Published online Dec 21, 2010. doi: 10.3748/wjg.v16.i47.5908
Revised: August 25, 2010
Accepted: September 1, 2010
Published online: December 21, 2010
Cholesterol is of vital importance for the human body. It is a constituent for most biological membranes, it is needed for the formation of bile salts, and it is the precursor for steroid hormones and vitamin D. However, the presence of excess cholesterol in cells, and in particular in macrophages in the arterial vessel wall, might be harmful. The accumulation of cholesterol in arteries can lead to atherosclerosis, and in turn, to other cardiovascular diseases. The route that is primarily thought to be responsible for the disposal of cholesterol is called reverse cholesterol transport (RCT). Therefore, RCT is seen as an interesting target for the development of drugs aimed at the prevention of atherosclerosis. Research on RCT has taken off in recent years. In this review, the classical concepts about RCT are discussed, together with new insights about this topic.
- Citation: Velde AEVD. Reverse cholesterol transport: From classical view to new insights. World J Gastroenterol 2010; 16(47): 5908-5915
- URL: https://www.wjgnet.com/1007-9327/full/v16/i47/5908.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i47.5908
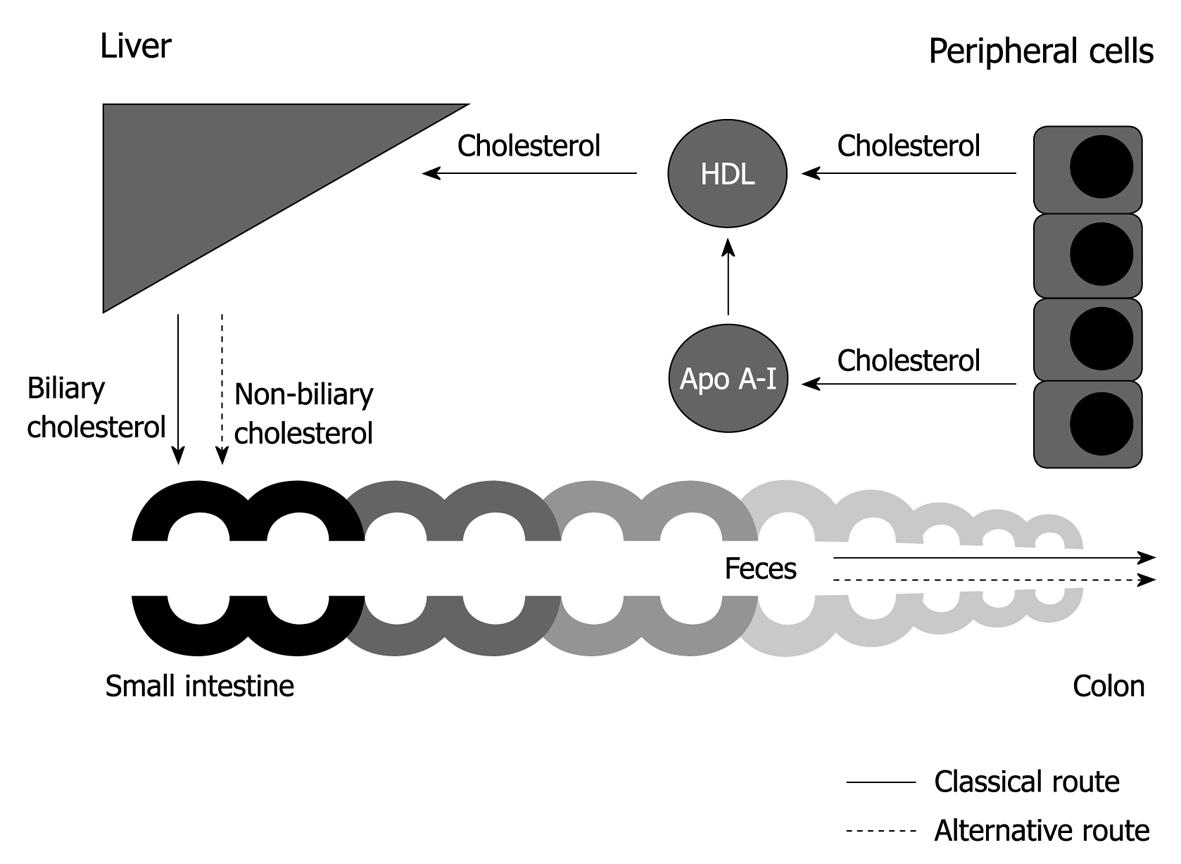
In order to dispose of cholesterol, it is transported from the periphery to the liver and intestine, and is finally excreted via the feces. This pathway has been traditionally referred to as reverse cholesterol transport (RCT) or centripetal cholesterol flux. In this review, the term RCT is used. Research on RCT has taken off in recent years. This review starts with introduction of the term RCT in the early 1970s. This is followed by a discussion of research in the following years that led to the classical view on RCT: high-density lipoprotein (HDL)-mediated transport of cholesterol from the periphery to the liver, the subsequent uptake of HDL cholesterol by the liver, hepatobiliary cholesterol secretion, and finally, excretion via the feces (Figure 1). Both free cholesterol and the esterified form are involved in RCT. Cholesteryl esters are much less amphiphatic than free cholesterol and appear to be the preferred form for transport in the plasma and for storage. The transport proteins involved in RCT receive special attention in this review. Finally, new insights in cholesterol excretion are discussed, which make the current concept of RCT questionable.
Glomset et al[1] introduced the term RCT in 1973, in a review that described the role of lecithin:cholesterol acyl transferase (LCAT) in metabolism. We nowadays know that LCAT is an enzyme that is synthesized in the liver and circulates in the blood plasma on the surface of lipoproteins, predominantly HDL. LCAT has the ability to catalyze the formation of cholesteryl esters on the surface of HDL by transferring fatty acids from phosphatidylcholine (PC, also known as lecithin) to the unesterified cholesterol[2]. It took several years after the discovery of LCAT before its physiological role was elucidated in more detail[3]. The in vitro testing of the possibility that LCAT plays a role in cholesterol transport was mainly hampered by the fact that most of the cholesterol of freshly prepared plasma lipoproteins is already esterified, that is, if the enzyme is able to alter the structure and physical properties of lipoproteins, most of the alterations have already occurred in vivo. The discovery of patients with familial LCAT deficiency[4-6] has provided a unique opportunity to circumvent these problems. By studying these patients, our knowledge about LCAT and cholesterol removal has increased.
Although tissue culture studies had favored HDL[7,8] as the principal vehicle for cholesterol transport from the periphery to the liver, it took a decade before in vivo evidence was presented by Miller et al[9]. When cholesterol-loaded macrophages are incubated in medium that contains plasma, cholesterol moves from the cells to HDL and is subsequently esterified by LCAT[10]. The accumulation of cholesteryl esters in these particles increases their size and decreases their density; enrichment with apoprotein E also occurs, which decreases electrophoretic mobility[11,12]. Miller et al[9] have shown that similar changes were present in the circulating HDL of rabbits, when their peripheral tissues were loaded with cholesterol by intravenous injection of acetylated or native human low-density lipoproteins (LDLs).
Evidence for the existence of receptors that recognize HDL in plasma membranes of hepatocytes has been presented by several groups[13-15]. It appears that the rate of uptake of HDL cholesteryl esters by the liver in rats is several times greater than that of HDL apolipoprotein A-I (apo A-l), which suggests that cholesteryl esters dissociate from HDL particles at the surface of hepatocytes. This process might be facilitated by transient binding of HDL to the plasma membranes of such cells[16]. A similar process has been studied in cultures of human hepatoma cells[17]. The pathways for delivery of cholesteryl ester to the liver clearly cannot operate in patients with familial LCAT deficiency. Nevertheless, such patients do not accumulate large quantities of cholesterol in their plasma, as might be expected if the delivery of cholesteryl ester to the liver were hampered[18].
Once cholesterol has reached the liver, conversion into bile salts is the final destination for most of the cholesterol[19]. In contrast to cholesterol, bile salts are amphiphatic. Bile salts function as signaling molecules, and they act as physiological detergents: they emulsify droplets of dietary lipids in the intestine, which makes them available for absorption. Synthesis of bile salts involves the action of multiple different enzymes and can follow two major pathways, named the “classic” or neutral pathway and the alternative or acidic pathway. The classic pathway accounts for the majority of bile salt synthesis. In the enterohepatic circulation, intestinal bacteria modify bile acid structures, which yields secondary bile salts, for example, lithocholate and deoxycholate[20]. As about 95% of the bile salts re-enter the enterohepatic circulation, the bile salt pool of the body consists of a mixture of primary and secondary bile salts. Only a small part of hepatic cholesterol is not converted to bile salts and is directly secreted into bile. The majority of this hepatobiliary secreted cholesterol will be re-absorbed in the small intestine[21].
The first evidence that HDL is not a homogeneous set of molecules, but rather a mixture of heterogeneous subclasses, arose in 1979. Gebhardt et al[22] have described the existence of a pre-β migrating subclass of apo A-l-containing particles when studying human amniotic fluid. Similar particles have been demonstrated in peripheral lymph of dogs[23,24] and in human lymphedema fluid[25]. Castro et al[26] have found that, when radiolabeled cholesterol effluxes from cultured human fibroblasts into medium that contains human serum, almost all of it enters a minor component of HDL that is composed of very small apo A-l-containing particles. These particles differ from the majority of plasma HDL in having pre-β electrophoretic mobility on agarose gel. It is rich in phospholipids, and contains little or no core lipid, and apo A-l as the only recognized apoprotein[26-28]. We know now that it is pre-β HDL cholesterol that is esterified by LCAT. After esterification, cholesteryl esters are sequestered into the core of the lipoprotein particle, eventually making spherical α-HDL. In this way, cholesterol is made ready for removal[2].
Some cholesteryl esters from HDL particles is transferred to LDL, very-low-density lipoproteins, or chylomicrons in exchange for triglycerides and phospholipids[29-35]. Triglyceride transfer is mediated by the cholesterol ester transfer protein (CETP) and phospholipid transfer is mediated by the phospholipid transfer protein (PLTP)[35,36]. Rodents do not express CETP, which might partly explain the high plasma HDL levels observed in these animals, in comparison to humans who express CETP. PLTP has not only been implicated in the transfer of phospholipids to HDL, but also in a process called HDL conversion[37]. In this process, PLTP mediates fusion of intermediate sized α-HDL particles to generate larger HDL particles with a concomitant release of lipid-poor apo A-I. These actions result in an enhanced capacity to take up cellular cholesterol.
In the 1990s, several proteins and receptors involved in RCT were identified, which has given new insights in the mechanisms behind RCT.
ABCA1: Tangier disease was originally described and named on the basis of kindred living in Tangier Island in Chesapeake Bay, USA. Assmann et al[38] and Brook et al[39] have linked Tangier disease with abnormal HDL levels. The inheritance of the disease was already described in 1964[40]. However, it took until 1999 before it became clear that mutations in the ABC1 (nowadays in humans and rodents referred to as ABCA1) gene were responsible for the severe HDL deficiency in Tangier disease[41-43]. We now know that ABCA1 is involved in the first step of RCT. The mechanism by which ABCA1 mediates cholesterol efflux has been a matter of intense investigation. Two distinct mechanisms have been proposed to explain ABCA1-mediated cholesterol efflux from macrophages to apo A-I. These models are described below.
One model argues that apo A-I binds ABCA1 at the plasma membrane and is subsequently internalized and targeted to intracellular compartments, where lipidation of apo A-I occurs as part of a retroendocytosis pathway[44-46]. Hassan et al[47] have shown that two-thirds of apo A-I is bound to the plasma membrane and one-third is found in intracellular compartments. It appears that the C-terminal region of apo A-I is important in the ABCA1-mediated lipid efflux pathway. Apo A-I dissociated four-fold faster from the intracellular compartments than from the plasma membrane, which suggests an important contribution of ABCA1 in the endocytic pathway to apo A-I lipidation. In contrast, Faulkner et al[48] have shown, by studying ABCA1-mediated cholesterol efflux from macrophages, that the internalized apo A-I is re-secreted as a degraded protein. Furthermore, they have demonstrated that lipid-free apo-A-I-mediated cholesterol efflux from macrophages could be pharmacologically uncoupled from apo A-I internalization into cells, which raises doubts as to the significance of the endocytic pathway in efflux. In addition, confocal microscopy and efflux assays of apo A-I internalization and lipidation as a function of ABCA1 expression have indicated that apo A-I lipidation occurs at the cell surface, whereas ABCA1-dependent apo A-I internalization leads to its lysosomal targeting and degradation[49]. Data of Azuma et al[50] have suggested that the retroendocytosis pathway of ABCA1/apo A-I contributes to HDL formation when excess lipoprotein-derived cholesterol has accumulated in cells.
The other model argues that apo A-I forms complexes with phospholipids and cholesterol at the plasma membrane in a process that is promoted by ABCA1 activity. There is abundant evidence that ABCA1-mediated cholesterol efflux to apo A-I can occur at the plasma membrane[51-53]. It has been shown that optimal cholesterol efflux in macrophages requires binding of the C-terminal domain of apo A-I to a cell-surface-binding site, and the subsequent translocation of intracellular cholesterol to an efflux-competent pool[54]. By studying the binding of wild-type and mutant forms of human apo A-I to mouse J774 macrophages, it has been shown that ABCA1 activity creates two types of high affinity apo A-I binding sites at the cell surface. Only 10% of cell-surface-bound apo A-I interacts directly with ABCA1, whereas the rest is bound to lipid domains via the C-terminal domain. The low capacity site formed by direct apo A-I/ABCA1 interaction functions in a regulatory role, whereas the much higher capacity site generated by apo A-I/lipid interactions functions in lipidation[55]. Vedhachalam et al[53] have proposed an apo A-I/ABCA1 reaction scheme that involves three steps. First, there is binding of a small regulatory pool of apo A-I to ABCA1, thereby enhancing net phospholipid translocation to the plasma membrane exofacial leaflet; this leads to unequal lateral packing densities in the two leaflets of the phospholipid bilayer. Second, the resultant membrane strain is relieved by bending and by creation of exovesiculated lipid domains. The formation of highly curved membrane surface promotes high affinity binding of apo A-I to these domains. Third, this pool of bound apo A-I spontaneously solubilizes the exovesiculated domain to create discoidal nascent HDL particles. These particles contain 2-4 molecules of apo A-I and a complement of membrane phospholipid classes, together with some cholesterol. A key feature of this mechanism is that membrane bending induced by ABCA1 lipid translocase activity creates the conditions required for nascent HDL assembly by apo A-I. Overall, this mechanism is consistent with the known properties of ABCA1 and apo A-I and reconciles many of the apparently discrepant findings in the literature. Recently, it has been shown that sera with similar HDL cholesterol levels or apo A-I levels differ in their ability to promote macrophage efflux due to the differences in the concentration of pre-β HDL[56].
ABCG1 and ABCG4: ABCA1 activity is needed for the predominant pathway for cholesterol efflux to apo A-I and the formation of pre-β HDL. However, it is doubtful whether ABCA1 operates alone in the formation of mature α-HDL. For example, HEK293 cells with ABCA1 overexpression, but without expression of HDL modifying factors like LCAT, PLTP, ABCG1, scavenger receptor class B type 1 (SR-BI), or apolipoprotein M, form pre-β HDL, but not α-HDL. In addition, it appears that the pre-β HDL that is formed is a poor substrate for subsequent lipidation by ABCA1, and presumably requires additional non-ABCA1-mediated lipidation for further maturation[57]. ABCG1 and ABCG4 or their heterodimers[58] might be good candidates for lipidation and maturation of HDL. Synergistic relationships between ABCA1 and ABCG1 and ABCG1/ABCG4 heterodimers have been demonstrated in vitro[59,60]. Wang et al[61] have shown that ABCG1 and ABCG4 mediate isotopic and net mass efflux of cellular cholesterol to HDL but not to lipid-poor apo A-I. In addition, mice with a targeted disruption of Abcg1 display impaired cholesterol efflux to mature HDL[62]. The exact mechanism behind ABCG1- and ABCG4-mediated cholesterol efflux is still a matter of debate.
SR-BI: SR-BI was identified as an HDL receptor (HDLR) in 1996. SR-BI is primarily expressed in liver and nonplacental steroidogenic tissues, and binds HDL with high affinity. The classic function of SR-BI is to mediate the selective uptake of HDL cholesterol by cells by a mechanism distinct from the classic LDL receptor (LDLR) pathway[63]. LDLR mediates endocytosis of the intact LDL particles via coated pits and vesicles, and their subsequent hydrolysis in lysosomes[64]. SR-BI mediates the selective uptake of HDL cholesterol by cells; primarily in the form of cholesteryl esters. This process involves the transfer of the cholesteryl esters from the hydrophobic core of the HDL particle to the cell, without transfer of the apolipoprotein at the surface of the particle. SR-BI-mediated selective lipid uptake appears to be a two-step process, in which high-affinity lipoprotein binding is followed by receptor-mediated transfer of lipids from the lipoprotein particle to the cell membrane. After lipid transfer, the lipid-depleted lipoprotein particle is released from the cells and re-enters the extracellular space[65]. SR-BI also mediates the bidirectional flux of unesterified cholesterol and phospholipids between HDL and cells[66].
Studies in genetically modified mice have revealed that SR-BI in the liver is particularly essential for RCT. In SR-BI-/- mice, plasma total cholesterol is elevated approximately twofold, and most of it circulates in abnormally large, heterogeneous, apolipoprotein-E-enriched HDL-like particles[67]. In addition, these mice exhibit impaired biliary cholesterol secretion, without concomitant changes in either biliary bile acid or phospholipid secretion[68]. Conversely, hepatic overexpression of SR-BI in mice results in the virtual disappearance of plasma HDL and a substantial increase in biliary cholesterol[69].
ABCG5 and ABCG8: At the beginning of the 21st century, two groups almost simultaneously identified mutations in genes ABCG5 and ABCG8, which underlie the disease sitosterolemia[70,71]. Sitosterolemia patients accumulate large amounts of plant sterols and exhibit complete abrogation of biliary plant sterol secretion. They also display increased cholesterol absorption and decreased biliary cholesterol secretion[72,73]. We now know that half-transporters ABCG5 and ABCG8 function as a heterodimer[70] in order to facilitate hepatobiliary cholesterol transport. Double knockout mice for Abcg5 and Abcg8 display extremely low biliary cholesterol concentrations in comparison to wild-type animals[74]. Overexpression of the human ABCG5 and ABCG8 in mice[75] and pharmacological induction of endogenous Abcg5 and Abcg8[76,77] both result in increased biliary cholesterol levels. Mice that lack either Abcg5[78], Abcg8[79], or both[74] do not show the same phenotype regarding physiology, which leaves room for discussion about the way these transporters function. Currently, two hypotheses exist. One argues that ABCG5/G8 act as a “flippase”. In this case, the heterodimer shuttles cholesterol from the inner leaflet of the canalicular membrane through a chamber formed by the two half-transporters. This is followed by ATP binding and hydrolysis, which results in a conformational change of the complex. Cholesterol is thereby flipped into the outer membrane leaflet in a configuration that favors its release. Then, cholesterol is ready to be picked up by acceptors[80]. The other hypothesis argues that ABCG5/G8 act as a “liftase”[81]. In this case, the heterodimer promotes an activated state of cholesterol so that acceptors can easily pick up cholesterol. ABCG5/G8 could form a channel that binds cholesterol. Thereafter, the heterodimer might push cholesterol partly into the lumen when ATP is hydrolyzed (activation of cholesterol). Once cholesterol is activated, mixed micelles and PC vesicles are able to serve as an acceptor for cholesterol.
In summary, the classical view describes RCT as follows: the transport of cholesterol from the periphery to apo A-I and HDL by processes that are mediated by ABCA1, ABCG1 and/or ABCG4. The subsequent uptake of HDL cholesterol by the liver involves SR-BI. Thereafter, hepatobiliary cholesterol secretion is mediated by ABCG5/G8 and final excretion is via the feces.
How solid is the classical definition of RCT? In recent years, new insights into RCT have been provided, which brings into question the current concept of RCT.
Studies that try to pinpoint the rate-controlling step in RCT often give rise to confusion. For example, Jolley et al[82] and Osono et al[83] has studied the importance of HDL in rate-control of RCT. They have used transgenic mice that expressed variable amounts of simian CETP in order to vary HDL levels. It appears that, in mice, neither the concentration of HDL cholesterol or apo A-I, nor the level of CETP activity dictate the magnitude of RCT[83]. Apo A-I-/- mice have very low HDL levels. When HDL is rate-controlling for RCT, then RCT should be hampered in apo A-I-/- mice. Nevertheless, Jolley et al[82] have demonstrated that the magnitude of the cholesterol flux from peripheral organs to the liver is virtually identical in mice that lacking apo A-I compared with control animals. They have suggested that the magnitude of RCT is probably ascribed to processes in peripheral organs. Although ABCA1 mediates cholesterol efflux from the periphery, it appears that Abca1-/- mice have unaltered hepatobiliary cholesterol secretion and fecal cholesterol excretion, while having virtually absent HDL levels[84]. Also, the proposed key player in the liver in HDL uptake, SR-BI, does not control RCT in mice[85]. Moreover, the exclusiveness of SR-BI as the sole high-affinity HDLR in the liver was compromised when Martinez et al[86] identified ecto-F1-ATPase as a high-affinity HDLR in hepatocytes. Furthermore, deficiency of Abcg5 and/or Abcg8 leads to mild[74,77] or no[78] decrease in fecal neutral sterol secretion in mice. This also questions the rate-controlling properties of these transporters on the magnitude of RCT. In addition, it has been shown that Abcg5/g8-independent, inducible routes exist that can significantly contribute to total hepatobiliary cholesterol output in mice[87]. Although most of the above-mentioned studies have been performed in mice and not in humans, it emphasizes the complexity of the mechanisms that underlie RCT.
In the classical concept of RCT, the liver plays a major role. When hepatobiliary cholesterol secretion is the primary route of cholesterol elimination, inhibition of ABCG5/G8, and hence, diminished hepatobiliary cholesterol secretion, should result in a drastic lowering of fecal neutral sterol excretion. Abcg5/Abcg8 double knockout mice do not show the expected low levels of fecal neutral sterol excretion[74]. In addition, Abcb4-/- mice, which also have almost no biliary cholesterol secretion, have the same fecal neutral sterol output as their wild-type littermates[88]. In addition, Kruit et al[88] have shown that, in these mice, intravenous radiolabeled cholesterol could be recovered in the feces. These findings indicate that hepatobiliary cholesterol secretion might not be the only route of cholesterol excretion. In mouse models with disturbed biliary secretion, there must be a direct transintestinal pathway for cholesterol excretion. In 2006, van der Velde et al[89] described significant direct intestinal cholesterol secretion in normal mice. This was later supported by van der Veen et al[90] who have measured cholesterol fluxes in mice. This route is nowadays referred to as transintestinal cholesterol efflux (TICE; see[91] for review) and continues to be elucidated[92-94]. Although quantitative cholesterol flux studies are warranted to quantify exactly the magnitude of TICE in humans, significant secretion of intestinal cholesterol in humans has been observed by Simmonds et al[21].
Although medical textbooks have taught us the classical view of RCT for several decades, it is desirable to open our eyes to the new insights into cholesterol excretion. Research on RCT has taken off in recent years. These new insights show us that the classical route for cholesterol excretion cannot be a strictly defined pathway. In addition, an alternative route for cholesterol disposal has come to light in the form of TICE (Figure 1). The new insights into RCT should make us question whether the classical view needs revision.
Peer reviewer: Dr. Richard A Rippe, Department of Medicine, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7038, United States
S- Editor Sun H L- Editor Kerr C E- Editor Zheng XM
| 1. | Glomset JA, Norum KR. The metabolic role of lecithin: cholesterol acyltransferase: perspectives form pathology. Adv Lipid Res. 1973;11:1-65. |
| 2. | Jonas A. Lecithin cholesterol acyltransferase. Biochim Biophys Acta. 2000;1529:245-256. |
| 3. | Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155-167. |
| 4. | Hamnström B, Gjone E, Norum KR. Familial plasma lecithin: cholesterol acyltransferase deficiency. Br Med J. 1969;2:283-286. |
| 5. | Norum KR, Gjone E. Familial serum-cholesterol esterification failure. A new inborn error of metabolism. Biochim Biophys Acta. 1967;144:698-700. |
| 6. | Norum KR, Borsting S, Grundt I. Familial lecithin: cholesterol acyltransferase deficiency. Study of two new patients and their close relatives. Acta Med Scand. 1970;188:323-326. |
| 7. | Glomset JA. Physiological role of lecithin-cholesterol acyltransferase. Am J Clin Nutr. 1970;23:1129-1136. |
| 8. | Nicoll A, Miller NE, Lewis B. High-density lipoprotein metabolism. Adv Lipid Res. 1980;17:53-106. |
| 9. | Miller NE, La Ville A, Crook D. Direct evidence that reverse cholesterol transport is mediated by high-density lipoprotein in rabbit. Nature. 1985;314:109-111. |
| 10. | Ho YK, Brown MS, Goldstein JL. Hydrolysis and excretion of cytoplasmic cholesteryl esters by macrophages: stimulation by high density lipoprotein and other agents. J Lipid Res. 1980;21:391-398. |
| 11. | Basu SK, Goldstein JL, Brown MS. Independent pathways for secretion of cholesterol and apolipoprotein E by macrophages. Science. 1983;219:871-873. |
| 12. | Gordon V, Innerarity TL, Mahley RW. Formation of cholesterol- and apoprotein E-enriched high density lipoproteins in vitro. J Biol Chem. 1983;258:6202-6212. |
| 13. | Bachorik PS, Franklin FA, Virgil DG, Kwiterovich PO Jr. High-affinity uptake and degradation of apolipoprotein E free high-density lipoprotein and low-density lipoprotein in cultured porcine hepatocytes. Biochemistry. 1982;21:5675-5684. |
| 14. | Hoeg JM, Demosky SJ Jr, Edge SB, Gregg RE, Osborne JC Jr, Brewer HB Jr. Characterization of a human hepatic receptor for high density lipoproteins. Arteriosclerosis. 1985;5:228-237. |
| 15. | Tamai T, Patsch W, Lock D, Schonfeld G. Receptors for homologous plasma lipoproteins on a rat hepatoma cell line. J Lipid Res. 1983;24:1568-1577. |
| 16. | Glass C, Pittman RC, Weinstein DB, Steinberg D. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein: selective delivery of cholesterol ester to liver, adrenal, and gonad. Proc Natl Acad Sci USA. 1983;80:5435-5439. |
| 17. | Rinninger F, Pittman RC. Regulation of the selective uptake of high density lipoprotein-associated cholesteryl esters by human fibroblasts and Hep G2 hepatoma cells. J Lipid Res. 1988;29:1179-1194. |
| 18. | Stokke KT, Bjerve KS, Blomhoff JP, Oystese B, Flatmark A, Norum KR, Gjone E. Familial lecithin:cholesterol acyltransferase deficiency. Studies on lipid composition and morphology of tissues. Scand J Clin Lab Invest Suppl. 1974;137:93-100. |
| 19. | Byers SO. Liver regulation of plasma cholesterol. Biochem Clin. 1964;4:157-168. |
| 20. | Bortolini O, Medici A, Poli S. Biotransformations on steroid nucleus of bile acids. Steroids. 1997;62:564-577. |
| 21. | Simmonds WJ, Hofmann AF, Theodor E. Absorption of cholesterol from a micellar solution: intestinal perfusion studies in man. J Clin Invest. 1967;46:874-890. |
| 22. | Gebhardt DO, Beintema A, Reman FC, van Gent CM. The lipoprotein composition of amniotic fluid. Clin Chim Acta. 1979;94:93-100. |
| 23. | Lefevre M, Sloop CH, Roheim PS. Characterization of dog prenodal peripheral lymph lipoproteins. Evidence for the peripheral formation of lipoprotein-unassociated apoA-I with slow pre-beta electrophoretic mobility. J Lipid Res. 1988;29:1139-1148. |
| 24. | Sloop CH, Dory L, Roheim PS. Interstitial fluid lipoproteins. J Lipid Res. 1987;28:225-237. |
| 25. | Reichl D, Forte TM, Hong JL, Rudra DN, Pflug J. Human lymphedema fluid lipoproteins: particle size, cholesterol and apolipoprotein distributions, and electron microscopic structure. J Lipid Res. 1985;26:1399-1411. |
| 26. | Castro GR, Fielding CJ. Early incorporation of cell-derived cholesterol into pre-beta-migrating high-density lipoprotein. Biochemistry. 1988;27:25-29. |
| 27. | Ishida BY, Frolich J, Fielding CJ. Prebeta-migrating high density lipoprotein: quantitation in normal and hyperlipidemic plasma by solid phase radioimmunoassay following electrophoretic transfer. J Lipid Res. 1987;28:778-786. |
| 28. | Kunitake ST, La Sala KJ, Kane JP. Apolipoprotein A-I-containing lipoproteins with pre-beta electrophoretic mobility. J Lipid Res. 1985;26:549-555. |
| 29. | Albers JJ, Tollefson JH, Chen CH, Steinmetz A. Isolation and characterization of human plasma lipid transfer proteins. Arteriosclerosis. 1984;4:49-58. |
| 30. | Chajek T, Fielding CJ. Isolation and characterization of a human serum cholesteryl ester transfer protein. Proc Natl Acad Sci USA. 1978;75:3445-3449. |
| 31. | Ihm J, Ellsworth JL, Chataing B, Harmony JA. Plasma protein-facilitated coupled exchange of phosphatidylcholine and cholesteryl ester in the absence of cholesterol esterification. J Biol Chem. 1982;257:4818-4827. |
| 32. | Marcel YL, Vezina C, Teng B, Sniderman A. Transfer of cholesterol esters between human high density lipoproteins and triglyceride-rich lipoproteins controlled by a plasma protein factor. Atherosclerosis. 1980;35:127-133. |
| 33. | Morton RE, Zilversmit DB. Purification and characterization of lipid transfer protein(s) from human lipoprotein-deficient plasma. J Lipid Res. 1982;23:1058-1067. |
| 34. | Pattnaik NM, Montes A, Hughes LB, Zilversmit DB. Cholesteryl ester exchange protein in human plasma isolation and characterization. Biochim Biophys Acta. 1978;530:428-438. |
| 35. | Tall AR, Abreu E, Shuman J. Separation of a plasma phospholipid transfer protein from cholesterol ester/phospholipid exchange protein. J Biol Chem. 1983;258:2174-2180. |
| 36. | Chung BH, Segrest JP, Franklin F. In vitro production of beta-very low density lipoproteins and small, dense low density lipoproteins in mildly hypertriglyceridemic plasma: role of activities of lecithin:cholester acyltransferase, cholesterylester transfer proteins and lipoprotein lipase. Atherosclerosis. 1998;141:209-225. |
| 37. | Settasatian N, Duong M, Curtiss LK, Ehnholm C, Jauhiainen M, Huuskonen J, Rye KA. The mechanism of the remodeling of high density lipoproteins by phospholipid transfer protein. J Biol Chem. 2001;276:26898-26905. |
| 38. | Assmann G, Simantke O, Schaefer HE, Smootz E. Characterization of high density lipoproteins in patients heterozygous for Tangier disease. J Clin Invest. 1977;60:1025-1035. |
| 39. | Brook JG, Lees RS, Yules JH, Cusack B. Tangier disease (alpha-lipoprotein deficiency). JAMA. 1977;238:332-334. |
| 40. | Fredrickson DS. The inheritance of high density lipoprotein deficiency (Tangier disease). J Clin Invest. 1964;43:228-236. |
| 41. | Bodzioch M, Orsó E, Klucken J, Langmann T, Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C, Porsch-Ozcürümez M. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347-351. |
| 42. | Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336-345. |
| 43. | Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352-355. |
| 44. | Neufeld EB, Remaley AT, Demosky SJ, Stonik JA, Cooney AM, Comly M, Dwyer NK, Zhang M, Blanchette-Mackie J, Santamarina-Fojo S. Cellular localization and trafficking of the human ABCA1 transporter. J Biol Chem. 2001;276:27584-27590. |
| 45. | Neufeld EB, Stonik JA, Demosky SJ Jr, Knapper CL, Combs CA, Cooney A, Comly M, Dwyer N, Blanchette-Mackie J, Remaley AT. The ABCA1 transporter modulates late endocytic trafficking: insights from the correction of the genetic defect in Tangier disease. J Biol Chem. 2004;279:15571-15578. |
| 46. | Takahashi Y, Smith JD. Cholesterol efflux to apolipoprotein AI involves endocytosis and resecretion in a calcium-dependent pathway. Proc Natl Acad Sci USA. 1999;96:11358-11363. |
| 47. | Hassan HH, Bailey D, Lee DY, Iatan I, Hafiane A, Ruel I, Krimbou L, Genest J. Quantitative analysis of ABCA1-dependent compartmentalization and trafficking of apolipoprotein A-I: implications for determining cellular kinetics of nascent high density lipoprotein biogenesis. J Biol Chem. 2008;283:11164-11175. |
| 48. | Faulkner LE, Panagotopulos SE, Johnson JD, Woollett LA, Hui DY, Witting SR, Maiorano JN, Davidson WS. An analysis of the role of a retroendocytosis pathway in ABCA1-mediated cholesterol efflux from macrophages. J Lipid Res. 2008;49:1322-1332. |
| 49. | Denis M, Landry YD, Zha X. ATP-binding cassette A1-mediated lipidation of apolipoprotein A-I occurs at the plasma membrane and not in the endocytic compartments. J Biol Chem. 2008;283:16178-16186. |
| 50. | Azuma Y, Takada M, Shin HW, Kioka N, Nakayama K, Ueda K. Retroendocytosis pathway of ABCA1/apoA-I contributes to HDL formation. Genes Cells. 2009;14:191-204. |
| 51. | Nandi S, Ma L, Denis M, Karwatsky J, Li Z, Jiang XC, Zha X. ABCA1-mediated cholesterol efflux generates microparticles in addition to HDL through processes governed by membrane rigidity. J Lipid Res. 2009;50:456-466. |
| 52. | Vaughan AM, Oram JF. ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J Lipid Res. 2003;44:1373-1380. |
| 53. | Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123-25130. |
| 54. | Burgess JW, Frank PG, Franklin V, Liang P, McManus DC, Desforges M, Rassart E, Marcel YL. Deletion of the C-terminal domain of apolipoprotein A-I impairs cell surface binding and lipid efflux in macrophage. Biochemistry. 1999;38:14524-14533. |
| 55. | Vedhachalam C, Ghering AB, Davidson WS, Lund-Katz S, Rothblat GH, Phillips MC. ABCA1-induced cell surface binding sites for ApoA-I. Arterioscler Thromb Vasc Biol. 2007;27:1603-1609. |
| 56. | de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796-801. |
| 57. | Mulya A, Lee JY, Gebre AK, Thomas MJ, Colvin PL, Parks JS. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler Thromb Vasc Biol. 2007;27:1828-1836. |
| 58. | Cserepes J, Szentpétery Z, Seres L, Ozvegy-Laczka C, Langmann T, Schmitz G, Glavinas H, Klein I, Homolya L, Váradi A. Functional expression and characterization of the human ABCG1 and ABCG4 proteins: indications for heterodimerization. Biochem Biophys Res Commun. 2004;320:860-867. |
| 59. | Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534-540. |
| 60. | Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433-2443. |
| 61. | Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci USA. 2004;101:9774-9779. |
| 62. | Kennedy MA, Barrera GC, Nakamura K, Baldán A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121-131. |
| 63. | Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518-520. |
| 64. | Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1-39. |
| 65. | Krieger M. Charting the fate of the "good cholesterol": identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523-558. |
| 66. | Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793-797. |
| 67. | Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA. 1997;94:12610-12615. |
| 68. | Mardones P, Quiñones V, Amigo L, Moreno M, Miquel JF, Schwarz M, Miettinen HE, Trigatti B, Krieger M, VanPatten S. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J Lipid Res. 2001;42:170-180. |
| 69. | Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414-417. |
| 70. | Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771-1775. |
| 71. | Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79-83. |
| 72. | Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53:1033-1043. |
| 73. | Miettinen TA. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur J Clin Invest. 1980;10:27-35. |
| 74. | Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99:16237-16242. |
| 75. | Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671-680. |
| 76. | Plōsch T, Kok T, Bloks VW, Smit MJ, Havinga R, Chimini G, Groen AK, Kuipers F. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J Biol Chem. 2002;277:33870-33877. |
| 77. | Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565-15570. |
| 78. | Plösch T, Bloks VW, Terasawa Y, Berdy S, Siegler K, Van Der Sluijs F, Kema IP, Groen AK, Shan B, Kuipers F. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology. 2004;126:290-300. |
| 79. | Klett EL, Lu K, Kosters A, Vink E, Lee MH, Altenburg M, Shefer S, Batta AK, Yu H, Chen J. A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med. 2004;2:5. |
| 80. | Wittenburg H, Carey MC. Biliary cholesterol secretion by the twinned sterol half-transporters ABCG5 and ABCG8. J Clin Invest. 2002;110:605-609. |
| 81. | Small DM. Role of ABC transporters in secretion of cholesterol from liver into bile. Proc Natl Acad Sci USA. 2003;100:4-6. |
| 82. | Jolley CD, Woollett LA, Turley SD, Dietschy JM. Centripetal cholesterol flux to the liver is dictated by events in the peripheral organs and not by the plasma high density lipoprotein or apolipoprotein A-I concentration. J Lipid Res. 1998;39:2143-2149. |
| 83. | Osono Y, Woollett LA, Marotti KR, Melchior GW, Dietschy JM. Centripetal cholesterol flux from extrahepatic organs to the liver is independent of the concentration of high density lipoprotein-cholesterol in plasma. Proc Natl Acad Sci USA. 1996;93:4114-4119. |
| 84. | Groen AK, Bloks VW, Bandsma RH, Ottenhoff R, Chimini G, Kuipers F. Hepatobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J Clin Invest. 2001;108:843-850. |
| 85. | Alam K, Meidell RS, Spady DK. Effect of up-regulating individual steps in the reverse cholesterol transport pathway on reverse cholesterol transport in normolipidemic mice. J Biol Chem. 2001;276:15641-15649. |
| 86. | Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezón E, Champagne E, Pineau T, Georgeaud V, Walker JE, Tercé F. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75-79. |
| 87. | Plösch T, van der Veen JN, Havinga R, Huijkman NC, Bloks VW, Kuipers F. Abcg5/Abcg8-independent pathways contribute to hepatobiliary cholesterol secretion in mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G414-G423. |
| 88. | Kruit JK, Plösch T, Havinga R, Boverhof R, Groot PH, Groen AK, Kuipers F. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128:147-156. |
| 89. | van der Velde AE, Vrins CL, van den Oever K, Kunne C, Oude Elferink RP, Kuipers F, Groen AK. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967-975. |
| 90. | van der Veen JN, van Dijk TH, Vrins CL, van Meer H, Havinga R, Bijsterveld K, Tietge UJ, Groen AK, Kuipers F. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284:19211-19219. |
| 91. | van der Velde AE, Brufau G, Groen AK. Transintestinal cholesterol efflux. Curr Opin Lipidol. 2010;21:167-171. |
| 92. | Brown JM, Bell TA 3rd, Alger HM, Sawyer JK, Smith TL, Kelley K, Shah R, Wilson MD, Davis MA, Lee RG. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J Biol Chem. 2008;283:10522-10534. |
| 93. | Gälman C, Bonde Y, Matasconi M, Angelin B, Rudling M. Dramatically increased intestinal absorption of cholesterol following hypophysectomy is normalized by thyroid hormone. Gastroenterology. 2008;134:1127-1136. |
| 94. | Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968-1978. |