Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5766
Revised: October 15, 2010
Accepted: October 22, 2010
Published online: December 7, 2010
AIM: To determine the safety and effectiveness of transarterial embolization ablation (TEA) of hepatocellular carcinoma (HCC) with a lipiodol-ethanol mixture.
METHODS: Between January 1 and December 31, 2009, 15 patients with HCC (13 men/two women, aged 38-75 years) accepted TEA treatment and were enrolled in this study, including five newly diagnosed patients and 10 with refractory disease. Two months after TEA, angiography and contrast computed tomography (CT) were performed, and responses were assessed using a modified version of Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). The follow-up period was to June 30, 2010.
RESULTS: Every new case was treated once. Angiography was performed immediately after TEA, and showed that the tumor-feeding vessels were completely embolized and that lipiodol was densely deposited inside tumors. Two months after treatment, contrast CT showed no enhanced lesions. Alpha fetoprotein levels returned to normal in four patients and markedly decreased in another. mean ± SD survival after treatment was 10.8 ± 4.5 mo. All five patients survived during the follow-up period. Ten patients with refractory disease were treated a total of 14 times. Angiography immediately after TEA showed that blood flow to the tumors was obviously decreased in all cases, and contrast CT showed obvious depositions of lipiodol. Two months after treatment, the tumors had shrunk (6/10) or were stable (3/10). One had progressed after 2 mo and died of tumor rupture 3 mo after TEA. mean ± SD survival after treatment was 8.6 ± 4.3 mo; two patients survived during the follow-up period. Adverse effects included reversible hepatic decompensation, upper abdominal pain, and fever.
CONCLUSION: TEA is an effective therapy for patients with HCC and might be more effective than transcather arterial chemoembolization for treating refractory disease.
- Citation: Gu YK, Luo RG, Huang JH, Si Tu QJ, Li XX, Gao F. Transarterial embolization ablation of hepatocellular carcinoma with a lipiodol-ethanol mixture. World J Gastroenterol 2010; 16(45): 5766-5772
- URL: https://www.wjgnet.com/1007-9327/full/v16/i45/5766.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i45.5766
Hepatocellular carcinoma (HCC) is the fifth most common tumor worldwide and the third most common cause of tumor-related death[1-3]. HCC is not sensitive to radiotherapy or chemotherapy, therefore, surgery is still the treatment of choice. Unfortunately, < 30% of patients with HCC benefit from surgical resection because of unfavorable tumor location, stage or extent of disease, limited liver functional reserve, or high operative risk[4,5].
Minimal invasive ablation, a new treatment for HCC, achieves complete responses in > 80% of tumors < 3 cm in diameter. However, in tumors of 3-5 cm in diameter, the complete response rate is only 50%[6]. Therefore, ablation is not recommended for tumors > 5 cm. HCC is a hypervascular tumor that receives mostly hepatic arterial blood[7]. Since its introduction by Yamada et al[8,9] in the 1980s, transcatheter arterial chemoembolization (TACE) has been widely used to treat patients with inoperable liver tumors. The theoretical basis of TACE for treating HCC is that chemotherapeutic agents mixed with embolic material and injected into the hepatic artery will embolize the arteries that supply the tumor and kill the tumor cells[8,9]. However, Liu et al[7] also have reported that many HCC tumors, especially those > 5 cm in diameter, receive blood from both the hepatic artery and the portal vein. Therefore, TACE must be administered more than once, and tumor necrosis might still be incomplete[10-12].
Ethanol can kill tumor cells, and percutaneous ethanol injections have been used to treat unresectable small HCC tumors[13]. Ethanol can also produce permanent microcirculatory embolization that inhibits tumor growth by denaturing protein, coagulating platelets, and dehydrating vascular endothelial cells in tumors[14,15].
Transarterial embolization ablation (TEA) has been used to treat HCC with lipiodol-ethanol mixture (LEM)[16-18]. Compared with TACE, TEA has the following potential advantages: (1) LEM can permanently embolize tumor-feeding vessels that come from the hepatic artery and portal vein[15,17,19]; therefore, TEA might have a stronger antitumor effect than TACE; (2) after TACE, tumor and peritumoral normal tissues are ischemic, hypoxic, and secrete angiogenic substances that provide new conditions for tumor proliferation and recurrence[20,21], whereas TEA leads to infarction of the entire tumor and of the peritumoral normal liver tissue without collateral circulation; and (3) embolization of tumor vessels by LEM helps ethanol diffuse into the tumor[18,22-26]. It has been reported that the mean ± SD lipiodol retention rate (89.5% ± 10.7%) after TEA was significantly higher than after TACE (47.5% ± 21.2%) in liver lesions, and 1- and 2-year survival after TEA (93.3% and 80.0%, respectively) was also significantly higher than that after TACE (73.3% and 43.3%, respectively). Furthermore, the 1- and 2-year incidence of extrahepatic metastasis after TEA (both 0%) was substantially lower than it was after TACE (35.5% and 39.2%, respectively)[18].
In the present small pilot study, we sought to determine further the safety and effectiveness of TEA in treating patients with newly diagnosed and refractory HCC tumors.
The protocol was approved by the Institutional Review Board of the Department of Medical Imaging and Interventional Radiology, Cancer Center, Sun Yat-sen University, Guangzhou, Guangdong, China. A total of 15 HCC patients who provided written informed consent before being enrolled in the study were treated with TEA from January 1 to December 31, 2009. Inclusion criteria included accurate proof of HCC, refusal of or contraindication to surgical resection (five cases), refractory disease (10 cases, progressed after TACE), Eastern Cooperative Oncology Group performance status not greater than 2. Exclusion criteria included total serum bilirubin ≥ 50 μmol/L, serum albumin level < 28 g/L, and evidence of extrahepatic disease at presentation.
The demographic data of these patients are shown in Table 1. There were 13 men and two women with an average age of 55.7 ± 10.3 years (range: 38-75 years). All 15 patients were positive for hepatitis B and had concomitant cirrhosis. With regard to liver function status, 13 had Child-Pugh classification grade A and two had grade B. Of the 15, five had been recently diagnosed with single-nodule HCC (patients with new tumors and tumor diameter ≤ 5 cm) and 10 patients with refractory tumors, including five with huge lesions (> 5 cm) and five with diffuse lesions. The five patients recently diagnosed with single-nodule HCC were all α fetoprotein (AFP)-positive and the lesion diameters ranged from 2.6 to 4.8 cm (mean ± SD, 3.7 ± 1.0 cm). The lesions were in the right lobule in two patients and in the left in three patients. Three of the five patients with huge HCC were AFP-positive, and all lesions were in the right lobule and the lesion diameters ranged from 8 to 15 cm (10.8 ± 3.1 cm). The five patients with diffuse lesions were all AFP-positive.
| n | |
| Features | |
| Sex (M/F) | 13/2 |
| Age (yr) | 55.7 ± 10.3 |
| Seropositive for HBsAg/HCV | 15/0 |
| Underlying cirrhosis (+/-) | 13/2 |
| Radiological/histological evidence of HCC | 14/1 |
| Newly diagnosed/refractory cases | 5/10 |
| Serum AFP level (μg/L) | |
| ≤ 200 | 2 |
| > 200 | 13 |
| Liver function status | |
| Grade A/B | 13/2 |
| Feature of tumors | |
| Single nodule1 ( ≤ 5 cm) | 5 |
| Huge lesion2 (> 5 cm) | 5 |
| Diffused lesion3 | 5 |
After puncturing the right femoral artery with the Seldinger technique, a Yashiro (5F, Terumo Heart, Inc., Japan) or R-H (5F, Terumo Heart) catheter was inserted into the common hepatic or superior mesenteric artery through a 5-F catheter sheath. Angiography was then performed to evaluate tumor blood supply and the surrounding vascular anatomy. Next, a 3F microcatheter (Terumo Heart) was inserted into the catheter (which remained in the proximal hepatic artery) and was advanced into the tumor-feeding arteries. For diffuse tumors, the microcatheter was inserted into segmental or subsegmental liver arteries to avoid the gastroduodenal and cystic arteries.
One percent lidocaine (from 2% lidocaine with saline, 1:1 by volume), 5-10 mL, was injected through a 2.5-mL syringe and followed by injection of a mixture of lipiodol (Guerbert S.A., Villepinte, France) and absolute ethanol (Department of Pharmacy, General Hospital of Guangzhou Military Command, China), 1:1 by volume. The injection of LEM was stopped when the tumor-feeding arteries were occluded, or when injection reflux was noted, or when the amount injected reached 60 mL.
Follow-up was to June 30, 2010 and measurements consisted of the degree of tumor embolization as determined visually immediately after TEA by angiography, judged by two skilled radiologists, liver function [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] for 1-7 d after TEA, and AFP levels, computed tomography (CT) or magnetic resonance imaging, and changes of postoperative symptoms and signs up to 8 wk after treatment. Responses were assessed using a modified version of Response Evaluation Criteria in Solid Tumors (RECIST version 1.1)[27].
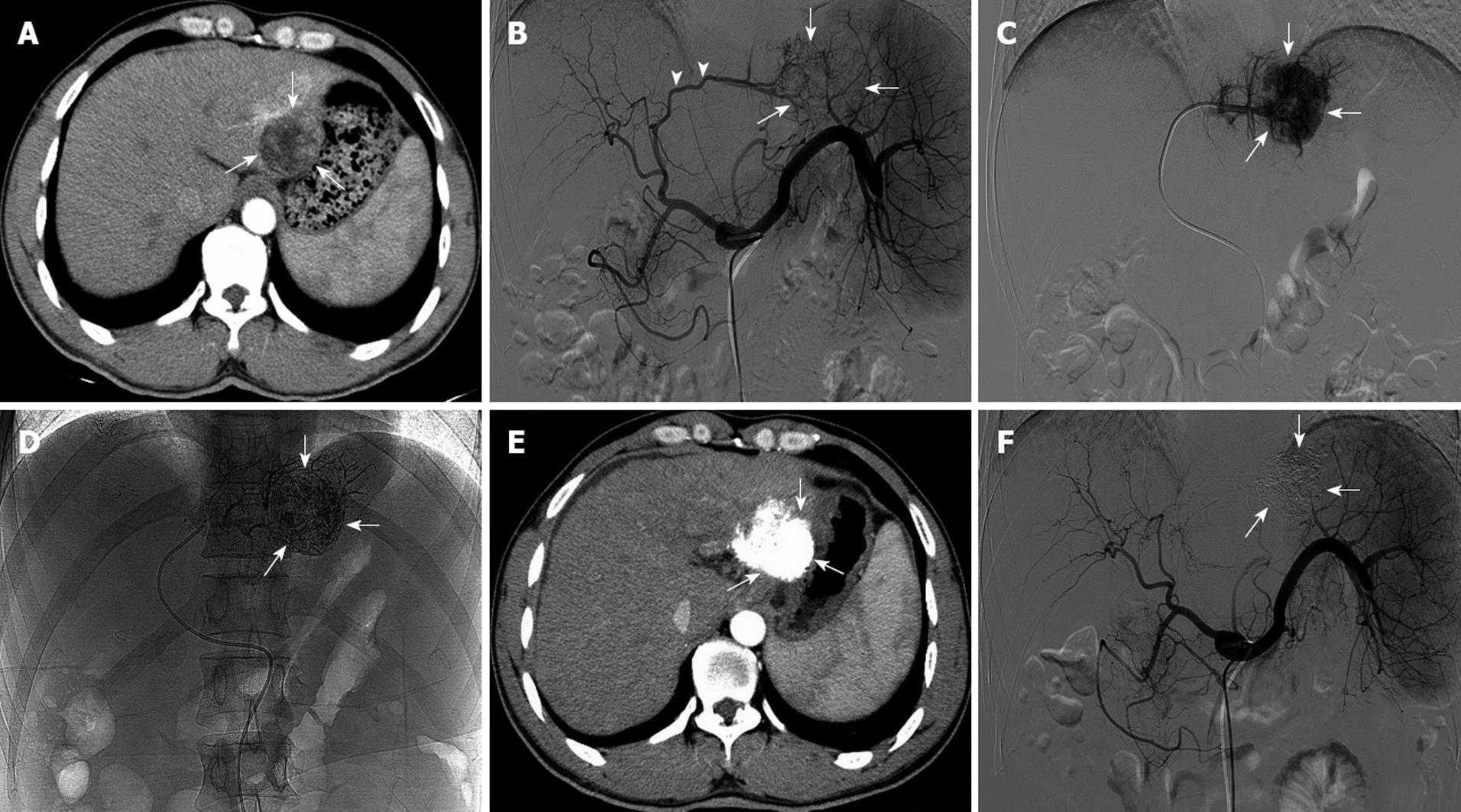
Every newly diagnosed patient was treated once. The maximum total dose of LEM was 54 mL (10.8 ± 5.4 mL). Angiography immediately after TEA showed that all tumor-feeding arteries were completely embolized and that lipiodol had been densely deposited inside tumors. CT images 2 mo after treatment showed lesions with non-enhancement that had been replaced by deposition of lipiodol (Figure 1). AFP levels returned to normal in four patients (before TEA: 2947.6 ± 3724.5 μg/mL; post-TEA: 13.7 ± 8.2 μg/mL; P = 0.04) and decreased substantially in another patient (from 37 625 μg/mL to 26 376 μg/mL). Mean survival after treatment was 10.8 ± 4.5 mo. All five patients survived during the follow-up period (Table 2).
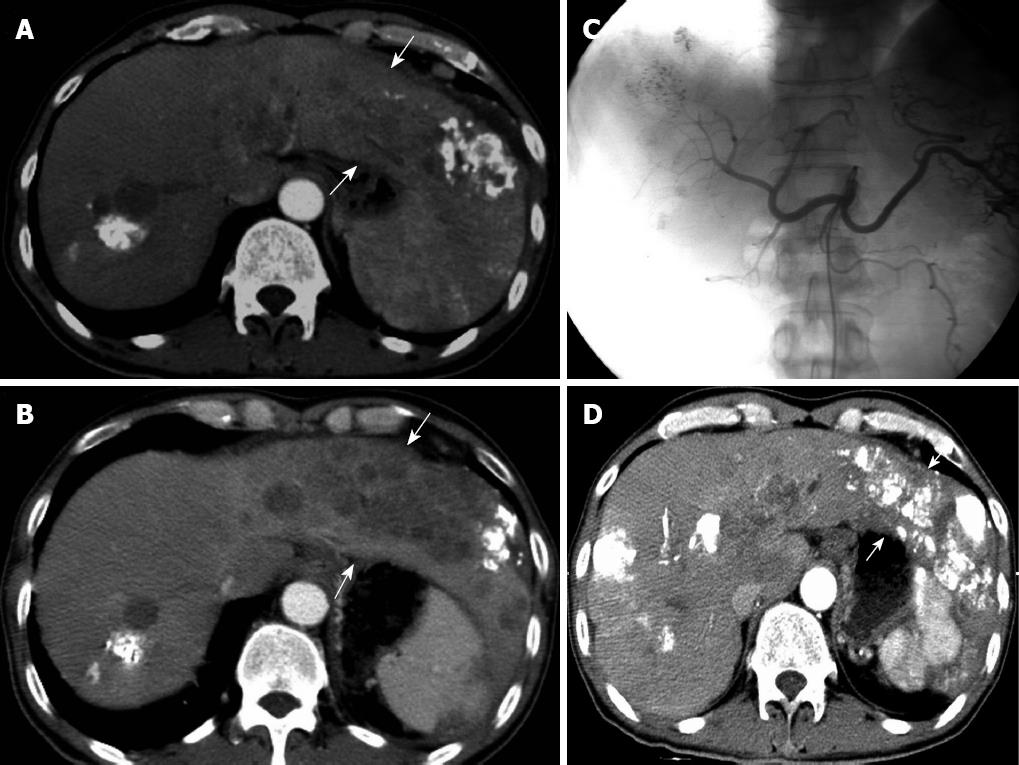
All refractory patients were treated a total of 14 times. The total dose of LEM was 256 mL (mean: 18.3 ± 13.5 mL). Angiography immediately after TEA showed that blood flow to the tumors was obviously decreased. Two months after treatment, CT showed that lipiodol was deposited more obviously than before and the tumors were smaller (6/10) or stable (3/10) in nine patients (Figure 2). For eight AFP-positive patients, AFP levels returned to normal in one patient, were decreased in six after a mean 2.5 ± 1.1 mo, and continued to rise in one. The mean survival was 8.6 ± 4.3 mo after treatment. Two patients survived during the follow-up period (Table 3).
Adverse events post-TEA are listed in Table 4. Of the 15 patients, 10 had transient elevations in transaminase after TEA, all of whom returned to normal after drug treatment to protect liver function. All patients had intraoperative or postoperative upper abdominal pain, which was relieved or eliminated after symptomatic treatment. Eleven patients had fever at 2-7 d after TEA but recovered with the administration of antipyretic drugs or dexamethasone (5-10 mg). There were no other serious adverse effects.
| Adverse events | n |
| Pain | 15 |
| Fever | 11 |
| Hepatic decompensation | 10 |
| Liver abscess | 0 |
| Biliary stricture or obstruction | 0 |
Currently, TACE has become an important palliative treatment for patients with inoperable HCC[5,11,12,28-30]. Embolization of tumor-feeding arteries also prolongs the exposure and concentration of tumor drugs and chemotherapeutic agents in the tumor microvascular bed, rather than allowing the drugs to reach the systemic circulation, which would reduce their effectiveness[5,8,9]. However, HCC tumors are occasionally partially supplied by the portal vein, especially when capsule formation around the tumor is incomplete[7,17,31]. After TACE, tumor blood supply from the portal vein increases, which makes achieving complete tumor necrosis difficult. In addition, embolization after TACE creates ischemia and hypoxia in the tumor and surrounding normal tissue, which leads tumor cells and peritumoral normal liver cells to secrete angiogenic factors, such as vascular endothelial growth factor and fibroblast growth factors, which promote collateral circulation and restoration of the tumor blood supply, which leads to tumor proliferation and recurrence[20,21]. To achieve complete tumor necrosis, the blood supply to the tumor from the liver artery and portal vein and fistula between them needs to be stopped, and the formation of collateral circulation needs to be prevented[32,33].
In animal experiments, Kan et al[15] have proved that transarterial injection of LEM achieves lobar ablation effects because it simultaneously embolizes the hepatic artery and portal vein. Ethanol is a strong protein coagulant that can inactivate tumor cells directly in situ and cause vascular endothelial injury and platelet cohesion, which results in permanent occlusion of tumor-feeding vessels and tumor infarction without causing collateral circulation[14,15].
In 1993, Matsui et al[17] described transarterial ethanol embolization of HCC. However, ethanol is not radio-opaque, and its flow and speed are difficult to visualize when it is administered. In contrast, LEM traces not only the ethanol, which is necessary to avoid regurgitation and ectopic embolization, but also enhances the effects of embolization. Therefore, we call the transarterial injection of LEM TEA to distinguish it from TACE by injection of gelatin sponge or other embolic materials.
We treated 15 HCC patients with a mixture of equal volumes of lipiodol and ethanol. The results showed that TEA could completely embolize tumor-feeding vessels, reduce the size of single-nodule HCC tumors, and decrease levels of AFP. Enhanced CT examinations of the liver showed no enhancement of lesions. For refractory HCC, TEA can also shrink tumors and decrease AFP levels.
Reports were varied about the effectiveness of different ratios of lipiodol to ethanol. Matsui et al[17] have treated 100 patients with 124 HCC tumors < 4 cm with equal volumes of lipiodol and ethanol through hepatic artery injection. Survival rates for 82 patients with Child class A or B disease at 1 and 4 years were 100% and 67%, respectively. Kan et al[15] have reported that embolic effects were better when the lipiodol:ethanol ratio was 5:1, 4:1, or 3:1 than if the ratio was 1:1. However, several studies have reported good results in treating HCC with transarterial injections with lipiodol:ethanol ratios between 3:1 and about 1:3[16,17,19,34-36].
We think that the lipiodol:ethanol ratio can be adjusted according to the type of HCC. For single-nodule HCC, a relatively high lipiodol ratio (such as 2-3:1) is appropriate because the tumors are relatively small, have a complete capsule, and have a blood supply that comes mainly from the hepatic artery, with few fistulae between the hepatic artery and the portal vein. The injury to small arteries is relatively slight because of the lower percentage of ethanol in the mixture, and accordingly, more LEM is deposited inside the tumor. Meanwhile, the uptake of lipiodol by liver cancer cells means that the mixture is retained longer in tumor cells and has more time to produce antitumor effects[15,37].
For refractory HCC, a relatively high ethanol ratio is appropriate to inhibit the double blood supply from the hepatic artery and the portal vein with fistulae between them. Ethanol can permanently embolize tumor-feeding vessels and block the fistulae, as well as reducing the loss of lipiodol and extending the exposure time in the tumor cells, which achieves a better therapeutic effect. However, further research is needed to determine the optimal lipiodol:ethanol ratios for different types of HCC.
The most common adverse events after TEA were liver dysfunction, fever, and upper abdominal pain. In this study, 10 of 15 patients experienced liver dysfunction, and mean levels of ALT and AST rose to 600-1200 U/L in serious cases; therefore, liver function needs to be monitored closely and protected after TEA. We usually review patient’s liver function the day after TEA and then every 2-3 d until it returns to normal or nearly normal. Antipyretic drugs or dexamethasone are usually effective in treating fever. Damage to the vascular wall caused by ethanol results in upper abdominal pain in all patients. We administer intramuscular injections of 10 mg diazepam at 10-30 min before treatment and 10 mg intramuscular morphine after catheterization of tumor-feeding arteries. We also inject 5-10 mL 1% lidocaine into the arteries before LEM. Patients generally tolerate TEA given this preparation. We stop injection of LEM for 1-2 min if patients feel pain and resume injection when the pain is relieved after injection of lidocaine through the catheter. For those who still feel pain after TEA, a fentanyl patch (4.2 mg from beginning) can be applied.
Ethanol is a strong protein coagulant and a permanent embolic material, but it can also injure normal liver tissue and vessels[4,16,27,35,36,38]. Therefore, tumor blood vessels must be selected and treated carefully to avoid severe liver damage, and even failure, and other serious complications caused by shallow intubation. On the other hand, excessively deep intubation can lead to incomplete embolization.
In addition, the speed of injection can also affect the efficacy of TEA. Blood dilutes ethanol and therefore will weaken the effect of ethanol on tumor cells if it is injected slowly, yet rapid injection might destroy blood vessel walls and form a vessel-casting mold. Injection of too much mixture can lead to reflux into normal liver tissues or to the blood vessels that supply the gastrointestinal tract. Therefore, research is needed into the effect of injection speed and on the dose of LEM.
Our study had some limitations. The sample size was small, but it was large enough to prove the concept. The follow-up after TEA was also short, and long-term efficacy needs to be studied further. In addition, some huge tumors had some necrosis before TEA, and the degree of tumor shrinkage was difficult to determine. Finally, we determined efficacy only on the basis of angiographic and CT imaging, without a postoperative pathological examination. These limitations can easily be avoided in larger, longer, and better-funded studies.
On the basis of our findings, we conclude that TEA is effective therapy for patients with HCC and might be better than TACE for treating refractory disease. Further studies, including randomized controlled trials, are warranted to confirm its role.
Transarterial embolization ablation (TEA) is a new treatment strategy for hepatocellular carcinoma (HCC). Some researchers have reported its effectiveness in HCC, especially in single-nodule HCC. However, its clinical effect for refractory HCC [not suitable for surgery and local ablation and with disease progression after transcatheter arterial chemoemblization (TACE)] remains unknown.
TACE is accepted widely in treating advanced HCC because of its confirmed clinical effect. Advanced HCC tumors often receive blood from the hepatic artery and portal vein. Therefore, TACE must be administered more than once, and tumor necrosis can still be incomplete. In the present study, the authors demonstrated that TEA with lipiodol-ethanol mixture was an effective therapy for patients with HCC and might be more effective than TACE for treating refractory disease.
TACE with a mixture of lipiodol and ethanol has been shown to be an effective treatment for intrahepatic lesions of HCC, although it has not been widely used or described. Recent reports have highlighted the importance of this new method for its good results in nodular or capsulated HCC. Furthermore, this study suggests that this new method can also be applied in advanced refractory HCC.
Due to the exciting results and acceptable adverse events, TEA could represent a future treatment strategy for advanced HCC.
Lipiodol is a very important embolization agent in TACE of HCC. Ethanol can kill tumor cells, and percutaneous ethanol injections have been used to treat unresectable small HCC tumors (chemical ablation). Ethanol can also produce permanent microcirculatory embolization that inhibits tumor growth by denaturing proteins, coagulating platelets, and dehydrating vascular endothelial cells in tumors. Transarterial lipiodol-ethanol mixture injection is called TEA.
The authors report on the prospective follow-up of 15 patients treated with lipiodol-ethanol injection. The results are interesting. They reveal that TEA is a effective and safe treatment for advanced HCC. A further comparative study with TACE is needed to re-evaluate this new method.
Peer reviewer: Michael A Fink, MBBS, FRACS, Department of Surgery, The University of Melbourne, Austin Hospital, Melbourne, Victoria 3084, Australia
S- Editor Sun H L- Editor Kerr C E- Editor Lin YP
| 1. | Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3:353-367. |
| 2. | Umemura T, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. Hepatol Res. 2007;37 Suppl 2:S95-S100. |
| 3. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. |
| 4. | Lin SM. Recent advances in radiofrequency ablation in the treatment of hepatocellular carcinoma and metastatic liver cancers. Chang Gung Med J. 2009;32:22-32. |
| 5. | Sutherland LM, Williams JA, Padbury RT, Gotley DC, Stokes B, Maddern GJ. Radiofrequency ablation of liver tumors: a systematic review. Arch Surg. 2006;141:181-190. |
| 6. | Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, Brú C, Bruix J. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352-1360. |
| 7. | Liu K, Lu J, Tan W. [Portal vein supply and embolization therapy for hepatocellular carcinoma]. Zhonghua Yixue Zazhi. 1995;75:403-405, 445. |
| 8. | Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397-401. |
| 9. | Yamada R, Nakatsuka H, Nakamura K, Sato M, Itami M, Kobayashi N, Minakuchi K, Onoyama T, Kanno T, Monna T. Hepatic artery embolization in 32 patients with unresectable hepatoma. Osaka City Med J. 1980;26:81-96. |
| 10. | Vogl TJ, Naguib NN, Nour-Eldin NE, Rao P, Emami AH, Zangos S, Nabil M, Abdelkader A. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol. 2009;72:505-516. |
| 11. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. |
| 12. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. |
| 13. | Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, Kondo F, Saisho H. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458-464. |
| 14. | Lin XD, Lin LW. Local injection therapy for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2006;5:16-21. |
| 15. | Kan Z, Wallace S. Transcatheter liver lobar ablation: an experimental trial in an animal model. Eur Radiol. 1997;7:1071-1075. |
| 16. | Yu SC, Hui EP, Wong J, Wong H, Mo F, Ho SS, Wong YY, Yeo W, Lai PB, Chan AT. Transarterial ethanol ablation of hepatocellular carcinoma with lipiodol ethanol mixture: phase II study. J Vasc Interv Radiol. 2008;19:95-103. |
| 17. | Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, Miyayama S, Takashima T, Unoura M, Kogayashi K. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79-83. |
| 18. | Yu SC, Hui JW, Hui EP, Mo F, Lee PS, Wong J, Lee KF, Lai PB, Yeo W. Embolization efficacy and treatment effectiveness of transarterial therapy for unresectable hepatocellular carcinoma: a case-controlled comparison of transarterial ethanol ablation with lipiodol-ethanol mixture versus transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2009;20:352-359. |
| 19. | Ito K, Kusunoki H, Okamoto E, Ozawa M, Ishikawa A, Matsuura M, Nakajima N. Intra-arterial alcoholization of advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33 Suppl:S42-S47. |
| 20. | Xiao EH, Guo D, Bian DJ. Effect of preoperative transcatheter arterial chemoembolization on angiogenesis of hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:4582-4586. |
| 21. | Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523-529. |
| 22. | Tanaka K, Okazaki H, Nakamura S, Endo O, Inoue S, Takamura Y, Sugiyama M, Ohaki Y. Hepatocellular carcinoma: treatment with a combination therapy of transcatheter arterial embolization and percutaneous ethanol injection. Radiology. 1991;179:713-717. |
| 23. | Bartolozzi C, Lencioni R, Caramella D, Vignali C, Cioni R, Mazzeo S, Carrai M, Maltinti G, Capria A, Conte PF. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812-818. |
| 24. | Hasuike Y, Okamura J, Furukawa J, Naoi M, Takata N, Maruyama H, Kinuta M, Yayoi E, Oi H, Okamoto S. Efficacy of combination treatment--(TAE with adriamycin and ethanol)--for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1992;31 Suppl:S30-S34. |
| 25. | Tanaka K, Nakamura S, Numata K, Okazaki H, Endo O, Inoue S, Takamura Y, Sugiyama M, Ohaki Y. Hepatocellular carcinoma: treatment with percutaneous ethanol injection and transcatheter arterial embolization. Radiology. 1992;185:457-460. |
| 26. | Lencioni R, Vignali C, Caramella D, Cioni R, Mazzeo S, Bartolozzi C. Transcatheter arterial embolization followed by percutaneous ethanol injection in the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1994;17:70-75. |
| 27. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. |
| 28. | Vogl TJ, Zangos S, Eichler K, Yakoub D, Nabil M. Colorectal liver metastases: regional chemotherapy via transarterial chemoembolization (TACE) and hepatic chemoperfusion: an update. Eur Radiol. 2007;17:1025-1034. |
| 30. | Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796-802. |
| 31. | Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493-497. |
| 32. | Matsui O, Kawamura I, Takashima T. Occurrence of an intrahepatic porto-arterial shunt after hepatic artery embolization with Gelfoam powder in rats and rabbits. Acta Radiol Diagn (Stockh). 1986;27:119-122. |
| 33. | Demachi H, Matsui O, Takashima T. Scanning electron microscopy of intrahepatic microvasculature casts following experimental hepatic artery embolization. Cardiovasc Intervent Radiol. 1991;14:158-162. |
| 34. | Cheung YC, Ko SF, Ng SH, Chan SC, Cheng YF. Survival outcome of lobar or segmental transcatheter arterial embolization with ethanol-lipiodol mixture in treating hepatocellular carcinoma. World J Gastroenterol. 2005;11:2792-2795. |
| 35. | Cheng Y, Kan Z, Chen C, Huang T, Chen T, Yang B, Ko S, Lee T. Efficacy and safety of preoperative lobar or segmental ablation via transarterial administration of ethiodol and ethanol mixture for treatment of hepatocellular carcinoma: clinical study. World J Surg. 2000;24:844-850; discussion 850. |
| 36. | Yu SC, Leung TW, Lau WY, Lee N, Hui EP, Yeo W, Lai PB, Mok TS. A comparison of three transarterial lipiodol-based formulations for hepatocellular carcinoma: in vivo biodistribution study in humans. Cardiovasc Intervent Radiol. 2008;31:289-298. |
| 37. | Kan Z, Ivancev K, Lunderquist A. Peribiliary plexa--important pathways for shunting of iodized oil and silicon rubber solution from the hepatic artery to the portal vein. An experimental study in rats. Invest Radiol. 1994;29:671-676. |
| 38. | Yu SC, Chan CT. Transarterial ethanol ablation of cirrhotic liver with lipiodol-ethanol mixture: safety and efficacy study in rats. Invest Radiol. 2006;41:609-617. |