Published online Dec 21, 2010. doi: 10.3748/wjg.v16.i47.5993
Revised: June 25, 2010
Accepted: July 2, 2010
Published online: December 21, 2010
AIM: To asses the value of computed tomography (CT)-perfusion in the detection of residual hepatocellular carcinoma (HCC) vascularization after transarterial chemoembolization (TACE).
METHODS: Thirty-two consecutive patients were prospectively included in this study. All patients had liver cirrhosis and a confirmed HCC lesion which was treated with TACE. One month after treatment, perfusion measurements of treated lesions were carried out. The CT-perfusion (CT-p) protocol was performed with 16 slice multidetector computed tomography which included the following parameters: 8 dynamic slices/scan per 40 scans after iv injection of 50 mL of iodinated contrast (350 mg/mL) at a flow rate of 6 mL/s. Treated lesions were evaluated using dedicated perfusion software, which generated a quantitative colour map of perfusion. The following parameters were considered: hepatic perfusion (HP), arterial perfusion (AP), blood volume (BV), hepatic perfusion index (HPI), and time to peak (TTP). Perfusion parameters were described with quartile values of their distribution and statistically analyzed.
RESULTS: Perfusion parameters of the treated lesions could be quantitatively assessed using CT-p analysis. The presence of residual tumor tissue was observed in 13 of the 32 patients. The values of the perfusion parameters measured within the relapse tissue were: HP (mL/100 g per minute): median = 44.4 (1stqt = 31.3, 3rdqt = 55.8); BV (mL/100 g): median = 18.7 (1stqt = 11.5, 3rdqt = 22.5); AP (mL/min): median = 39.0 (1stqt = 36.5, 3rdqt = 61.3); HPI (%): median = 34.0 (1stqt = 30.4, 3rdqt = 38.9); TTP (s): median = 17.3 (1stqt = 15.8, 3rdqt = 26.5). With the use of the univariate paired Wilcoxon signed rank test, HP, AP and HPI were shown to be significantly higher (P < 0.001) in the relapse site than in the primary lesion. The BV and TTP parameters showed a tendency to be greater and lower, respectively, in the relapse site than in the primary lesion.
CONCLUSION: In patients with HCC treated with TACE, CT-p provides measurement of flow parameters related to residual arterial structures in viable tumor, thus helping in the assessment of therapeutic response.
- Citation: Ippolito D, Bonaffini PA, Ratti L, Antolini L, Corso R, Fazio F, Sironi S. Hepatocellular carcinoma treated with transarterial chemoembolization: Dynamic perfusion-CT in the assessment of residual tumor. World J Gastroenterol 2010; 16(47): 5993-6000
- URL: https://www.wjgnet.com/1007-9327/full/v16/i47/5993.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i47.5993
The prognosis of patients with untreated hepatocellular carcinoma (HCC) is poor, with a median survival of less than 6 mo after diagnosis. Although surgery remains the only hope for cure, very few patients (10%-15%) are candidates[1]. For large HCCs (> 3 cm), transarterial chemoembolization (TACE) remains the sole approach to inhibit cancer growth in most patients, and has been used extensively to treat unresectable HCCs[2]. The rationale for chemoembolization stems from the observation that unlike normal liver tissue, HCC receives most of its blood supply from the hepatic artery, therefore, it is logical to use the hepatic artery as a means to target the tumor while preserving normal liver[1]. The goal of TACE is to determine tumor necrosis and control tumor growth while preserving as much functional liver tissue as possible[1], therefore, the early detection of residual or recurrent tumor after TACE is a critical point and can facilitate successful retreatment at an early stage. Usually the presence of residual viable tumor or recurrence after TACE is assessed using multiphasic multidetector computed tomography (MDCT) scanning, but this technique can be affected by artefacts produced by high concentrations of lipiodol, making it difficult to evaluate the characteristics of the lesion. Response may be better assessed by noting alterations in vascular tumor perfusion rather than size, and therefore functional measurements may be an appropriate method to assess tumor response.
Perfusion computed tomography (CT) is a technique that enables depiction of tumor vascular physiology, being, also, non-invasive and fast; perfusion-CT can be repeated sequentially to assess temporal changes in tumor blood flow, which is of clinical importance for monitoring tumor response to antiangiogenic agents and other treatments[3].
The utility of hepatic perfusion characterization relies on the resolution of each component of its dual blood supply-the portal vein and the hepatic artery-because contributions from each are altered predictably in many diseases and could be used in monitoring therapeutic effectiveness[4]. TACE is a liver-directed therapy that takes advantage of the relatively selective vascularization of hepatic arterial tumors. HCCs can derive approximately 80% to 85% of their blood supply from the hepatic artery, whereas the portal vein as well as the hepatic artery supplies the normal hepatic parenchyma[4].
Quantitative measurement of hepatic perfusion has the potential to provide important information about tumor vascularization, useful in the assessment and management of various liver diseases and in the determination of their treatment outcome[5].
The purpose of this study was to determine the value of functional CT with perfusion imaging in the quantitative assessment of blood flow changes related to the effects of TACE treatment in patients with HCC lesions.
According to the Barcelona staging classification[6] TACE was performed in 35 consecutive cirrhotic patients with otherwise untreatable HCC, and included 28 men and 7 women (mean age, 65 years; range, 45-76 years). The cause of cirrhosis was hepatitis infection in 24 patients (hepatitis B in 7 patients, hepatitis C in 17 patients), alcohol related in 4 patients, and both hepatitis C and alcohol related in 7 patients.
To be included in the study, patients had to meet the following criteria: (1) be older than 18 years; (2) Child-Pugh class A or B; (3) have focal or multifocal HCC; and (4) not have any contraindications to CT imaging. Excluded from the study were patients who had: (1) a life expectancy of less than 6 mo; (2) Child-Pugh class C; (3) uncorrectable coagulopathy (international normalized ratio > 1.5); (4) thrombosis of the main portal vein branches (portal venous thrombosis was excluded prior to TACE by means of Doppler US); (5) a total bilirubin level higher than 4.0 mg/dL; (6) a serum creatinine level higher than 1.7 mg/dL; and (7) thrombocytopenia (platelet count < 50 000/μL). The diagnosis of HCC was established with FNAB or on the basis of the presence of a tumor larger than 2 cm in diameter with typical imaging findings[6] in the setting of cirrhosis.
Before being enrolled, all subjects gave their informed consent after the nature of the procedure had been fully explained, in accordance with the regulations of the institutional review board that approved our study.
According to the current literature[7-9], CT is commonly used as the standard imaging technique for evaluating the therapeutic response in patients with HCC after TACE[8], and was performed 4 wk after TACE treatment, in order to assess the intralesional deposition of iodized oil and evaluate the presence of the residual viable portion of the treated lesion[6,8].
All patients underwent CT-perfusion 1 d after the MDCT study and subsequently a selective conventional DSA study to correlate the angiographic findings with those of the CT-perfusion examination.
After initial mapping, visceral conventional DSA with a selective 5-F visceral catheter [either: Simmons (Cook, Bloomington, IN, USA) or Cobra (Terumo Medical, Somerset, NJ, USA) catheters were used], mesenteric arteriography was performed to check for presence of the right hepatic artery. Indirect portography was performed next to outline the portal circulation in the venous phase. Depending on the size, location, and arterial supply of the tumor and its satellites, the tip of the catheter was advanced further into segmental arteries for selective embolization, typically we coaxially inserted a 2.8-F microcatheter (Renegade Hi-Flow; Boston Scientific, Natick, MA, USA) over a 0.016-inch-diameter guidewire (Headliner; Terumo, Tokyo, Japan) to super select the hepatic lobar or segmental hepatic artery supplying the targeted tumor. We performed conventional DSA following an injection of Iobitridol (Xenetix 350; Guerbet, Aulnay, France).
Once the operator selected the final catheter position for TACE, intraarterial chemotherapy was performed by injection of 10-12 mL of iodized oil (Lipiodol Ultra Fluide; Laboratoire Guerbet, Roissy, France) mixed with an emulsion of 40 mg of doxorubicin hydrochloride into the hepatic artery. Embolization was performed by means of a mixture of Iobitridol (Xenetix 350; Guerbet, Aulnay, France) and 1-mm-diameter absorbable gelatin sponge particles (Spongostan; Ferrosan, Søborg, Denmark).
Under direct radiographic monitoring, we injected the solution (mean dose, 9.4 mL; range, 2-18 mL) until some slowing of antegrade blood flow to the targeted tumor was identified with conventional fluoroscopy. The amount of injected chemoembolic material was chosen at the discretion of the attending interventional radiologist. After the TACE procedure, the patients recovered with 20-24 h bed rest.
Perfusion-CT was performed using a multi-detector 16-slice CT scanner (Brilliance, Philips Medical Systems, Eindhoven, The Netherlands). For selection of the appropriate transverse level, an unenhanced CT scan of the liver, performed during a single breath hold, was obtained before initiation of perfusion CT scanning. The perfusion study of the selected area (maximal tumor diameter) was performed in a single breath hold at the end of expiration. Single location (2.4 cm width) cine CT scanning (40 scans; eight slices/scan) was obtained using the following parameters: 120 kV, 120 mA, 512 × 512 matrix, 3-mm slice thickness, and 1-s scan time. The radiation dose delivered to each patient with our CT perfusion technique was 8 mSv. Oxygen inhalation (4 mL/min) was given to help the patients hold their breath during the examination. Scanning was initiated after a 7-s delay from the start of the intravenous bolus injection of 50 mL of non-ionic iodinated contrast agent (Xenetix 350; Guerbet, Aulnay, France) at a flow rate of 6 mL/s, using an 18-gauge catheter positioned in the antecubital vein. Dynamic CT images were acquired for a total duration of 40 s.
To avoid motion artefacts, patients were clearly informed of a possible flushing sensation commonly associated with a rapid bolus of iodinated contrast agent. Moreover, as image quality may be degraded in patients breathing deeply during acquisition resulting in inaccurate perfusion parameters values, a band compressing the abdomen that limited breath-related liver excursions was used.
The functional computed tomographic images were then transferred to an image workstation, and the data were analyzed using dedicated perfusion software (Philips Brilliance Workspace 2.0; Philips Medical Systems), which generated a quantitative map of liver perfusion displayed on the monitor using a colour scale. The parametric map images were created using the highest spatial resolution pixel-by-pixel calculation technique. Perfusion was assessed by the dedicated computed tomographic software based on the maximum slope model: perfusion was therefore calculated as the average slope of the tissue enhancement divided by the peak enhancement in the aorta, as described initially by Miles and Griffiths[4,10].
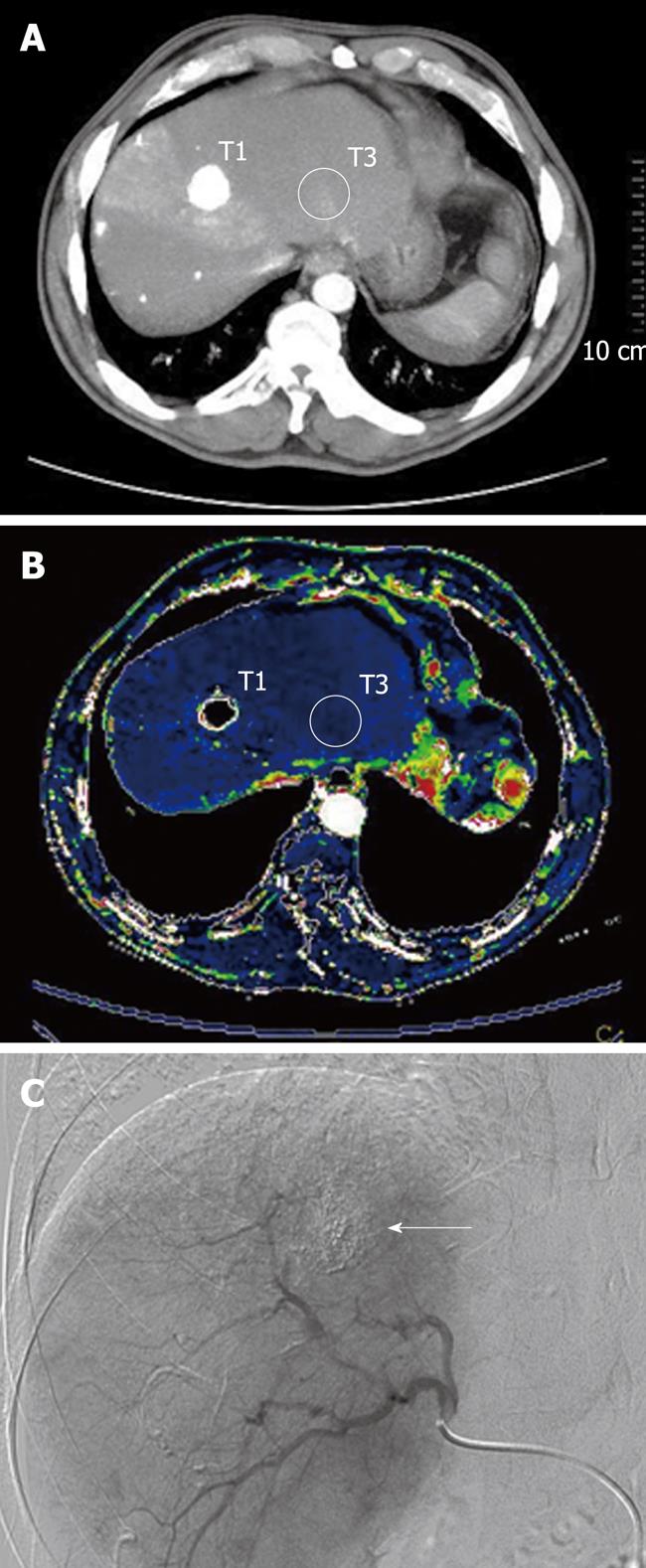
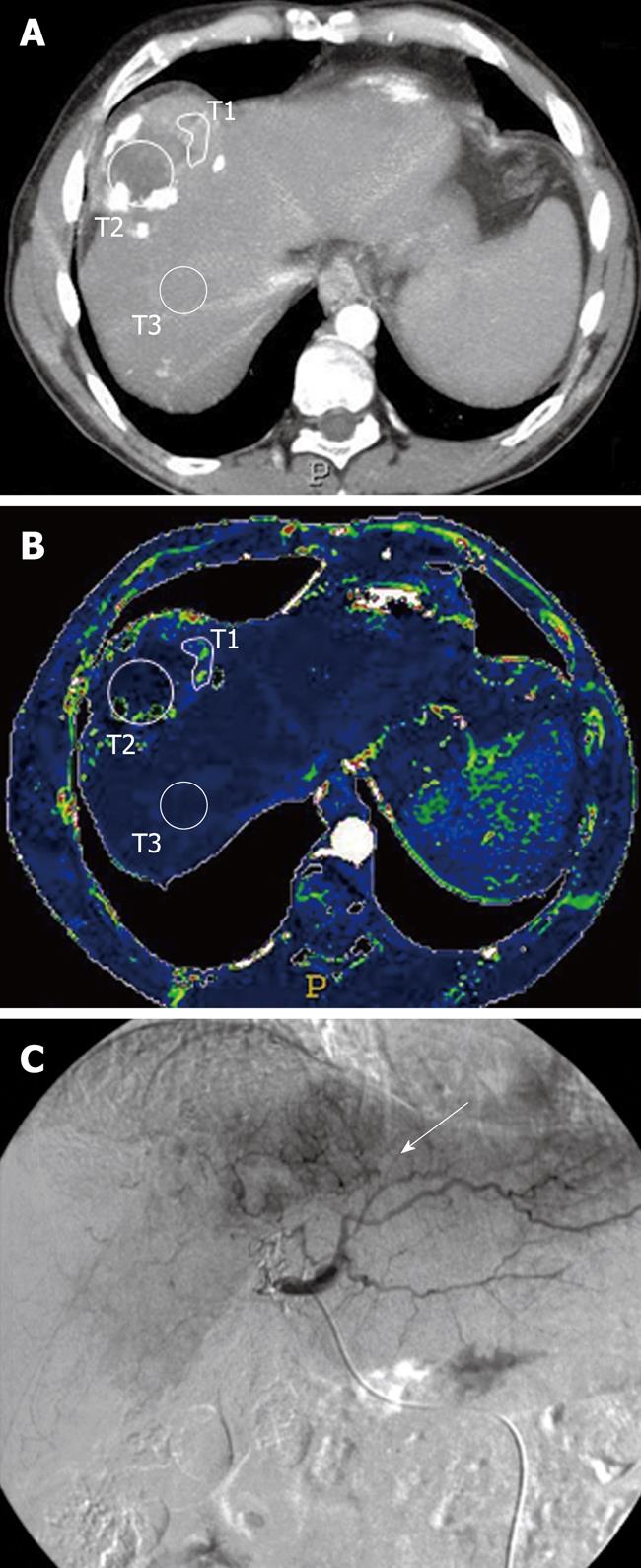
The region of interest (ROI) was hand drawn in a selected portion of the treated lesion where a hypervascular area had been detected on the colour map (Figures 1 and 2); another ROI was positioned within the intralesional deposition of iodized oil (Figures 1 and 2); further ROIs were also placed in definite areas of the surrounding cirrhotic liver parenchyma (Figures 1 and 2). In those patients in whom the presence of a focal hypervascular area, suspected as residual HCC, had not been seen at the CT-perfusion study, ROI was positioned only on the site of deposition of iodized oil and in the surrounding liver parenchyma, and the perfusion parameters were calculated. The resulting temporal changes in contrast enhancement were then analyzed to quantify a range of parameters that reflected the functional status of tissue perfusion.
The absolute values of the following five perfusion parameters were calculated for treated HCC, and for cirrhotic liver parenchyma: (1) Hepatic perfusion (HP), mL/100 g per minute), which is the blood flow per unit volume/mass of tissue per minute; (2) Blood volume (BV, mL/100 g), which is the blood volume contained in 100 g of tissue; (3) Arterial perfusion (AP, mL/min), which is the arterial fractional blood flow; (4) Hepatic perfusion index (HPI, %), which represents, of the total blood liver flow [arterial perfusion (AP) + portal perfusion (PP)], the percentage of arterial origin (AP/AP + PP); and (5) Time to peak (TTP, s) which is the time to reach the maximum value of contrast material concentration.
In only three of 35 patients, the perfusion study was not performed due to the fact that the tumor and portal trunk, or main portal branches, were not on the same transverse plane.
The perfusion parameters HP, BV, AP, HPI, TTP were described by the quartile values of their distribution and calculated: for the ROI in the area of iodized oil deposition (T1) in all patients (with or without residual disease); for the ROI in the hypervascular area (T2) at the colour map in 13 patients who presented residual neoplastic disease; for the ROI placed in the surrounding liver parenchyma (T3) in all patients.
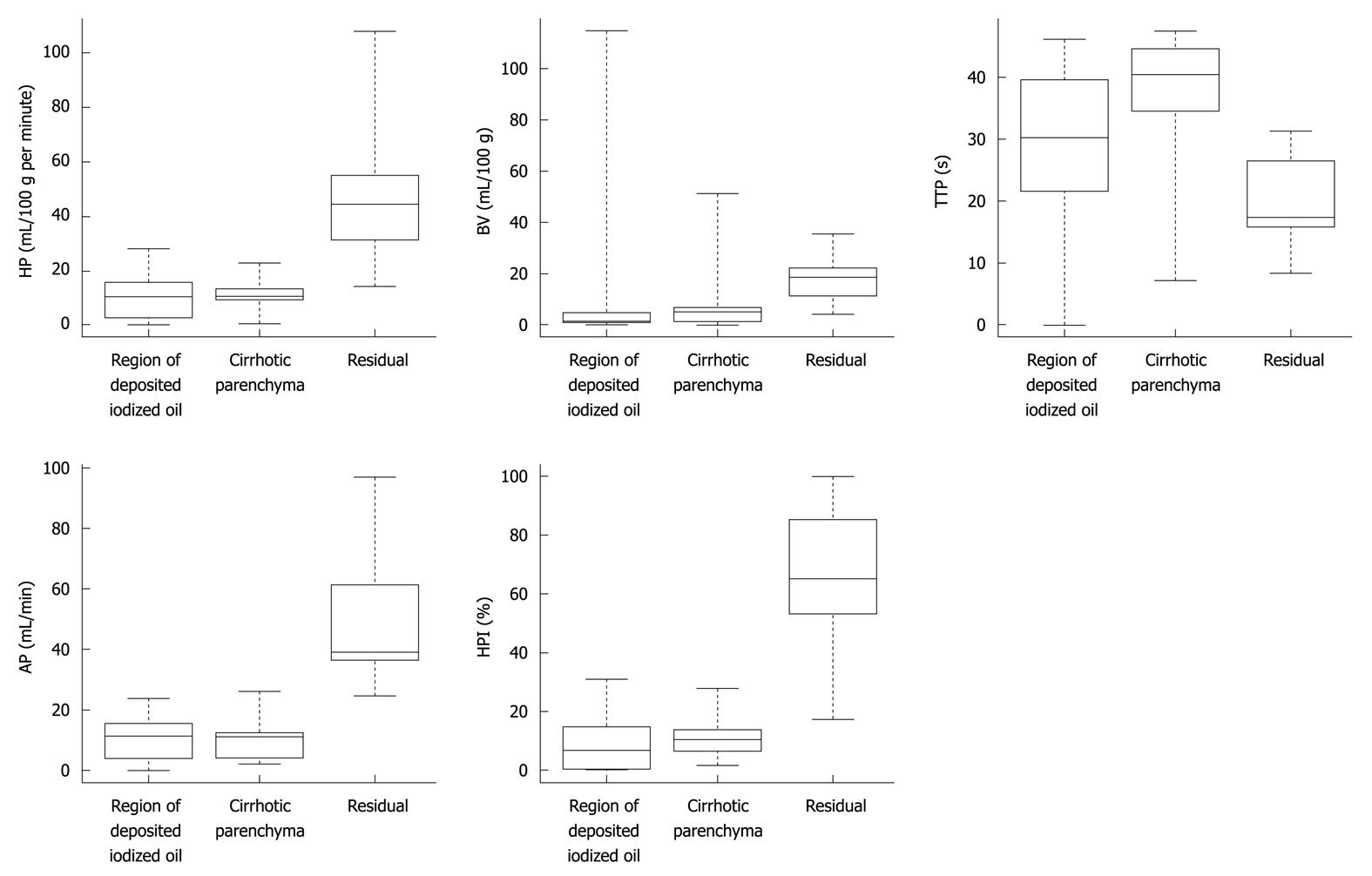
The distributions of the differences in the parameters between the residual disease, deposition of iodized oil and surrounding cirrhotic liver tissue were graphically represented by means of box-plot representations (Figure 3).
The univariate unpaired Wilcoxon signed rank test was used to assess whether there were differential expressions of each single perfusion parameter in the area of deposition of iodized oil between both groups of patients (those who did and did not present residual HCC). While, the paired version of the Wilcoxon test was used to assess whether, in patients who had residual disease, there were differential expressions of the perfusion parameters between the area of deposition of iodized oil and the residual site.
Bonferroni correction was used to take multiple comparisons into account, considering P-values less or equal to 0.05/10 were indicative of a statistically significant difference[11].
In the comparison between the area of deposition of iodized oil and the residual site for patients who had residual disease, the alternative hypothesis postulated a greater value for, HP, BV, AP and HPI in the residual disease than in the area of deposition of iodized oil or cirrhotic parenchyma, due to the presence of residual arterial vascular structures. For TTP, the alternative hypothesis postulated a lower value for the residual disease site than the primary lesion, due to a possible increase in the arterial flow within the tumor.
The statistical analysis was performed using the R statistical package (http://cran.r-project.org).
A total of 32 lesions (mean size: 4.1 ± 1.6 cm), located on both hepatic lobes were evaluated with the CT-Perfusion (CTp) technique.
For all patients, the descriptive analysis of perfusion parameters measured within the treated tumor tissue with deposition of iodized oil (T1) showed the following results.
HP (mL/100 g per minute): median = 10.5 (1stqt = 9.3, 3rdqt = 13.7); BV (mL/100 g): median = 5.2 (1stqt = 1.3, 3rdqt = 6.7); AP (mL/min): median = 11.2 (1stqt = 4.0, 3rdqt = 12.5); HPI (%): median = 10.7 (1stqt = 6.4, 3rdqt = 13.7); TTP (s): median = 40.3 (1stqt = 34.6, 3rdqt = 44.6) (Table 1, Figure 3).
| Parameter | Primary treated lesion site in region of deposited iodized oil | Background cirrhotic liver parenchyma | Residual hepatocellular carcinoma | ||||||
| Median | 1stqt | 3rdqt | Median | 1stqt | 3rdqt | Median | 1stqt | 3rdqt | |
| HP (mL/100 g per minute) | 10.5 | 9.3 | 13.7 | 10.4 | 9.3 | 13.2 | 44.4 | 31.3 | 55.8 |
| BV (mL/100 g) | 5.2 | 1.3 | 6.7 | 11.7 | 9.5 | 11.9 | 18.7 | 11.5 | 22.5 |
| HPI (%) | 10.7 | 0.3 | 14.9 | 16.4 | 13.8 | 18.3 | 65 | 53.2 | 85.0 |
| AP (mL/s) | 11.2 | 4 | 12.5 | 10.4 | 9.5 | 11.9 | 39 | 36.5 | 61.3 |
| TTP (s) | 40.3 | 34.6 | 44.6 | 44.6 | 40.3 | 51.8 | 17.3 | 15.8 | 26.5 |
In the 13 patients who presented residual disease, the same descriptive analysis of perfusion parameters was performed within the residual tumor tissue (T2) and showed the following results: HP (mL/100 g per minute): median = 44.4 (1stqt = 31.3, 3rdqt = 55.8); BV (mL/100 g): median = 18.7 (1stqt = 11.5, 3rdqt = 22.5); AP (mL/min): median = 39.0 (1stqt = 36.5, 3rdqt = 61.3); HPI (%): median = 65.0 (1stqt = 53.2, 3rdqt = 85.0); TTP (s): median = 17.3 (1stqt = 15.8, 3rdqt = 26.5) (Table 1, Figure 3).
The corresponding perfusion values calculated also in the cirrhotic liver parenchyma surrounding the treated HCC lesion were: HP (mL/100 g per minute): median = 10.4 (1stqt = 9.3, 3rdqt = 13.2); BV (mL/100 g): median = 11.7 (1stqt = 9.5, 3rdqt = 11.9); HPI (%): median = 16.4 (1stqt = 13.8, 3rdqt =18.3); AP (mL/min): median = 10.4 (1stqt = 9.5, 3rdqt = 11.9); TTP (s): median = 44.6, (1stqt = 40.3, 3rdqt = 51.8) (Table 1, Figure 3).
With the use of the univariate paired Wilcoxon signed rank test, HP, AP and HPI were shown to be significantly higher (P < 0.001) in the site of residual disease than in the region with iodized oil deposition (Figure 3). The BV parameter showed a tendency to be greater in the residual site than in the region of iodized oil deposition, but significance according to the Bonferroni correction was not reached (P = 0.03) (Figure 3). The TTP values showed a tendency to be lower in the site of residual disease than in the region of iodized oil deposition, but again significance according to the Bonferroni correction was not reached P = 0.01) (Figure 3).
Use of the univariate unpaired Wilcoxon signed rank test showed that no significant differences were found for each parameter, calculated in the region of the lesion with deposition of iodized oil, between the two groups of patients with or without relapse of HCC (Figure 3).
In our series no significant correlation was found between CT perfusion parameters in cirrhotic parenchyma and in the successfully treated lesion, although in lesions with deposition of iodized oil mean BV value was moderately lower than those in the surrounding cirrhotic parenchyma.
The survival rate at 6, 12 and 18 mo was 94% (30/32), 82% (26/32) and 72% (23/32), respectively.
TACE is the most widely used therapy in patients with HCC who are considered unsuitable candidates for surgery, and should be considered not only the best possible therapeutic approach in patients with advanced HCC but also an appropriate step before surgical resection or liver transplantation[12].
The mixture of chemotherapeutic agents and iodized oil is almost completely retained in neoplastic nodules and can remain in HCC tissue for a long time. Subsequent mechanical embolization of the artery feeding the neoplasm causes ischemic damage to the tumor and prolongs the duration of the effects of chemotherapeutic agents[13].
For a definite assessment of the therapeutic efficacy of interventional procedures, histological examination using percutaneous needle biopsy may be the most definite assessment of the therapeutic efficacy of interventional therapy. However, it is an invasive procedure, and the specimen retrieved does not always represent the entire lesion owing to sampling errors.
Similar to the findings in most previously reported investigations[7,8], the presence of residual viable tumor or recurrence after TACE is assessed using multiphasic MDCT scanning, which represents the standard imaging technique for monitoring the effectiveness of TACE.
Generally, HCC tends to recur at the site adjacent to the original tumor, however, after TACE treatment it can be difficult to evaluate contrast enhancement in a tumor with partial retention of iodized oil on contrast-enhanced CT due to beam hardening artefacts produced by the high attenuation of iodized oil[12].
In general, the viable portion of malignant tumors exhibits increased perfusion, and in the primary tumors, therefore, the radiologic estimation of perfusion in liver tumors is useful for both diagnosis and selection of the therapeutic strategy[14].
After TACE, tumor response may be better assessed by alterations in vascular perfusion rather than tumor size, and functional measurements may therefore be more appropriate[15].
In this regard, perfusion-CT could be considered suitable for monitoring tumor response after treatment.
Perfusion CT techniques typically require a baseline image acquisition without contrast enhancement followed by a series of images acquired over time after an intravenous bolus of conventional iodinated contrast material. The resulting temporal changes in contrast enhancement are subsequently analyzed to quantify a range of parameters that reflect the functional status of the vascular system[16].
In the current study, we investigated the value of several tissue perfusion parameters obtained by functional CT, with the perfusion technique, for the quantitative assessment of HCC-related residual vascularization after TACE treatment.
Our results show that the values of the perfusion parameters calculated were significantly different in residual tumor compared to the area of iodized oil deposition, in particular, HP, AP, BV and HPI were found to be higher (P < 0.001) in HCC than in the surrounding cirrhotic liver tissue, due to tumor-associated neovascularization, whereas TTP values were lower in areas of HCC relapse. In the assessment of residual neovascularization related to tumor regrowth, AP - which represents the arterial fractional blood flow - may be considered the most relevant perfusion parameter, as it can specifically demonstrate the presence of new arterial blood vessels in partial or incomplete treated lesions. Also, the higher HPI values could explain the reduction of portal perfusion inflow in residual HCC disease, with a simultaneous increase in arterial fractional inflow.
Findings in the current work are also in line with those recently reported in experimental studies where perfusion CT was used for assessing tumor response to treatment by evaluating perfusion changes[17-19]. Those studies validated the idea that functional CT can provide quantification of residual viable tumor perfusion. In an animal model, Kan et al[17] found that functional CT enabled accurate quantification of changes in liver tumor perfusion during and after an embolization procedure thus helping optimize therapeutic outcomes. In another study[18], the same researchers reported on the ability of functional CT to assess changes in liver tumor perfusion in response to antiangiogenic treatment.
In our study, we found that the zone of increased perfusion appeared as a hypervascular area on the colour map, representing an area in which the arterial blood continued to sustain tumor growth.
Our findings are in line with a previous study by Chen et al[19] who correlated the changes of CT-perfusion parameters to different responses of tumors to TACE, supporting the idea that CT-perfusion could be used in the assessment of TACE efficacy, by evaluating quantitative parameters before and after chemoembolization.
Our data confirmed the recent experimental work of Sabir et al[15], who demonstrated in a murine xenograft tumor model receiving antiangiogenic therapy, that perfusion MDCT was able to identify changes in tumor blood flow, and therefore indicate the early reversal of tumor responsiveness to antiangiogenetic therapy. This also demonstrated, therefore, that perfusion MDCT is able to identify focal areas of new tumor perfusion before clinically measurable changes in tumor size.
Our study findings are consistent with those of Li et al[20] who demonstrated that CT perfusion parameters can be reliable indicators for evaluating tumor necrosis and angiogenesis, relating the dynamic contrast-enhanced measures with outcome of histopathologic (microvessel density) studies in lung carcinoma. Evaluating the CT perfusion parameters of the necrotic lung carcinomas, they found that necrotic tumors exhibited significantly lower perfusion.
The main limitation of the present study is related to the fact that a liver tissue section of only 2.4 cm in thickness could be examined in each patient. This limitation, however, could be overcome by the use of multi-detector CT scanners enabling the acquisition of 64 or more sections which can produce isotropic spatial resolution, while simultaneously delivering exceptional temporal resolution with excellent z-axis coverage (4-8 cm)[21]. A further limitation was that CT perfusion parameters were not compared with established markers of tumor vascularity, such as histologic examination, which is the most reliable method for assessing therapeutic efficacy of the TACE procedure. Such a method, however, is invasive and the specimen retrieved does not always represent the entire lesion owing to sampling errors[6].
In conclusion, our preliminary study in humans has shown that perfusion CT may be helpful in monitoring the outcome of TACE in cirrhotic patients with HCC. We believe that perfusion-CT provides non-invasive, reliable information on residual tumor vascularization and could be part of the current CT protocols aimed at following-up such patients.
Transcatheter arterial chemoembolization (TACE) is the most widely used therapy in patients with hepatocellular carcinoma (HCC) who are considered unsuitable candidates for surgery. Chemoembolization involves delivery of chemotherapy combined with arterial embolization to destroy tumor cells. Such a treatment makes use of the hypervascular nature of HCC: antineoplastic agents are directly injected into the hepatic artery allowing high intratumoral concentrations of drugs and thereby reducing systemic side effects. After TACE, tumor response may be better assessed by alterations in vascular perfusion rather than tumor size, and functional measurements may therefore be more appropriate. Thus, quantitative measurement of hepatic perfusion has the potential to provide important information in the assessment and management of HCC lesions and in the determination of their outcome.
Perfusion computed tomography is a recent technique that allows depiction of tumor vascular physiology and has the ability to detect regional and global alterations in organ blood flow, in addition to being non-invasive and fast; perfusion computed tomography (CT) can be repeated sequentially to assess temporal changes in tumor blood flow, which is of clinical importance for monitoring tumor response to antiangiogenic agents and other treatments like TACE.
This new technique may better define the assessment of therapeutic efficacy of interventional procedures without using histological examination, evaluate tumor progression and monitor response to treatment providing a quantitative measurement of flow parameters related to residual arterial structures in viable tumor. As it is a relatively simple imaging technique, perfusion CT could be integrated into the current computed tomographic protocols, providing an in vivo marker of tumor-related angiogenesis.
This preliminary study in humans has shown that perfusion CT may be used to successfully monitor therapeutic response after TACE in cirrhotic patients with HCC lesions. In addition, the follow-up protocol along with conventional multidetector computed tomography may include a CT-perfusion protocol in order to better depict residual tumor after TACE treatment, by providing quantitative information about tumor vascularization.
The authors assess the value of CT-perfusion technique in detection of residual HCC vascularization after TACE. It’s a nice imaging clinical study to follow up patients with HCC.
Peer reviewers: Assy Nimer, MD, Assistant Professor, Liver Unit, Ziv Medical Centre, Box 1008, Safed 13100, Israel; Naoaki Sakata, MD, PhD, Division of Hepato-Biliary Pancreatic Surgery, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8574, Japan
S- Editor Wang JL L- Editor Webster JR E- Editor Zheng XM
| 1. | Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S211-S221. [Cited in This Article: ] |
| 2. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. [Cited in This Article: ] |
| 3. | Hakimé A, Peddi H, Hines-Peralta AU, Wilcox CJ, Kruskal J, Lin S, de Baere T, Raptopoulos VD, Goldberg SN. CT perfusion for determination of pharmacologically mediated blood flow changes in an animal tumor model. Radiology. 2007;243:712-719. [Cited in This Article: ] |
| 4. | Miles KA. Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol. 2003;76 Spec No 1:S36-S42. [Cited in This Article: ] |
| 5. | Materne R, Van Beers BE, Smith AM, Leconte I, Jamart J, Dehoux JP, Keyeux A, Horsmans Y. Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci (Lond). 2000;99:517-525. [Cited in This Article: ] |
| 6. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [Cited in This Article: ] |
| 7. | Guan YS, Sun L, Zhou XP, Li X, Zheng XH. Hepatocellular carcinoma treated with interventional procedures: CT and MRI follow-up. World J Gastroenterol. 2004;10:3543-3548. [Cited in This Article: ] |
| 8. | Lim HS, Jeong YY, Kang HK, Kim JK, Park JG. Imaging features of hepatocellular carcinoma after transcatheter arterial chemoembolization and radiofrequency ablation. AJR Am J Roentgenol. 2006;187:W341-W349. [Cited in This Article: ] |
| 9. | Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, Sato M, Yamanaka N, Shimamura Y, Ohto M. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699-704. [Cited in This Article: ] |
| 10. | Miles KA, Griffiths MR. Perfusion CT: a worthwhile enhancement? Br J Radiol. 2003;76:220-231. [Cited in This Article: ] |
| 11. | Hollander M, Wolfe DA. Nonparametric statistical methods. 2nd ed. New York: John Wiley and Sons 1999; . [Cited in This Article: ] |
| 12. | Lee KH, Sung KB, Lee DY, Park SJ, Kim KW, Yu JS. Transcatheter arterial chemoembolization for hepatocellular carcinoma: anatomic and hemodynamic considerations in the hepatic artery and portal vein. Radiographics. 2002;22:1077-1091. [Cited in This Article: ] |
| 13. | Caturelli E, Siena DA, Fusilli S, Villani MR, Schiavone G, Nardella M, Balzano S, Florio F. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue-long-term prospective study. Radiology. 2000;215:123-128. [Cited in This Article: ] |
| 14. | Tsushima Y, Funabasama S, Aoki J, Sanada S, Endo K. Quantitative perfusion map of malignant liver tumors, created from dynamic computed tomography data. Acad Radiol. 2004;11:215-223. [Cited in This Article: ] |
| 15. | Sabir A, Schor-Bardach R, Wilcox CJ, Rahmanuddin S, Atkins MB, Kruskal JB, Signoretti S, Raptopoulos VD, Goldberg SN. Perfusion MDCT enables early detection of therapeutic response to antiangiogenic therapy. AJR Am J Roentgenol. 2008;191:133-139. [Cited in This Article: ] |
| 16. | Ippolito D, Sironi S, Pozzi M, Antolini L, Ratti L, Alberzoni C, Leone EB, Meloni F, Valsecchi MG, Fazio F. Hepatocellular carcinoma in cirrhotic liver disease: functional computed tomography with perfusion imaging in the assessment of tumor vascularization. Acad Radiol. 2008;15:919-927. [Cited in This Article: ] |
| 17. | Kan Z, Kobayashi S, Phongkitkarun S, Charnsangavej C. Functional CT quantification of tumor perfusion after transhepatic arterial embolization in a rat model. Radiology. 2005;237:144-150. [Cited in This Article: ] |
| 18. | Kan Z, Phongkitkarun S, Kobayashi S, Tang Y, Ellis LM, Lee TY, Charnsangavej C. Functional CT for quantifying tumor perfusion in antiangiogenic therapy in a rat model. Radiology. 2005;237:151-158. [Cited in This Article: ] |
| 19. | Chen G, Ma DQ, He W, Zhang BF, Zhao LQ. Computed tomography perfusion in evaluating the therapeutic effect of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2008;14:5738-5743. [Cited in This Article: ] |
| 20. | Li Y, Yang ZG, Chen TW, Chen HJ, Sun JY, Lu YR. Peripheral lung carcinoma: correlation of angiogenesis and first-pass perfusion parameters of 64-detector row CT. Lung Cancer. 2008;61:44-53. [Cited in This Article: ] |