria participates in the regulation of both cell proliferation and death, including apoptosis, and are thus potential mediators of the PI3K/Akt signaling pathway.
This is a well written paper with well thought out, well-controlled data. The paper evaluates the effect of a high-fat diet and correlates it with similar findings seen with blocking the P13K or Akt inhibitors, and thereby implicated this pathway as the mechanism for high-fat diet hepatic injury.
Peer reviewer: Dr. Shawn David Safford, Department of Surgery, Duke University Medical Center, 994 West Ocean View Avenue, Norfolk, VA 23503, United States
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
| 1. | Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817-824. |
| 2. | Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311-357. |
| 3. | Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147-152. |
| 4. | Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360-369. |
| 5. | Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739-745. |
| 6. | Uddin S, Hussain AR, Siraj AK, Manogaran PS, Al-Jomah NA, Moorji A, Atizado V, Al-Dayel F, Belgaumi A, El-Solh H. Role of phosphatidylinositol 3'-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood. 2006;108:4178-4186. |
| 7. | Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360-369. |
| 8. | Caldwell SH, de Freitas LA, Park SH, Moreno ML, Redick JA, Davis CA, Sisson BJ, Patrie JT, Cotrim H, Argo CK. Intramitochondrial crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2009;49:1888-1895. |
| 9. | Lee DH, Szczepanski MJ, Lee YJ. Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt signaling pathway in human prostate cancer cells. J Cell Biochem. 2009;106:1113-1122. |
| 10. | Pang Y, Zheng B, Fan LW, Rhodes PG, Cai Z. IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia. 2007;55:1099-1107. |
| 11. | Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497-509. |
| 12. | Chen CH, Lai JM, Chou TY, Chen CY, Su LJ, Lee YC, Cheng TS, Hong YR, Chou CK, Whang-Peng J. VEGFA upregulates FLJ10540 and modulates migration and invasion of lung cancer via PI3K/AKT pathway. PLoS One. 2009;4:e5052. |
| 13. | Petrosillo G, Portincasa P, Grattagliano I, Casanova G, Matera M, Ruggiero FM, Ferri D, Paradies G. Mitochondrial dysfunction in rat with nonalcoholic fatty liver Involvement of complex I, reactive oxygen species and cardiolipin. Biochim Biophys Acta. 2007;1767:1260-1267. |
| 14. | Shen W, Hao J, Tian C, Ren J, Yang L, Li X, Luo C, Cotma CW, Liu J. A combination of nutriments improves mitochondrial biogenesis and function in skeletal muscle of type 2 diabetic Goto-Kakizaki rats. PLoS One. 2008;3:e2328. |
| 15. | Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587-3593. |
| 16. | Mullauer FB, Kessler JH, Medema JP. Betulinic acid induces cytochrome c release and apoptosis in a Bax/Bak-independent, permeability transition pore dependent fashion. Apoptosis. 2009;14:191-202. |
| 17. | Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637-5644. |
| 18. | Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358-1362. |
| 19. | Shroff EH, Snyder CM, Budinger GR, Jain M, Chew TL, Khuon S, Perlman H, Chandel NS. BH3 peptides induce mitochondrial fission and cell death independent of BAX/BAK. PLoS One. 2009;4:e5646. |
| 20. | Ekert PG, Jabbour AM, Manoharan A, Heraud JE, Yu J, Pakusch M, Michalak EM, Kelly PN, Callus B, Kiefer T. Cell death provoked by loss of interleukin-3 signaling is independent of Bad, Bim, and PI3 kinase, but depends in part on Puma. Blood. 2006;108:1461-1468. |
| 21. | Brown GC, Borutaite V. Regulation of apoptosis by the redox state of cytochrome c. Biochim Biophys Acta. 2008;1777:877-881. |
| 22. | Rose P, Armstrong JS, Chua YL, Ong CN, Whiteman M. Beta-phenylethyl isothiocyanate mediated apoptosis; contribution of Bax and the mitochondrial death pathway. Int J Biochem Cell Biol. 2005;37:100-119. |
| 23. | Almeida S, Brett AC, Góis IN, Oliveira CR, Rego AC. Caspase-dependent and -independent cell death induced by 3-nitropropionic acid in rat cortical neurons. J Cell Biochem. 2006;98:93-101. |
| 24. | Hans G, Malgrange B, Lallemend F, Crommen J, Wislet-Gendebien S, Belachew S, Robe P, Rogister B, Moonen G, Rigo JM. Beta-carbolines induce apoptosis in cultured cerebellar granule neurons via the mitochondrial pathway. Neuropharmacology. 2005;48:105-117. |
| 25. | Sharifi AM, Eslami H, Larijani B, Davoodi J. Involvement of caspase-8, -9, and -3 in high glucose-induced apoptosis in PC12 cells. Neurosci Lett. 2009;459:47-51. |