THE CLINICAL IMPACT OF HEPATIC ISCHEMIA-REPERFUSION INJURY
The rate of liver failure is increasing in the UK population[1]. Liver transplantation is an effective treatment for patients with end-stage disease, giving an average of 17-22 years of additional life[2,3]. Access to liver transplantation is limited by donor availability; several innovations, including split liver, living donor transplantation, non-heart beating donation (NHBD) and the expansion of the donor criteria, have been attempted to tackle this disparity[4].
Ischemia-reperfusion injury (IRI) causes a spectrum of early organ dysfunction after transplantation; the most severe form, termed primary non-function, may result in patient death. “Marginal organs”, including those from older donors, those affected by steatosis and those from donors with long pre-donation intensive care unit stay, may be judged to pose an excessive risk of IRI and be discarded, placing additional pressure on the already scarce donor resource[5].
Due to shifting patterns of organ donation, there is a tendency towards the increased use of marginal organs. Year on year, the mean donor age is increasing, in part due to improvements in road safety and declining numbers of traumatic deaths. Furthermore, NHBD (also termed donation after cardiac death) is becoming an increasingly important component of the donor resource[6]. Compared with the “gold standard” of heart beating donation (HBD), NHBD is associated with a decreased quantity of donated organs (2.1 organs per donor compared with 3.4 organs per HBD). Albeit in small studies, NHBD liver transplantation is also associated with a higher risk of IRI leading to elevated incidence of primary non-function[7].
Our group has previously estimated that negating the effects of IRI in HBD would lead to a 6% increase in the donor supply through recruitment of these marginal organs back into the donor pool[4]. In the current climate of rapidly increasing NHBD and increasing donor age, the imperative to better understand and avert hepatic ischemia is becoming ever stronger.
HEME OXYGENASE-1
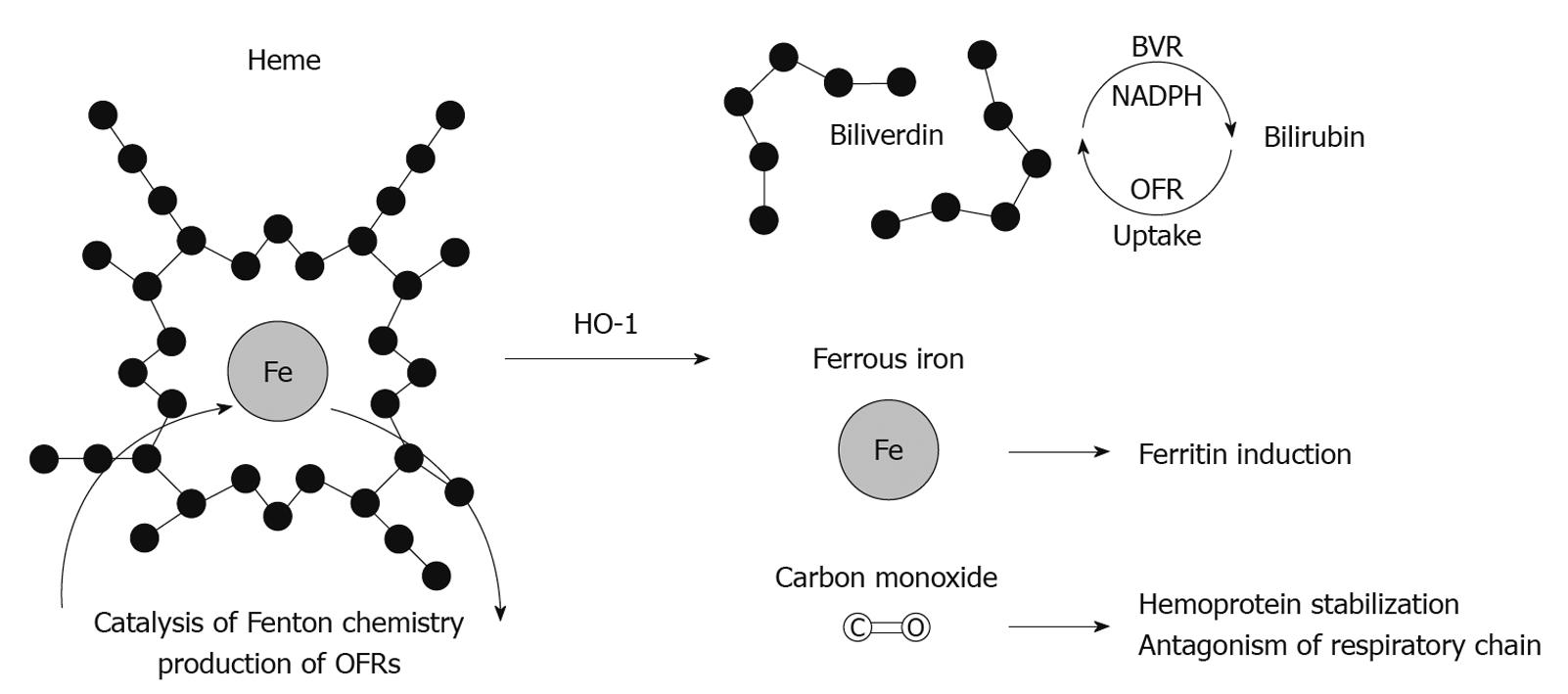
Heme oxygenase (HO) is a nicotinamide adenine dinucleotide phosphate (NADPH)-dependent microsomal enzyme, which catalyzes the breakdown of heme to biliverdin, iron and carbon monoxide (CO)[10] (Figure 1). Biliverdin is subsequently reduced to bilirubin by biliverdin reductase while free iron is sequestrated by ferritin. Of the three heme oxygenase isoforms (HO-1, HO-2 and HO-3), only HO-1 (also known as heat shock protein 32) is inducible[11]. HO-1 is a 32 kDa enzyme encoded by the hmox1 gene. It has been found to be upregulated during states of oxidative and cellular stress and plays an important role in maintaining oxidative/antioxidant homeostasis[12].
Figure 1 Putative mechanisms of heme oxygenase-1 antioxidant effect.
HO-1: Heme oxygenase-1; BVR: Biliverdin; OFR: Oxygen free radicals; NADPH: Nicotinamide adenine dinucleotide phosphate.
Induction of HO-1 and its metabolites is protective in a large number of seemingly unrelated pathologies, including sepsis, malaria, endotoxic shock, IRI, organ transplant rejection, induction of tolerance, myocardial infarction, type 2 diabetes and obesity[13]. This spectrum of protection is attributed to multi-level mechanisms of cytoprotection and inflammatory modulation.
Polymorphism in the (GT)n microsatellite of the HMOX1 promoter is thought to be responsible for the variations seen in the human response of HO-1 to stimuli[14]. This may account for the differences in the susceptibility of individuals to certain pathologies and in the apparent longevity associated with increased HO-1 expression[15,16].
HO-1 in hepatic IRI
In the context of IRI and transplantation, HO-1 induction or supplementation with its metabolites has been shown repeatedly to be protective in both the liver and other organs.
In early experiments, HO-1 was induced by heat shock in donor livers prior to 44 h of cold ischemia preceding liver isogeneic transplantation in rats, resulting in marked improvements in recipient survival[17]. HO-1 upregulation with adenoviral HO-1 or cobalt protoporphyrin (CoPP) resulted in improved portal venous flow on ex-vivo perfusion, while on transplantation into syngeneic hosts, recipient survival doubled, histological injury was ameliorated and influx of macrophages and T cells was reduced[18]; findings confirmed in similar experiments using transplantation[19,20] and hepatic warm ischemia models[21]. More recently, targeted deletion of Bach-1, which normally suppresses HO-1 transcription, led to HO-1 upregulation and protection from myocardial ischemia[22], although these experiments have yet to be repeated in models of hepatic ischemia.
In our own laboratory we have shown that HO-1 is upregulated in Kupffer cells during ischemic preconditioning (IPC)[23], and that targeted deletion of hmox-1 resulted in aberrant Kupffer cell differentiation and susceptibility to ischemia-reperfusion insults[24]. Our work has suggested that Kupffer cells are a likely site of HO-1 action: recent work from Kupiec Weglinski’s laboratory has demonstrated that adoptive transfer of HO-1 overexpressing bone marrow-derived macrophages was capable of protecting mice from hepatic ischemic insults[25]. Developing this theme, other experiments have shown HO-1 induction with CoPP to protect mice from hepatic ischemia arising from liver transplantation: Kupffer cells recovered from HO-1-induced animals produced less tumor necrosis factor α (TNFα) and interleukin (IL)-6 under stimulation in ex vivo culture[26].
HO-1 in hepatic IPC
IPC is a surgical maneuvre in which an organ is paradoxically protected by a brief period of controlled ischemia and reperfusion immediately prior to a longer index ischemic event, which by itself would normally lead to injury[27,28]. Several small randomized controlled trials have looked at the effectiveness of IPC in both liver transplantation and resection; subsequent Cochrane reviews conclude that further trials are still required to evaluate its role in hepatic and transplant surgery[29,30].
Kanoria et al[31] demonstrated in their rodent model that the application of a hindleg tourniquet (remote IPC) led to a protective phenotype from hepatic IRI. Ischemic post-conditioning has also been described, in which injury is abrogated by modified reperfusion. This emphasizes that tissue damage from ischemia-reperfusion continues following the end of the ischemic insult[32].
The mechanisms behind IPC are poorly understood. Numerous candidate molecules, including nitric oxide, adenosine, protein kinase C, tyrosine kinase and mitogen-activated-protein kinase, have been identified as potential mediators of protection[33]. Given that IPC can act remotely, it may involve an immunomodulatory mechanism.
HO-1 is upregulated following IPC and may be responsible for the observed protection[23]. HO-1 is also upregulated in sites distant to the site of preconditioning. In a remote preconditioning model, renal ischemic insults led to cardiac HO-1 induction[34]. Likewise, HO-1 induction occurred in the liver following four 10 min episodes of femoral artery occlusion, and protection afforded by remote preconditioning was lost when HO-1 was inhibited with SnPP[35].
Given that HO-1 is upregulated in IPC and is known to be powerfully protective when upregulated, it is likely that HO-1 has a role in IPC together with other up- and downstream molecules.
Mechanisms of HO-1-mediated cytoprotection
As described above, it is now beyond doubt that HO-1 induction is protective in the context of hepatic ischemia and transplantation. HO-1’s cytoprotective effects can be credited to a combination of removal of toxic metabolites (heme), and production of protective second messenger molecules in the forms of biliverdin (and subsequently bilirubin), CO, and free iron (which induces ferritin)[36]. HO-1 may also have other mechanisms of protection, independent of its enzyme activity[37].
Catabolysis of free heme
Heme consists of an iron atom contained within a porphyrin ring: heme moieties are usually contained within the “heme pockets” of hemoproteins. Although the most abundant hemoproteins are hemoglobin and myoglobin, heme moieties are contained within many other ubiquitous enzymes, for example iNOS and the mitochondrial electron transfer chain.
Under conditions of oxidative stress, hemoproteins may be oxidized, leading to the release of free heme which causes cellular injury by multiple mechanisms. Firstly, the central iron ion of the heme moiety catalyzes the production of free radicals by Fenton chemistry as follows: Step 1: Fe2+ + H2O2→ Fe3+ + OH· + OH-; Step 2: Fe3+ + H2O2→ Fe2+ + OOH· + H+.
Heme-dependent free radical generation can cause direct cytotoxicity, including damage to the cytoskeleton, lipid bilayer, intermediary metabolic enzymes and DNA[38,39], while circulating heme can cause LDL oxidation leading to endothelial cell toxicity[40]. Heme sensitizes cells to pro-death signals including TNFα and Fas, an effect abrogated by treatment with antioxidants, implying dependence upon free radical production[36].
HO-1 reduces free heme concentrations by two mechanisms. Firstly, catabolysis of heme into metabolites biliverdin, iron and CO directly removes free heme. Secondly, binding of CO to hemoglobin, forming carboxyhemoglobin, prevents its oxidation to methemoglobin and ensuing release of free heme moieties[36].
Some authors have argued strongly that neutralization of free heme during cellular injury can account for a large proportion of the protection offered by HO-1. In experimental malaria, it was found that C57/Bl6J mice had reduced HO-1 induction compared with BALB/c animals, and consequently had higher levels of circulating free heme and greater disease severity. Administering exogenous heme to BALB/c animals caused worsening of their disease severity whereas conversely, CO treatment of C57Bl6J mice was protective[41]. In the same model, HO-1 protected animals from hepatic failure induced by overwhelming circulating heme[42].
Hepatic HO-1 expression is focused in Kupffer cells. This cell type is highly adapted to detect hemolysis: the hemoglobin-haptoglobin complex receptor CD163 is able to induce HO-1 in an IL-10-dependent manner[43,44].
Production of biliverdin
Biliverdin is produced by catalysis of the heme porphyrin ring and is almost immediately converted by biliverdin reductase to unconjugated bilirubin. This, in turn, is glucuronidated to render it water soluble. Bilirubin is a powerful antioxidant believed to be responsible for much of the antioxidant activity of serum[45]. At micromolar concentrations it is capable of scavenging large volumes of reactive oxygen species (ROS), protecting cells from high concentrations of peroxide, with enhanced efficacy in hypoxic conditions, making this effect particularly relevant in the context of ischemia[46]. Bilirubin’s extraordinary antioxidant capacity may arise from an active cycle in which biliverdin reductase undertakes NADPH-dependent reduction of biliverdin to bilirubin: in turn bilirubin is oxidized by free radicals back to biliverdin, before enzymatic reduction back to bilirubin. Although controversial[47], this cycling mechanism has been proposed as a highly adapted mechanism through which cells and whole organisms maintain redox status[45].
Bilirubin’s effects may extend beyond its antioxidant action. It has been identified as an endogenous ligand of the aryl hydrocarbon receptor (AHR), present on many immune cell populations[48]. In some experiments AHR ligands have suppressed T cell proliferation through expansion of T regulatory (Treg) cells[49], although expansion of either Treg or their polar opposite, Th17 cells, has been shown depending upon which experimental AHR ligand is used[50].
Patients with persistent hyperbilirubinemia (Gilbert’s syndrome) have been observed to have significantly lower incidence of atherosclerotic disease in several studies, an effect attributed to enhanced antioxidant potential of serum[51]. The effect of chronic hyperbilirubinemia upon T cell phenotype is unclear, although immunosuppression has been reported in patients with cholestatic jaundice[52].
Work using ex vivo liver perfusion models in which explanted livers were stored for 16[53] or 24 h[54] at 4°C in University of Wisconsin solution, prior to mechanical perfusion, found that addition of low concentrations of bilirubin to the graft perfusate mimicked the protective effect of heme oxygenase induction. Graft function was improved in terms of portal venous flow and bile production, and hepatocellular injury was ameliorated in terms of histological injury scoring and transaminase release. Biliverdin has also been shown to have an immunomodulatory effect. In an MHC-mismatched cardiac allograft model, twice or three times daily injections of biliverdin for 2 wk increased graft survival, and led to complete allograft tolerance in 50% of animals[55].
To test whether bilirubin was protective in the clinical setting, our group hypothesized that hyperbilirubinemia would protect transplant recipients from IRI, in which case there would be an inverse correlation between pre-operative bilirubin and post-operative transaminase measurements. In a small retrospective study, no relationship was observed, although given the heterogeneity of the study population an effect could not be ruled out[56].
Production of Fe2+
Paradoxically for an antioxidant enzyme, one of HO-1’s reaction products, free iron, is a powerful oxidant. Free iron catalyzes the generation of OFR by Fenton chemistry in a manner comparable to free heme as described above. However, it is more effectively neutralized than when contained within a heme ring, being actively exported from the cell[57] and chelated by iron-binding proteins, including ferritin. Ferritin sequesters Fe2+ by oxidation and placement of iron ions within a “core” in which they are unable to catalyze Fenton reactions[58]. HO-1 induction leads to increased ferritin expression[59], through a mechanism dependent upon the production of free iron[60,61].
Since other HO-1 metabolites had been shown to be capable of substituting for HO-1 induction in protecting animals from IRI, Berberat et al[62] hypothesized that ferritin overexpression could also confer protection. Using an ex vivo perfusion model of hepatic IRI, this group identified that transfection with adenoviral heavy chain ferritin conferred protection in terms of bile flow, portal blood flow, histological injury scoring and transaminase release, and improved survival of syngeneic liver recipients.
Ferritin induction has been observed in retinal[63] and cardiac IPC[64] in a manner dependent upon an iron signal[65]: although there are no published studies of ferritin expression in hepatic preconditioning, it would be reasonable to hypothesize that similar results would be obtained.
Although ferritin is capable of protecting cells and organs from oxidative stress, it is worth noting that its induction is unlikely to be the only mechanism of cellular protection by HO-1. Sheftel et al[61] compared heme and sodium arsenite (a non-heme inducer of HO-1) in vitro, and found sodium arsenite to confer protection through HO-1 induction without parallel ferritin induction.
Production of CO
CO is best known for its toxicity, causing death at atmospheric concentrations of 500-1000 ppm, however, at lower doses CO has been shown to have important cytoprotective and immunomodulatory functions: it is released by HO-1 during the catabolysis of heme and can substitute for the protective effect of HO-1.
CO cannot have a specific receptor since it binds only to transition metals and is not thought to be capable of direct interaction with amino acids. Therefore, its pharmacology as a signaling molecule is necessarily novel, and subject to ongoing debate. An important hypothesis is that CO effects must be mediated by interactions with proteins which contain transition metal cores, for example in heme rings contained within a range of enzymes including soluble guanylate cyclase (sGC), cytochromes, hemoglobin, myoglobin and nitric oxide synthase[66]. It has been suggested that CO protects from oxidative stress by preventing hemoprotein oxidation and subsequent release of heme rings[36], diminishing production of free radicals and subsequent apoptosis[67]. Others have suggested that the protection may be mediated by antagonism of respiratory chain enzymes, reducing cellular oxygen requirements[66], by inducing vasodilatation via sGC[68] or by opening calcium-sensitive ion channels[69].
Using an ex-vivo liver perfusion model, Amersi et al[70] demonstrated enhanced portal blood flow and bile production, and amelioration of hepatic IRI, in terms of histological injury score and transaminase release when perfusate was supplemented with CO at 300 ppm. Blockade of heme oxygenase using zinc protoporphyrin (ZnPP) did not obliterate the protective effect, indicating that CO was capable of substituting for HO-1 function. Inhibitor studies have demonstrated that the CO effect was independent of iNOS and cGMP but dependent upon p38 MAPK.
In a cardiac transplantation model, no hearts stored at 4°C for 24 h prior to implantation into syngeneic recipients functioned after implantation, whereas 5 out of 6 grafts survived when HO-1 was induced with CoPP, an effect lost with HO-1 inhibition. Administration of 400 ppm CO to the heart donor during cold storage resulted in survival equivalent to that achieved with HO-1 induction[71]. In xenotransplant models, HO-1 inhibition with tin protoporphyrin (SnPP) caused rejection, whereas this effect was overcome by recipient CO inhalation[72].
Although the finding that CO can be protective at low doses is scientifically exciting, the potential therapeutic use of inhaled CO can be limited by practical difficulties, both in control of dosage, and spillage of the gas into the environment around the patient. For this reason, Motterlini has developed transition metal carbonyl “CO releasing compounds” (CORMs). Of these (CORM-A1, CORM-2, CORM-3), the most commonly used has been CORM-3 [tricarbonylchloro(glycinato)ruthenium(II)], which releases CO when dissolved in saline but not water[73]. To control for the presence of the ruthenium compound, a CO depleted substance (iCORM3) can be prepared by dissolving CORM-3 in PBS prior to the experiment.
Using a coronary occlusion model in which mice were subjected to 30 min of ischemia followed by 24 h of reperfusion, investigators administered CORM-3 during the first hour of reperfusion, which halved the area of myocardial infarct compared to iCORM control[74]. In a further study, the same group compared the effects of CORM-3 with a late-phase IPC protocol. Animals received either IPC or intravenous CORM-3 infusion lasting 60 min, with appropriate controls. Animals then underwent 30 min coronary ischemic insults 24 to 120 h later. The reperfusion phase lasted for 24 h post-operatively before animals were killed and hearts examined for infarct size. Both IPC and CORM-3 infusion resulted in cardioprotection at between 24 and 72 h compared with appropriate control groups[75].
In the context of transplantation, the potential for using CO to enhance graft function has been explored in various transplantation models. In the kidney, several studies have successfully protected grafts using storage media supplemented with CORM-3/CORM-A1[76] or CORM-2[77] for extended periods of cold ischemia prior to isogenic transplantation. In a vascular allograft model, donor HO-1 induction or CORM treatment reduced subsequent CD8 influx and intimal hyperplasia lasting until animals were sacrificed at 6 wk[78].
In the liver, CO persufflation of University of Wisconsin (UW) storage solution improved subsequent graft function[79], a result replicated using CORM-3 supplemented UW[80,81] after cold ischemic times of 48 h. Use of CO treatment in experimental liver transplant recipients has also been explored. Tomiyama et al[82] transplanted livers from wild-type donors into E-GFP transgenic rat recipients who received inhaled CO prior to and for the 24 h post transplantation. Grafts within CO-treated recipients were found to have reduced numbers of infiltrating CD45+ host leucocytes, while purified donor CD68+ Kupffer cells produced less IL-6 and TNFα: primary cultured ex vivo Kupffer cells from CO-treated animals secreted less IL-6 and TNFα in response to lipopolysaccharide (LPS) stimulus.
CO clearly has powerful antioxidant and anti-inflammatory effects in a variety of systems. For this reason, despite the difficulties presented by administering it in its inhaled form, phase 2 clinical trials are underway in renal transplantation (http://www.clinicaltrials.gov identifier NCT00531856), chronic obstructive pulmonary disease (NCT00094406) and post-operative intestinal ileus (NCT01050712). Further development of CORMs should make CO therapies more convenient, thereby widening their potential clinical applications: on the basis of the preclinical data presented above such applications would be expected to include hepatic IRI and transplantation.
Other mechanisms of HO-1-mediated cytoprotection
The bulk of data exploring mechanisms of HO-1-induced cytoprotection supports the concept that it is its antioxidant function which protects cells and animals from injury. Two separate pieces of data suggest that other mechanisms should be considered. Firstly, Ponka’s laboratory have demonstrated that the amounts of intracellular heme available for degradation may be insufficient to account for HO-1’s powerful antioxidant effects[61]. Secondly, Dennery’s laboratory has shown HO-1 translocation to the nucleus after cellular hypoxia or heme treatment, suggesting a role in transcriptional regulation[37]. Developing this work, this group has published research using a reporter system with catabolically inactive HO-1 which suggests HO-1 may have a forward-acting positive effect upon its own transcription[83], although as yet there has been no work demonstrating a protective effect of catabolically inactive HO-1.
HO-1 AND IMMUNOMODULATION IN IRI
It is likely that HO-1 protects organs from IRI by modulation of both direct cellular injury and both the innate and adaptive immune systems[84]. Deficiency of HO-1 in both humans and mice is associated with a pro-inflammatory phenotype[85,86].
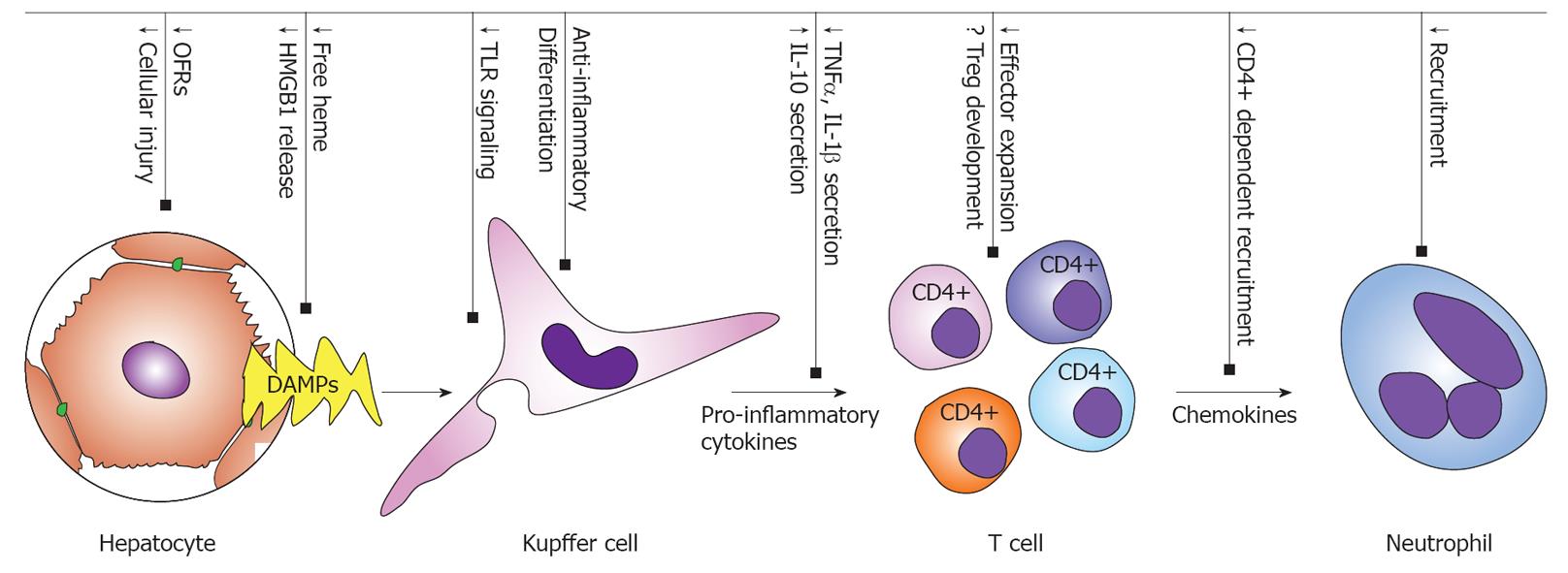
According to Matzinger’s “Danger Model” of the initiation of immune responses to injured organs, injured parenchymal cells release “Danger-Associated Molecular Patterns” (DAMPs) which stimulate antigen presenting cell (APC) activation through pattern recognition receptors[87,88]. Activated APCs subsequently activate and recruit immune effector cells through the secretion of pro-inflammatory mediators (cytokines, chemokines and adhesion molecules) and antigen presentation[9], resulting in the activation and recruitment of T cells and neutrophils (Figure 2). This recruitment of leucocytes into the tissue is further facilitated by increased endothelial permeability and adhesion molecule expression resulting from endothelial activation.
Figure 2 Heme oxygenase-1-mediated suppression of the inflammatory response in hepatic ischemia-reperfusion injury.
HMGB1: High Mobility Group Box-1; TLR: Toll-like receptor; DAMPs: Danger-associated molecular patterns; TNFα: Tumor necrosis factor α; IL: Interleukin; OFR: Oxygen free radicals.
Danger-associated molecular patterns
Hypoxic cellular injury can be regarded as the initiating event in a cascade of immunological activation. “Danger-Associated Molecular Patterns” or “alarmins” spilt by dying cells act on tissue resident immune populations via various molecular sensors including the toll-like receptor (TLR) family and purinergic receptors. A prototypic “alarmin”, High Mobility Group Box-1 (HMGB1) is released passively from injured cells, a signal amplified by active secretion from surrounding viable immune and non-immune cells[89]. Several authors have published studies demonstrating that circulating HMGB1 levels rise after experimental hepatic ischemia and that anti-HMGB1 neutralizing antibodies are capable of lowering circulating TNFα and IL-6 levels, and ameliorating injury[90-92]. Human clinical studies have confirmed the synthesis of HMGB1 during early reperfusion after liver transplantation, an effect exaggerated in steatotic livers, and with levels correlating with injury severity as measured by peak ALT[93]. Recombinant HMGB1 has been found to worsen the severity of experimental hepatic ischemic injury, an effect neutralized by targeted deletion of its receptor TLR-4[92].
HO-1 is capable of modulating the HMGB1 alarmin system. In sepsis[94] and acute lung injury[95] models, macrophage LPS-mediated HMGB1 release was suppressed by HO-1 induction by CORMs resulting in improved survival. Oxidative stress can initiate macrophage HMGB1 synthesis which occurs in a dose response relationship with peroxide stress in vitro[96]. It is therefore unclear whether HO-1’s modulation of HMGB1 secretion arises simply by reducing the extent of parenchymal cell injury or whether it is dependant upon another mechanism.
Free heme is another potential DAMP, being released from damaged cells and acting on TLRs[97]. Heme has also been shown to induce neutrophil chemotaxis using in vitro transmigration assays and after intraperitoneal injection in vivo[98]. By metabolising free heme, HO-1 induction would be expected to reduce this immune stimulus, further contributing to the possible mechanisms of protection from injury.
Intriguingly, HO-1 and CO also modulate TLR signaling upon which many DAMPs converge[99]. CO treatment of cultured RAW264.7 macrophages was found to reduce TLR4 signaling by reducing TLR4/Myd88 interactions and movement of TLR4 receptors to the cell surface[100].
It is likely that much of the immune modulation offered by HO-1 is owed to its antioxidant action which quietens the initial immune stimulus by reducing cellular injury and spillage of DAMPs.
Kupffer cells
Hepatic tissue resident macrophages (Kupffer cells) are the first immune cells to be activated by IRI, being acted upon by DAMPs released from surrounding parenchymal cells as well as being subject themselves to cellular hypoxia. Subsequently, they coordinate an appropriate influx of other immune cells by secretion of chemokines and cytokines. Kupffer cells have the ability to harm surrounding parenchymal cells by secretion of pro-inflammatory cytokines including TNFα, IL-6 and IL-1, which may be directly toxic[101]. On the other hand, Kupffer cells may protect the tissues by secretion of anti-inflammatory cytokines including IL-10. Furthermore, as the principle HO-1-expressing cells of the liver[24] they may secrete diffusible CO which may act in a paracrine manner upon surrounding cells to protect them from oxidative stress.
In our work, we have identified that HO-1 acts as a powerful switch on resting macrophage differentiation. We found HO-1-deficient Kupffer cells in vivo and bone marrow-derived monocytes (BMDMs) in vitro to differentiate down a Ly6c+ MARCO+ F4/80- pathway associated with macrophage inflammatory protein (MIP)-1 responsive pro-inflammatory monocytes[102]. HO-1 deficiency was associated with marked susceptibility to hepatic IRI measured in terms of ALT release and histological injury score[24]. Parallel work elsewhere using an in vitro migration assay has demonstrated enhanced migration of Hmox-1-/- BMDMs towards MIP-1α[95]. In an experimental liver transplant model, inhaled CO conferred protection from injury and reduced secretion of pro-inflammatory cytokines from recovered CD68+ cells cultured ex vivo[82], reducing the subsequent influx of CD45+ leucocytes. In vitro and in vivo, low dose CO downregulates the production of macrophage pro-inflammatory cytokines [TNFα, IL1β and MIP-1β (CCL4)] and increases the expression of the anti-inflammatory cytokine IL10[103].
Data from injury models showing modulation of pro-inflammatory cytokine secretion by HO-1 induction is subject to the criticism that the changes observed are merely the downstream effects of parenchymal cellular protection, and suppression of DAMPs. However, data from our laboratory has shown HO-1 effects upon the resting state of macrophage differentiation implying that HO-1 may modulate immune responses themselves.
Adaptive immune system
There is growing evidence from a variety of animal IRI models (T cell-deficient nude rats, severe combined immunodeficiency mice, RAG 1-/- mice, CD4+/CD8+-/- mice and CD4+ depleted mice) that there is a significant decrease in biochemical and histopathological evidence of injury in the absence of T cells (reviewed by Linfert et al[9]). More specifically, Khandoga et al[104] demonstrated it was a CD4+ rather than CD8+ T cell-dependent phenomenon; depletion of CD4+ T cells leads to a significant reduction in the observed injury. This influx of CD4+ cells on reperfusion is rapid and may determine the mode of neutrophil activation and subsequently the extent of the observed tissue damage[105,106]. In a series of elegant adoptive transfer experiments with CD4+ cells in a renal model, Burne et al[107] found IRI to be an interferon-γ-dependent process; this may imply the importance of the T helper 1 lineage in the pathology of IRI.
HO-1 has a number of immunomodulatory effects on effector and Treg cells. HO-1 and CO appear to inhibit T cell proliferation and activation through the suppression of IL-2 secretion[108]. HO-1 induction has also been shown to induce the apoptosis of activated T helper cells through activation-induced cell death[109].
Treg cells suppress the activation of the immune system and inhibit the activation of autoreactive T cells[110]. In clinical studies, Treg/Th17 “imbalance” has been associated with acute coronary syndromes[111]. HO-1 is induced by FoxP3: inhibition of HO-1 function in vivo reduced the suppressive ability of naturally occurring Tregs[112]. Furthermore, studies from Fritz Bach’s group have shown tolerogenesis to require HO-1[109]. Recently, however, these studies have been challenged by showing that Hmox-1-/- mice have normal numbers of Treg cells[113] and that HO-1 is not necessary or sufficient for T reg function[114]. These controversies may be resolved by work from the Agarwal group which showed that although Hmox-1-/- mice had normal (or elevated) numbers of circulating Treg cells, their suppressive function was dependant upon HO-1 expression by antigen-presenting cells[115].
In the context of hepatic ischemia, further work is needed firstly to establish which T cell populations are responsible for IRI and IPC, and secondly to resolve the controversy concerning the role of HO-1 in the expansion of different T cell subsets.
Neutrophils
Severe IRI is associated with significant influx of neutrophils[9]. Their recruitment from the vascular compartment into the liver is mediated by the expression of chemokines, cytokines and adhesion molecules[9]. Accumulation of activated neutrophils directly injures hepatocytes and the vascular epithelium through the release of proteases and the generation of ROS[116]. The initial recruitment of neutrophils appears to be a CD4+-dependent process[105]; continued neutrophil recruitment and activation may be dependent on the neutrophil production of IL-17A, which is important in a positive feedback mechanism[117]. As described above, heme is also capable of initiating neutrophil chemotaxis[98].
Although neutrophil influx is enhanced in Hmox-1-/- animals after ischemia-reperfusion insults[24], it is unclear whether this is due to a direct effect upon neutrophils or merely as a result of reduced stimulation due to upstream effects.