Published online Dec 21, 2010. doi: 10.3748/wjg.v16.i47.5975
Revised: November 2, 2010
Accepted: November 9, 2010
Published online: December 21, 2010
AIM: To investigate the correlation between cyclooxygenase-2 (COX-2) and cell cycle-regulatory proteins in patients with esophageal squamous cell carcinoma (ESCC).
METHODS: One hundred and two surgically obtained specimens of ESCC were randomly collected. All specimens were obtained from patients who had not received chemo- or radiotherapy prior to surgical resection. Twenty-eight specimens of normal squamous epithelium served as controls. The expression of COX-2, Ki-67, cyclin A and p27 was examined by immunohistochemistry. The Pearson test was used to analyze the relationship between groups.
RESULTS: The protein level of COX-2, Ki-67 and cyclin A was significantly higher in ESCC than in normal squamous epithelium (74.7 ± 61.2 vs 30.2 ± 43.4, 64.0 ± 51.6 vs 11.6 ± 2.3, 44.2 ± 32.2 vs 11.7 ± 5.0, respectively, all P < 0.01). In contrast, the protein level of p27 was significantly lower in ESCC than in normal squamous epithelium (182.0 ± 69.0 vs 266.4 ± 28.0, P < 0.01). In ESCC, COX-2 expression was correlated with T stage, the score of T1-T2 stage was lower than that of T3-T4 stage (55.0 ± 42.3 vs 83.0 ± 66.5, P < 0.05), and Ki-67, cyclin A and p27 expressions were correlated with the tumor differentiation (43.8 ± 31.7 vs 98.4 ± 84.8, 32.0 ± 19.0 vs 54.1 ± 53.7, 206.2 ± 61.5 vs 123.5 ± 68.3, respectively, all P < 0.01). COX-2 expression was positively correlated to Ki-67, cyclin A and negatively correlated to p27 expression in ESCC (r = 0.270, 0.233 and -0.311, respectively, all P < 0.05).
CONCLUSION: The expression of COX-2 is correlated with tumor cell invasion and is closely related to the cell proliferation in patients with ESCC.
- Citation: Huang JX, Xiao W, Chen WC, Lin MS, Song ZX, Chen P, Zhang YL, Li FY, Qian RY, Salminen E. Relationship between COX-2 and cell cycle-regulatory proteins in patients with esophageal squamous cell carcinoma. World J Gastroenterol 2010; 16(47): 5975-5981
- URL: https://www.wjgnet.com/1007-9327/full/v16/i47/5975.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i47.5975
Esophageal cancer is one of the most frequently occurring malignancies and the seventh leading cause of cancer-related deaths in the world[1]. Esophageal squamous cell carcinoma (ESCC) remains the most common tumor histologically worldwide. A high incidence of ESCC has been observed in northern China, whereas adenocarcinoma of the esophagus is increasing in some areas of the United States and other Western countries. The prognosis for patients with locally advanced esophageal cancer treated with the standard approaches of surgery or radiotherapy is poor. Treatment failure is mainly due to both a high incidence of local-regional failure and early systemic dissemination of the disease[2].
Several studies have focused on molecular markers in this cancer to elucidate their relation to clinicopathologic features and prognosis of esophageal carcinoma[3-5]. Cyclooxygenase-2 (COX-2) may be involved in an early stage of squamous cell carcinogenesis of the esophagus and plays a non-redundant role in the regulation of cellular proliferation and tumorigenesis of esophageal epithelial cells[6], meanwhile, a significant correlation of COX-2 inhibitor nonsteroidal anti-inflammatory drugs was found with esophageal cancer[7]. COX-2 expression was also associated with the tumor volume response to radiotherapy in patients with cervical squamous cell carcinoma[8], and the use of COX-2 inhibitors could reduce the risk of bone metastases in stage II-III breast cancer[9].
Ki-67 is known as an indicator of cell proliferation, which is present in all phases of the cell cycle except in G0. Moreover, Ki67 expression in localized prostate cancer could often predict postoperative progression[10]. In humans, several types of cyclin, including A-type, B-type, and C-type, have been isolated[11-15]. Cyclin A and B1-2 reach maximum levels in the S-phase and G2. Cyclin D1 over-expression indicated a poor prognosis of ESCC[16]. p27, one of the cell cycle-regulatory proteins, is a negative factor of cell proliferation, which is a cyclin-dependent kinase inhibitor[17,18]. Previously, we found that the p27 expression in the nuclei in well-differentiated ESCC was higher than in the other tumor types[19], and Miyabe et al[17] thought that p27 might be the most useful prognostic factor in mucoepidermoid carcinoma. Therefore, a molecular research into cancers has resulted in a better understanding of tumor biologic behavior, which has helped improve the survival of cancer patients, on the other hand, clinical application of immunohistochemical methodology may be cost-effective[20]. To our knowledge, the relationship between COX-2 expression and cell cycle-regulatory protein in ESCC has not been reported to date.
The aim of the present study was to investigate the correlation between COX-2 and cell cycle-regulatory proteins and explore the relationship between proliferative activity of cancer cells and COX-2 in patients with ESCC.
We determined the expression of COX-2, Ki-67, cyclin A and p27 in 102 surgically resected ESCC specimens were formalin fixed and paraffin embedded and in 28 normal esophageal mucosa by immunohistochemical method. One hundred and two patients (86 males and 16 females) with ESCC were treated surgically at the Department of Thoracic Surgery, People’s Hospital of Taizhou (Taizhou Medical School, Yangzhou and Nantong University) between August 2005 and September 2007. All the patients underwent subtotal or total esophagectomy and radical lymph node dissection.
Histopathological specimens were fixed in 10% buffered formalin, processed routinely, and embedded in paraffin. All specimens were obtained from patients who had not received chemo- or radiotherapy prior to surgery. All hematoxylin and eosin stained sections were reviewed and reexamined by pathologists. The grade of tumor differentiation was determined according to the classification of the World Health Organization[21], and staging according to the TNM classification of American Joint Committee on Cancer[22].
The patients were 35-76 years of age with a median age of 58.0 years. The location of the tumor was as follows: upper intra-thoracic esophagus in 11 cases (10.8%), middle intra-thoracic esophagus in 55 cases (53.9%), and lower intra-thoracic esophagus in 36 cases (35.3%). There were 32 (31.4%) well-differentiated cases, 50 (49%) moderately-differentiated cases (49%), and 20 (19.6%) poorly-differentiated cases. There were 5 stage I cases, 47 stage II, 33 stage III and 17 stage IV cases. Normal esophageal mucosal samples were taken from the areas > 5 cm from the cancer, as non-tumor control samples.
The following antibodies were used in this study: rabbit monoclonal antibody anti-human COX-2 (Maixin Biotechnology Co., Ltd., Fuzhou, China); mouse monoclonal antibody anti-human Ki-67, p27 and PV-9000 test kit (Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing, China); mouse monoclonal antibody anti-human cyclin A (Thermo Fisher Scientific, CA). The final diluted concentration was 1:75.
The specimens of adjacent non-cancerous esophageal mucosa were cut into 4-5-μm thick sections and mounted onto slides, deparaffinized with xylene, and re-hydrated with graded concentrations of ethanol. Endogenous peroxidase activity was blocked with hydrogen peroxide (H2O2) in deionized water for 10 min. The slides were washed three times with Tris buffered saline (TBS) buffer (10 mmol/L Tris-HCl, 100 mmol/L NaCl, pH 7.5) for 2 min. An antigen retrieval technique was used before application of the primary antibody (10 mmol/L sodium citrate solution, pH 6.0 in a rice cooker, at 640 W for 30 min). After three washes with TBS, an aliquot of 100 μL of primary antibody was applied to each section and incubated at 4°C overnight. After washing 3 times with TBS, agent one and then agent two (including the kit) were applied for 20 min at room temperature. Finally, the sections were washed 3 times with TBS, and the immunoreactions were visualized with 0.0067% diaminobenzidine as the substrate with 0.03% H2O2 in 100 mmol/L Tris-HCl buffer for 3 min. The sections were lightly counterstained in Haris hematoxylin solution for microscopic examination. Simultaneously, each section was incubated with TBS instead of the primary antibody as an internal negative control. The positive control included: gastric cancer tissue for COX-2, breast cancer tissue for Ki-67 and p27, carcinoma of tonsil for cyclin A.
The immunostained specimens were analysed by two independent pathologists. Cytoplasm and/or nuclear staining (brown reaction product) was regarded as a positive result for COX-2. The staining of Ki-67, p27, cyclin A (brown reaction product) was confined to the nuclei of the cells. Five fields in each tumor and non-tumor section were evaluated at high power (× 400) to determine the proportion of tumor cells and the staining intensity of the cytoplasm and/or nuclei in the entire sections. A score (0-300) was calculated for each marker by multiplying the intensity (none 0, weak 1, moderate 2, strong 3) by the percentage of expression (range 0-100), as reported previously[23]. At least five fields were observed, the average score in each tumor and non-tumor sections served as the result. To confirm the reproducibility of the results, all sections were scored twice, and the highest score from the two observers were thus reported.
The frequency of COX-2, Ki-67, cyclin A and p27 staining was expressed as mean ± SE. The correlations between the expression of these biologic molecular and clinicopathological factors were determined using t test (two groups had a same range of variance) and analysis of variance. The Pearson test was used to analyze the relationship between groups. SPSS 16.0 (SPSS Inc., USA) software package was used for statistical analysis, and P values less than 0.05 were considered statistically significant.
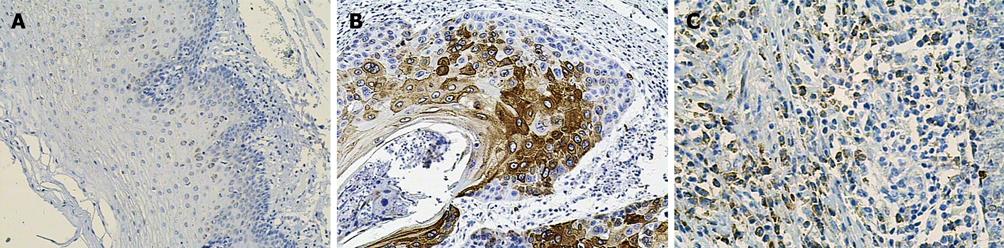
The mean of COX-2 staining in normal esophageal mucosa and ESCC was 30.2 and 74.7, respectively. This result suggested that the expression level of COX-2 in ESCC was higher than in normal human esophageal mucosa, with a significant difference (t = 5.042, P < 0.01, Figure 1).
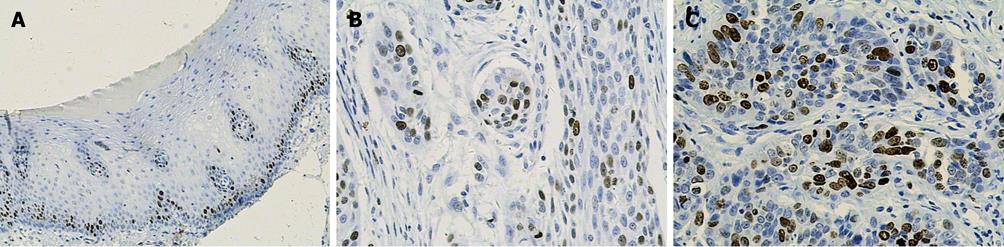
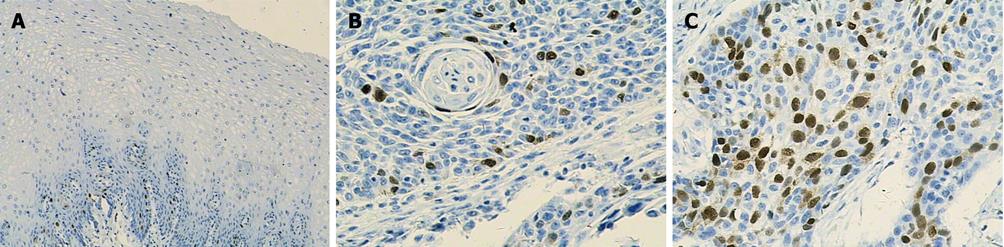
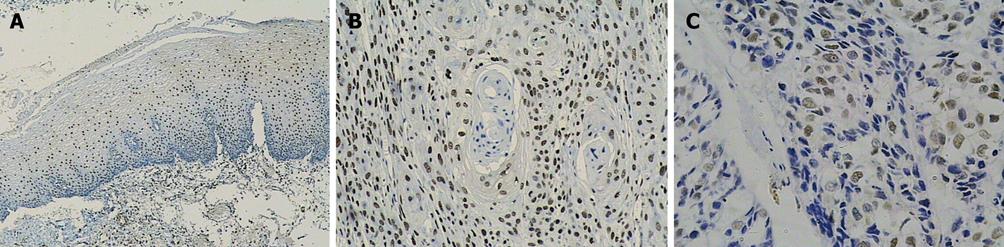
Ki-67 and cyclin A were only expressed in base cells of normal esophageal mucosa. The mean staining rate of the two markers was significantly higher in ESCC (64.0 and 44.2) than in normal esophageal mucosa (11.6 and 11.7, t = 5.272 and t = 5.021, respectively, P < 0.01). The staining of Ki-67 was confined to the nuclei of the cells, while the staining of cyclin A was concentrated mainly in the nuclei of cells, and occasionally in the cytoplasm (Figures 2 and 3). In contrast, p27 staining was observed in all the cells except the base cells of normal esophageal mucosa, the mean staining rates in normal esophageal mucosa and in ESCC were 266.4 and 182, respectively (Figure 4). There was a significantly decreased p27 expression in ESCC (t = 4.247, P < 0.01).
The correlations between the expression of COX-2, Ki-67, p27 and cyclin A and the clinicopathologic features of ESCC are summarized in Table 1. Statistically, the expression of COX-2 was not associated significantly with the age of the patients, tumor size, lymph node metastasis and the TNM stage, but its expression level was significantly higher in T3-T4 than in T1-T2 (t = 2.62, P < 0.05). For the clinicopathologic features of Ki-67, p27 and cyclin A in ESCC, the expression levels of KI-67 and cyclin A were significantly higher in poorly-differentiated squamous cell carcinoma than in well-differentiated ESCC (F = 7.839, P < 0.01; F = 3.519, P < 0.05). The p27 positive immunostaining rate in the nuclei in well-differentiated ESCC was higher than in the other tumor types (F = 5.49, P < 0.01).
| Factors | n | COX-2 | P | Ki-67 | P | Cyclin A | P | p27 | P |
| ESCC | 102 | 74.7 ± 61.2 | 0.00 | 64.0 ± 51.6 | 0.00 | 44.2 ± 32.2 | 0.00 | 182.0 ± 69.0 | 0.00 |
| Normal | 28 | 30.2 ± 43.4 | 11.6 ± 2.3 | 11.7 ± 5.0 | 266.4 ± 28.0 | ||||
| Age (yr) | |||||||||
| ≥ 60 | 44 | 77.8 ± 56.4 | NS | 66.7 ± 48.7 | NS | 44.6 ± 30.1 | NS | 185.4 ± 63.3 | NS |
| < 60 | 58 | 70.6 ± 67.2 | 60.5 ± 55.6 | 43.6 ± 37.2 | 179 ± 73.3 | ||||
| Gender | |||||||||
| Male | 86 | 72.8 ± 62.0 | NS | 64.5 ± 51.3 | NS | 43.3 ± 32.5 | NS | 183.6 ± 68.1 | NS |
| Female | 16 | 85.0 ± 57.6 | 61.5 ± 54.7 | 48.7 ± 37.3 | 173.1 ± 67.2 | ||||
| Location | |||||||||
| Upper | 11 | 59.5 ± 45.9 | NS | 49.6 ± 27.4 | NS | 48.3 ± 24.3 | NS | 205.4 ± 57.8 | NS |
| Middle | 55 | 76.7 ± 62.2 | 63.3 ± 48.0 | 45.3 ± 37.0 | 181.5 ± 67.8 | ||||
| Lower | 36 | 76.3 ± 64.6 | 69.6 ± 61.8 | 41.3 ± 29.7 | 175.5 ± 74.5 | ||||
| Differentiation | |||||||||
| Well | 32 | 68.7 ± 48.9 | NS | 43.8 ± 31.7 | 0.001 | 32.0 ± 19.0 | 0.03 | 206.2 ± 61.5 | 0.00 |
| Moderate | 50 | 82.3 ± 63.0 | 63.2 ± 36.2 | 47.9 ± 27.8 | 189.9 ± 61.4 | ||||
| Poor | 20 | 65.3 ± 74.0 | 98.4 ± 84.8 | 54.1 ± 53.7 | 123.5 ± 68.3 | ||||
| T stage | |||||||||
| T1-2 | 32 | 55.0 ± 42.3 | 0.028 | 70.2 ± 46.2 | NS | 40.6 ± 30.7 | NS | 169.3 ± 75.1 | NS |
| T3-4 | 70 | 83.0 ± 66.5 | 61.2 ± 54.0 | 45.8 ± 34.4 | 187.8 ± 65.8 | ||||
| N stage | |||||||||
| N0 | 55 | 77.9 ± 65.2 | NS | 63.8 ± 49.8 | NS | 41.5 ± 28.4 | NS | 189.8 ± 63.4 | NS |
| N1 | 47 | 70.9 ± 56.7 | 64.3 ± 54.2 | 47.4 ± 38.1 | 172.0 ± 74.7 | ||||
| M stage | |||||||||
| M0 | 85 | 74.3 ± 63.2 | NS | 63.9 ± 52.8 | NS | 41.8 ± 33.4 | NS | 181.7 ± 65.1 | |
| M1 | 17 | 76.8 ± 52.0 | 64.7 ± 46.4 | 54.9 ± 30.4 | 183.2 ± 75.6 | ||||
| Overall TNM stage | |||||||||
| I | 5 | 62.8 ± 48.3 | NS | 56.0 ± 34.8 | NS | 38.4 ± 31.7 | NS | 201.2 ± 61.5 | NS |
| II | 48 | 52.0 ± 40.5 | 75.7 ± 49.5 | 41.3 ± 29.6 | 187.3 ± 66.9 | ||||
| III | 32 | 85.0 ± 66.5 | 59.3 ± 53.7 | 43.0 ± 39.4 | 170.3 ± 71.1 | ||||
| IV | 17 | 81.5 ± 67.8 | 64.5 ± 55.3 | 55.9 ± 30.4 | 183.2 ± 75.6 |
The correlations between the frequency of COX-2 and Ki-67, cyclin A and p27 in ESCC are summarized in Table 2. Positive correlations were observed between the staining frequency of COX-2 and Ki-67 and cyclin A (r = 0.270 and 0.233, P < 0.01), but negative correlations between the staining frequency of COX-2 and p27 in ESCC (r = -0.311, P < 0.01).
COX-2 expression gradually increased from normal human esophageal mucosa to ESCC. One of the molecular alterations in esophageal carcinomatous change is high COX-2 expression. The staining frequency of COX-2 increased with the tumorigenesis, and its upregulation in ESCC was significantly associated with tumor progression. These results gave additional information about the possibility that COX-2 may be involved in an early stage of squamous cell carcinogenesis of the esophagus[6]. Our study demonstrated that the staining frequency of COX-2 was significantly higher in ESCC than in normal esophageal mucosa (P < 0.01), suggesting that over-expression of COX-2 was one of the important phenotypes and characteristics in ESCC carcinomatous change.
Apart from the over-expression of COX-2 in patients with ESCC, the present study suggested that tumor with high COX-2 expression was easy to invade. Although there were different conclusions on the relationship between COX-2 and the clinicopathologic features in ESCC, many researchers found that over-expression of COX-2 was associated with infiltrating field of tumor. Nozoe et al[24] reported that the prognosis of ESCC patients with a strong COX-2 expression was significantly poorer than that of ESCC patients with a weak COX-2 expression. Based on the data from Liu et al[25], the expression of COX-2 was not significantly correlated with gender or age, but increased expression of COX-2 was correlated with invasion and lymph node metastasis statistically. These studies have helped understand the molecular mechanisms of carcinogenesis and progression of ESCC. Yu et al[26] suggested that elevated COX-2 expression was not associated with clinicopathological features including age, sex, tumor size, histological grade, lymph node metastasis, and TNM stage. Our study found that expression of COX-2 was associated with T stage (tumor invasion) in ESCC patients, and there was a significantly lower expression in T1-T2 than in T3-T4 (P < 0.05)[24,25].
The levels of expression of Ki-67, cyclin A and p27 may suggest the proliferative activity of cancer cells in patients with ESCC. Ki-67, cyclin A over-expression and low p27 expression were associated with poorly-differentiated ESCC, compared with ESCC which had low Ki-67 and cyclin A expression and high p27 expression[3].
Previous studies had revealed the following mechanisms of COX-2 promoting cell proliferation: (1) COX-2 can contribute to inhibition of apoptosis and proliferation, increased angiogenesis, adhesion and invasion and modulation of inflammation; and (2) COX-2 may be involved in an early stage of squamous cell carcinogenesis of the esophagus and plays a non-redundant role in the regulation of cell proliferation and tumorigenesis of esophageal epithelial cells[6,27]. However, these findings are obtained from animal or in vitro, and the relationship between COX-2 and tumor cell proliferation in patients with cancer has not been completely elucidated. In this study, the samples were obtained from ESCC patients, and the expression of COX-2 was positively correlated to Ki-67 and cyclin A, which promotes cell proliferation, and was negatively correlated to p27 expression, which is a cell-proliferating suppressor in ESCC. This indicated the higher expression of COX-2 and the stronger tumor cell proliferation in ESCC, and also supported the theory that COX-2 is an important regulatory enzyme in cell proliferation.
In conclusion, our results showed that COX-2 over-expression not only was common in ESCC, but also correlated to tumor cell invasion, and COX-2 had the potential to become a molecular target in the treatment of ESCC. We will investigate the relationship between expression of COX-2 and curative effect of chemotherapy and survival rate of ESCC patients in the future studies. Ki-67 and cyclin A staining was positively correlated while p27 staining was negatively correlated with COX-2 expressions, which suggested that the over-expression of COX-2 was closely related to the cell proliferation in patients with ESCC.
Esophageal cancer is one of the most frequently occurring malignancies and the seventh leading cause of cancer-related deaths in the world. Treatment failure results from both a high incidence of local-regional failure and early systemic dissemination of the disease. Cyclooxygenase-2 (COX-2) may be involved in an early stage of squamous cell carcinogenesis of the esophagus and plays a non-redundant role in the regulation of cellular proliferation and tumorigenesis of esophageal epithelial cells. The relationship between COX-2 expression and cell cycle-regulatory protein in esophageal squamous cell carcinoma (ESCC) has not been reported to date.
COX-2 plays a non-redundant role in the regulation of cellular proliferation and tumorigenesis of esophageal epithelial cells. Ki-67 is known to be an indicator of cell proliferation. Cyclin A reaches maximum levels in the S-phase and G2. p27 is a negative factor of cell proliferation. This study investigated the correlation between COX-2 and cell cycle-regulatory proteins and explore the relationship between the proliferative activity of cancer cells and COX-2 in patients with ESCC.
Many studies have shown recently that not only there was a significant correlation between COX-2 inhibitor nonsteroidal anti-inflammatory drugs (NSAIDs) and esophageal cancer, but also a correlation between the COX-2 expression and the tumor volume response to radiotherapy. Moreover, Ki67 expression, cyclin D1 and p27 might be the most useful prognostic factor in different carcinomas. This study reported that COX-2 expression was positively correlated to Ki-67 and cyclin A and negatively correlated to p27 expression in ESCC. The results may help reveal the molecular feature of ESCC.
By better understanding tumor biologic behavior, the survival of cancer patients can be improved. COX-2 over-expression not only was common in ESCC, but also correlated to tumor cell invasion, and COX-2 had the potential to become a molecular target in the treatment of ESCC.
The authors analyzed the correlation between COX-2 and cell cycle-regulatory proteins in patients with ESCC using immunohistochemistry. They found that COX-2 expression had a significant correlation with T stage and was positively correlated to Ki-67, cyclin A and negatively correlated to p27 expression in ESCC. Considering the samples were from patients, the results are interesting and may help to reveal the molecular feature of ESCC.
Peer reviewer: Hitoshi Tsuda, MD, PhD, Diagnostic Pathology Section, Clinical Laboratory Division, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
S- Editor Sun H L- Editor Ma JY E- Editor Zheng XM
| 1. | Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Effect of neoadjuvant chemoradiotherapy on prognosis and surgery for esophageal carcinoma. World J Gastroenterol. 2009;15:4962-4968. |
| 2. | Ilson DH. Esophageal cancer chemotherapy: recent advances. Gastrointest Cancer Res. 2008;2:85-92. |
| 3. | Huang JX, Yan W, Song ZX, Qian RY, Chen P, Salminen E, Toppari J. Relationship between proliferative activity of cancer cells and clinicopathological factors in patients with esophageal squamous cell carcinoma. World J Gastroenterol. 2005;11:2956-2959. |
| 4. | Huang JX, Li FY, Xiao W, Song ZX, Qian RY, Chen P, Salminen E. Expression of thymidylate synthase and glutathione-s-transferase pi in patients with esophageal squamous cell carcinoma. World J Gastroenterol. 2009;15:4316-4321. |
| 5. | Okamura S, Fujiwara H, Suchi K, Okamura H, Umehara S, Todo M, Kuriu Y, Ikoma H, Kubota T, Nakanishi M. [The IL-6 and COX-2 expression related to sensitivity of chemoradiotherapy and prognosis in esophageal carcinoma]. Gan To Kagaku Ryoho. 2009;36:1979-1981. |
| 6. | Zhi H, Wang L, Zhang J, Zhou C, Ding F, Luo A, Wu M, Zhan Q, Liu Z. Significance of COX-2 expression in human esophageal squamous cell carcinoma. Carcinogenesis. 2006;27:1214-1221. |
| 7. | Ranka S, Gee JM, Johnson IT, Skinner J, Hart AR, Rhodes M. Non-steroidal anti-inflammatory drugs, lower oesophageal sphincter-relaxing drugs and oesophageal cancer. A case-control study. Digestion. 2006;74:109-115. |
| 8. | Noh JM, Park W, Huh SJ, Cho EY, Choi YL, Lee JH, Bae DS. Correlation between tumor volume response to radiotherapy and expression of biological markers in patients with cervical squamous cell carcinoma. J Gynecol Oncol. 2009;20:215-220. |
| 9. | Valsecchi ME, Pomerantz SC, Jaslow R, Tester W. Reduced risk of bone metastasis for patients with breast cancer who use COX-2 inhibitors. Clin Breast Cancer. 2009;9:225-230. |
| 10. | Inoue T, Segawa T, Shiraishi T, Yoshida T, Toda Y, Yamada T, Kinukawa N, Kinoshita H, Kamoto T, Ogawa O. Androgen receptor, Ki67, and p53 expression in radical prostatectomy specimens predict treatment failure in Japanese population. Urology. 2005;66:332-337. |
| 11. | Pines J, Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989;58:833-846. |
| 12. | Wang J, Chenivesse X, Henglein B, Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;343:555-557. |
| 13. | Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts JM. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217-1228. |
| 14. | Lew DJ, Dulić V, Reed SI. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197-1206. |
| 16. | Fukuchi M, Fukai Y, Kimura H, Sohda M, Miyazaki T, Nakajima M, Masuda N, Tsukada K, Kato H, Kuwano H. Prolyl isomerase Pin1 expression predicts prognosis in patients with esophageal squamous cell carcinoma and correlates with cyclinD1 expression. Int J Oncol. 2006;29:329-334. |
| 17. | Miyabe S, Okabe M, Nagatsuka H, Hasegawa Y, Inagaki A, Ijichi K, Nagai N, Eimoto T, Yokoi M, Shimozato K. Prognostic significance of p27Kip1, Ki-67, and CRTC1-MAML2 fusion transcript in mucoepidermoid carcinoma: a molecular and clinicopathologic study of 101 cases. J Oral Maxillofac Surg. 2009;67:1432-1441. |
| 18. | Ge XQ, Blow JJ. The licensing checkpoint opens up. Cell Cycle. 2009;8:2320-2322. |
| 19. | Huang JX, Song ZX, Qian RY, Xu GW. [Expression of cell cycle-regulatory proteins in squamous cell carcinoma of the esophagus]. Ai Zheng. 2003;22:277-281. |
| 20. | Takeno S, Noguchi T, Takahashi Y, Fumoto S, Shibata T, Kawahara K. Assessment of clinical outcome in patients with esophageal squamous cell carcinoma using TNM classification score and molecular biological classification. Ann Surg Oncol. 2007;14:1431-1438. |
| 21. | Gabbert HE, Nakamura Y, Shimoda T, Field JK, Hainaut P, Inoue H. Squamous cell carcinoma of the oesophagus. World Health Organization classification of the tumors. 1st ed. Lyon: IARC Press 2000; 16-17. |
| 22. | Huang J. Esophageal cancer. Manual medical oncology. 5th ed. Beijing: the people's medical publishing house 2007; 466-475. |
| 23. | Griffiths EA, Pritchard SA, McGrath SM, Valentine HR, Price PM, Welch IM, West CM. Hypoxia-associated markers in gastric carcinogenesis and HIF-2alpha in gastric and gastro-oesophageal cancer prognosis. Br J Cancer. 2008;98:965-973. |
| 24. | Nozoe T, Ezaki T, Kabashima A, Baba H, Maehara Y. Significance of immunohistochemical expression of cyclooxygenase-2 in squamous cell carcinoma of the esophagus. Am J Surg. 2005;189:110-115. |
| 25. | Liu WK, Fu Q, Li YM, Jiang XY, Zhang MP, Zhang ZX. The relationship between cyclooxygenase-2, CD44v6, and nm23H1 in esophageal squamous cell carcinoma. Onkologie. 2009;32:574-578. |
| 26. | Yu HP, Xu SQ, Liu L, Shi LY, Cai XK, Lu WH, Lu B, Su YH, Li YY. Cyclooxygenase-2 expression in squamous dysplasia and squamous cell carcinoma of the esophagus. Cancer Lett. 2003;198:193-201. |
| 27. | Hseu YC, Chen SC, Tsai PC, Chen CS, Lu FJ, Chang NW, Yang HL. Inhibition of cyclooxygenase-2 and induction of apoptosis in estrogen-nonresponsive breast cancer cells by Antrodia camphorata. Food Chem Toxicol. 2007;45:1107-1115. |