Published online Nov 14, 2010. doi: 10.3748/wjg.v16.i42.5367
Revised: August 2, 2010
Accepted: August 9, 2010
Published online: November 14, 2010
AIM: To investigate the feasibility of radionuclide therapy of colon tumor cells by baculovirus vector-mediated transfer of the sodium/iodide symporter (NIS) gene.
METHODS: A recombinant baculovirus plasmid carrying the NIS gene was constructed, and the viruses (Bac-NIS) were prepared using the Bac-to-Bac system. The infection efficiency in the colon cancer cell line SW1116 of a green fluorescent protein (GFP) expressing baculovirus (Bac-GFP) at different multiplicities of infection (MOI) with various concentrations of sodium butyrate was determined by flow cytometry. An in vitro cytotoxicity assay was also conducted after infection of SW1116 cells with Bac-NIS. Iodine uptake of Bac-NIS infected SW1116 cells and inhibition of this uptake by sodium perchlorate was examined, and the effect of Bac-NIS-mediated 131I in killing tumor cells was evaluated by cell colony formation tests.
RESULTS: Infection and transgene expression in SW1116 with Bac-GFP were significantly enhanced by sodium butyrate, as up to 72% of SW1116 cells were infected with the virus at MOI of 400 and sodium butyrate at 0.5 mmol/L. No obvious cytotoxicity was observed under these conditions. Infection of SW1116 with Bac-NIS allowed uptake of 131I in these tumor cells, which could be inhibited by sodium perchlorate. The viability of SW1116 cells infected with Bac-NIS was significantly lower than with Bac-GFP, suggesting that NIS gene-mediated 131I uptake could specifically kill tumor cells.
CONCLUSION: Baculovirus vector-mediated NIS gene therapy is a potential approach for treatment of colon cancer.
- Citation: Yin HY, Zhou X, Wu HF, Li B, Zhang YF. Baculovirus vector-mediated transfer of NIS gene into colon tumor cells for radionuclide therapy. World J Gastroenterol 2010; 16(42): 5367-5374
- URL: https://www.wjgnet.com/1007-9327/full/v16/i42/5367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i42.5367
Colon cancer is one of the most common malignant gastrointestinal tumors with a high morbidity and mortality. Currently, surgery and chemotherapy are the primary methods for treating colon cancer. However, surgical removal of the tumor is not an option for 30%-40% of these patients upon diagnosis because of metastasis; meanwhile, chemotherapy shows a relatively high incidence of severe side effects. Therefore, it is important to find alternative ways to improve the prognosis of patients with colon cancer.
The cloning of the sodium/iodide symporter (NIS) gene and subsequent studies of its properties have resulted in a new approach to targeted radiotherapy of malignant cancers. NIS is a transmembrane glycoprotein located in the basal membrane of thyroid follicular cells. It can effectively co-transport two Na+ and one I- into cells[1], and this transportation of iodine is an active process against the chemical gradient. The uptake of radioactive 131I can be achieved by the expression of the NIS protein in tumor cells via vector-mediated gene transfer, and the radiotherapeutic destruction of the tumor occurs by emission of β rays from 131I.
Recently, several researchers have treated tumors by delivering the NIS gene via vectors based on retroviruses[2], adenoviruses[3] and lentiviruses[4], and achieved a certain level of therapeutic effect. For the purpose of gene transfer, an ideal vector would have high transduction efficiency and robust protein expression so that the uptake of radioactive 131I in tumor cells will reach an effective radiotherapeutic dose. The adenovirus vector system does in fact have high transduction efficiency, but its high immunogenicity and cytotoxicity prevent its further application in vivo. The major concern regarding the retroviral or lentiviral system is that the transgenes are easily integrated into the genomic DNA of host cells, which may cause changes in the target genome expression and result in oncogenicity in vivo. Moreover, long-term gene expression is not necessary in therapeutic gene-mediated radiotherapy.
We previously performed a pilot study using a baculovirus vector to deliver the NIS gene as a reporter[5]. Our results showed that the large NIS gene could be inserted into the recombinant baculovirus. A follicular thyroid cancer cell line, FTC-133, infected with recombinant green fluorescent protein (GFP) baculovirus previously showed a high expression of GFP, which lasted approximately seven days[5]. Considering the above characteristics, we reasoned that a recombinant NIS gene baculovirus may be beneficial for mediating 131I uptake and the killing of tumor cells. Furthermore, since baculoviruses can only infect arthropods, this vector is highly safe for mammals. In fact, recombinant baculoviruses have been widely used in mammalian cells as a gene transfer vector because of these advantages[6]. However, no reports are available on baculovirus vector-mediated radiotherapy for cancer.
In this study, the recombinant sodium/iodide symporter gene baculovirus (Bac-NIS) was generated and used to infect colon cancer cells to investigate the expression and function of NIS in these cells and to provide an experimental basis for the NIS gene-mediated targeted radiotherapy of colorectal cancer.
The baculovirus vector pFBGFPR (kindly provided by the Molecular Institute of the University of Hong Kong) was digested with restriction endonucleases Acc I and Age I. The digested ends of the vector were filled in with Klenow fragment, yielding a 6 kb pFB fragment which was then purified. The target gene was prepared by digesting the recombinant plasmid pCDNA-NIS (kindly provided by Professor Sissy Jhiang of Ohio University, USA) with restriction endonucleases BamHI and XhoI and filled in with Klenow kinase to obtain the 2.3 kb NIS gene fragment. The purified NIS target gene fragment was ligated to the phosphatase-treated pFB vector using T4 bacteriophage DNA ligase. The resulting plasmid was transformed into DH5α competent bacteria (Tiangen Biotech Co., Ltd., Shanghai) and screened on ampicillin Luria Burtani (LB) culture plates. White colonies were selected and screened for the correct orientation of the inserted target gene fragment by sequencing. The confirmed recombinant baculovirus pFBNIS plasmid DNA was amplified and purified.
The baculovirus vector plasmid pFBGFPR and the constructed recombinant plasmid pFBNIS were transformed into DH10 competent bacteria (Invitrogen). The successfully transpositioned white colonies were selected and amplified. Bacmid plasmids expressing the NIS gene were extracted and transfected into Spodoptera frugiperda (sf9) cells using Lipofectamine 2000 (Invitrogen). The supernatant of the transfected cells containing the virus was collected 5-7 d later. Sf9 cells were infected at a multiplicity of infection (MOI) of 0.1 and harvested after 5-7 d in culture by centrifuging at 10 000 ×g for 15 min. The resulting supernatant was collected, centrifuged again at a high speed of 10 000 ×g at 4°C for 5 min, sterilized with a 0.45 μm filter and preserved at 4°C in the dark. Plaque assays were used to determine the titer of baculovirus stocks.
SW1116 (human Caucasian colon adenocarcinoma) was cultured in DMEM (Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL) and 1% penicillin/streptomycin (Gibco-BRL) in a humidified environment with 5% CO2 at 37°C.
SW1116 cells were seeded in 6-well or 24-well plates with DMEM with 10% FBS at least 24 h before infection. The medium was changed to fresh serum-free DMEM just before infection. After infection with the baculovirus vector at indicated MOIs, the cells were incubated with serum-free DMEM for 4 h, followed by addition of FBS to the final concentration of 10%. All infections were performed in triplicate.
SW1116 cells were seeded in 6-well plates at a density of 1 × 105 cells/well and cultured with DMEM without serum for 24 h. Recombinant green fluorescent protein gene baculovirus (Bac-GFP) was then added at an MOI of 400, and sodium butyrate was added at different concentrations (0, 0.5, 1 and 2 mmol/L) simultaneously. All experiments were performed in triplicate. Cells were incubated at 37°C for 4 h after treatment, and FBS was added to the final concentration of 10% and incubated for another 24 h. The expression of GFP and the mean fluorescence intensity were determined by flow cytometry. The excitation wavelength of blue laser was 488 nm, and the detection wavelength was 520 nm.
SW1116 cells were seeded in 6-well plates at a density of 1 × 105 cells/well, and cultured with serum-free DMEM for 24 h. Bac-GFP was added at the MOI of 0, 100, 200, or 400. The final concentration of sodium butyrate was 0.5 mmol/L, and cells without sodium butyrate were used as negative controls at various MOI of Bac-GFP. All experiments were performed in triplicate. After treatment, the cells were incubated at 37°C for 4 h, and FBS was added to reach the final concentration of 10% and incubated at 37°C for another 24 h. The expression of GFP was observed under an inverted phase-contrast fluorescence microscope. The expression of GFP and the mean fluorescence intensity were detected by flow cytometry.
SW1116 cells were seeded in 6-well plates at a density of 1 × 104 cells/well and cultured with serum-free DMEM for 24 h. Bac-GFP was added at the MOI of 0, 50, 100, 200 or 400. For testing the effect of sodium butyrate, the final concentration was 0, 0.5, 1, and 2 mmol/L with Bac-GFP at an MOI of 400, and wells without sodium butyrate were used as the control well at an MOI of 400. After treatment, the cells were incubated at 37°C for 4 h, FBS was then added to reach the final concentration of 10% and incubated at 37°C for another 24 h. Twenty microliters of 5 g/L MTT (Beyotime Co., Ltd., Shanghai) were added to each well and incubated for another 4 h. Subsequently, 100 μL DMSO was added, and the plate was gently shaken to completely dissolve the blue-violet precipitate. The absorption at 490 nm (A490) was determined using a microplate reader. Cell viability = test well A490/control well A490× 100%.
SW1116 cells were seeded in 6-well plates at a density of 5 × 104 cells/well and cultured with serum-free DMEM for 24 h. Bac-NIS was added at the MOI of 0, 50, 100, 200 or 400. The final concentration of sodium butyrate was 0.5 mmol/L, and cells without sodium butyrate were used as negative controls at various MOI of Bac-NIS. All experiments were performed in triplicate. After treatment, cells were incubated at 37°C for 4 h, FBS was then added to reach the final concentration of 10% and incubated at 37°C for another 24 h. The cells were washed twice with bHBSS solution (HBSS solution buffered with HEPES solution to achieve a pH 7.3), and then 0.5 mL bHBSS solution containing 0.1 μCi Na 125I (Shanghai Hinko Co.) with 1 μmol/L NaI were added into each well as reported by Weiss et al[7]. Cells were incubated at 37°C for 30 min, washed with cold bHBSS solution three times and incubated for another 20 min with 1 mL 100% cold ethanol. The samples were then collected to determine the radioactive counts per minute (cpm) by a well γ-counter.
SW1116 cells were seeded in 6-well plates at a density of 5 × 104 cells/well and cultured with serum-free DMEM for 24 h. Bac-NIS and Bac-GFP were added at the MOI of 400 respectively. The final concentration of sodium butyrate was 0.5 mmol/L, and Bac-GFP was used as negative controls. All experiments were performed in triplicate. After treatment, cells were incubated at 37°C for 4 h, FBS was then added to reach the final concentration of 10% and incubated at 37°C for another 24 h. Cells were washed twice with bHBSS solution, and 0.5 mL bHBSS solution containing 0.1 μCi Na 125I with 1 μmol/L NaI were added into each well, incubated for 5, 10, 15, 30, 60, 90, or 120 min, washed with cold bHBSS solution three times, and incubated for another 20 min with 1 mL 100% cold ethanol. The samples were then collected to determine the radioactive cpm by a well γ-counter.
SW1116 cells were seeded in 6-well plates at a density of 5 × 104 cells/well and cultured with serum-free DMEM for 24 h. Bac-NIS was added at the MOI of 400. The final concentration of sodium butyrate was 0.5 mmol/L. All experiments were performed in triplicate. After treatment, cells were incubated at 37°C for 4 h, and FBS was added to reach the final concentration of 10% and incubated at 37°C for another 24 h. Cells were washed twice with bHBSS solution. Subsequently, 0.5 mL bHBSS solution containing 0.1 μCi Na 125I was added into each well with NaClO4 at concentrations of 0, 30, or 300 μmol/L. After incubation for 30 min, the cells were washed with cold bHBSS solution three times and incubated for another 20 min with 1 mL 100% cold ethanol. The samples were then collected to determine the radioactive cpm by a well γ-counter.
SW1116 cells were inoculated in 6-well plate at a density of 5 × 104 cells/well and cultured with serum-free DMEM for 24 h. Bac-NIS and Bac-GFP were added at an MOI of 400 with sodium butyrate at 0.5 mmol/L respectively. Bac-GFP was used as the negative control. The same volume of bHBSS solution was added into a blank well as a control. All experiments were performed in triplicate. After treatment, cells were incubated at 37°C for 4 h, and FBS was added to reach the final concentration of 10% and incubated at 37°C for another 24 h. Cells were washed twice with bHBSS solution. After 0.5 mL bHBSS solution containing 0.1 μCi Na 125I was added into each well and incubated for another 6 h, the cells were washed twice with bHBSS solution, digested, counted, seeded in a 6-well plate (1000 cells/well) and incubated at 37°C for additional 7 d. Cells were then washed three times with bHBSS solution and stained with crystal violet solution (Sigma). Cell viability was determined in the infected cells by counting colonies visible to the naked eyes and calculated as a percentage of the colonies that were treated with only bHBSS[8].
All data are presented as mean ± SD. Differences were considered significant at P < 0.05. Statistical analysis was performed using SPSS 11.0 software.
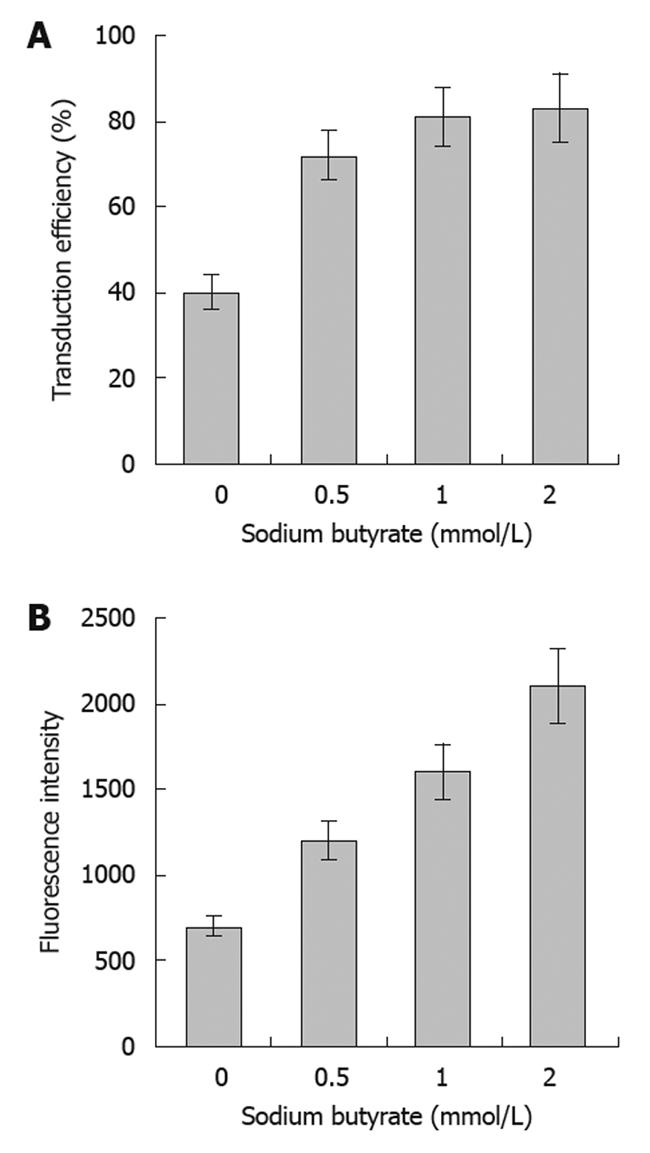
The transduction efficiencies of baculovirus in SW1116 cells were 39%, 71%, 80% and 83% at an MOI of 400 with concentrations of sodium butyrate of 0, 0.5, 1 or 2 mmol/L, respectively. This result indicated that sodium butyrate could significantly improve the transduction efficiency of baculovirus in SW1116 cells, achieving high levels of transduction at concentrations above 0.5 mmol/L. The fluorescence intensity of the infected SW1116 cells increased along with sodium butyrate in a dose-dependent manner, suggesting that the expression of the reporter protein was enhanced by the sodium butyrate (Figure 1).
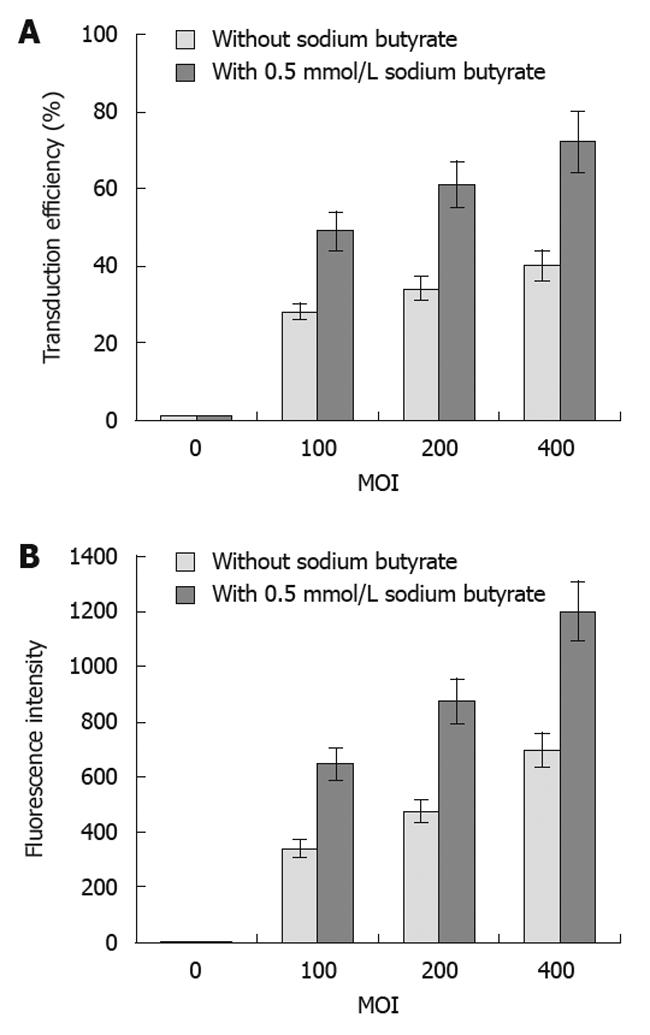
The transduction efficiency (Figure 2A) of baculovirus (Bac-GFP) in the SW1116 tumor cells gradually increased, and the fluorescence intensity (Figure 2B) was also enhanced with increasing MOI. Additionally, sodium butyrate could significantly improve the transduction efficiency and fluorescence intensity of tumor cells by Bac-GFP at different MOI (Figure 2). The sodium butyrate-mediated transduction efficiency of baculovirus inoculated at the MOI of 400 in SW1116 cells was 72%.
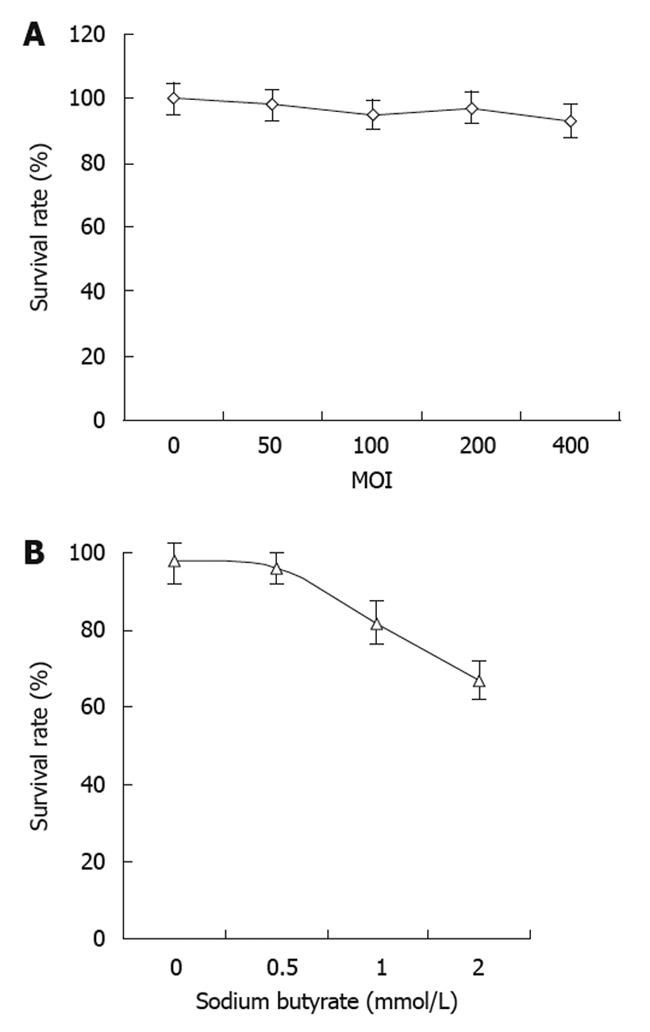
There was no obvious change in cell viability with the dose escalation of recombinant baculovirus (Figure 3A). There was no significant cell death observed at the highest MOI of 400. These results suggested that the baculovirus itself had no obvious cytotoxic effects on the tumor cells. On the other hand, the tumor cell viability decreased with increasing concentrations of sodium butyrate with virus at the MOI of 400 (Figure 3B). However, the cell viability did not change significantly when the concentration of sodium butyrate was lower than 0.5 mmol/L.
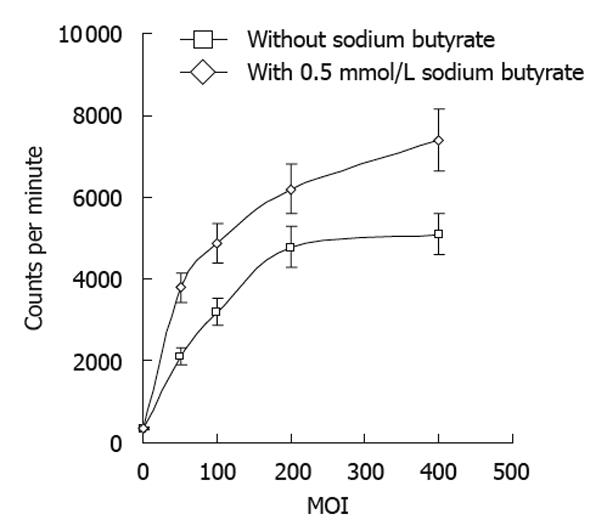
The radioiodine uptake of tumor cells increased with increasing doses of Bac-NIS, and this uptake was significantly enhanced by sodium butyrate (Figure 4).
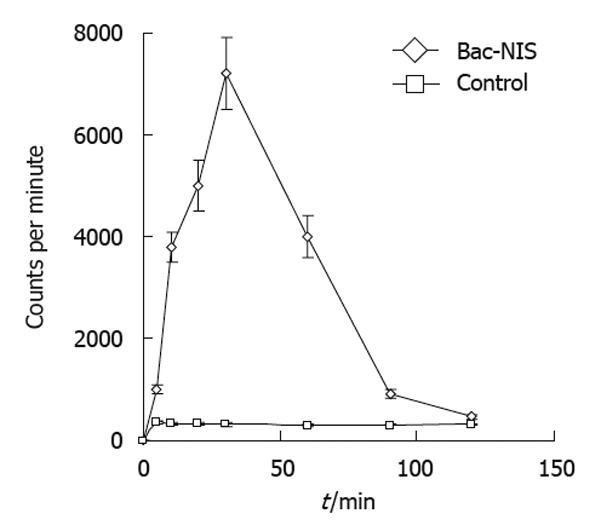
The radioiodine uptake of infected tumor cells increased over the incubation time with radioiodine which reached the peak at 30 min with Bac-NIS at the MOI of 400 and sodium butyrate at 0.5 mmol/L (Figure 5). This result suggested that the NIS protein expressed in transfected tumor cells could perform its normal function in iodine uptake.
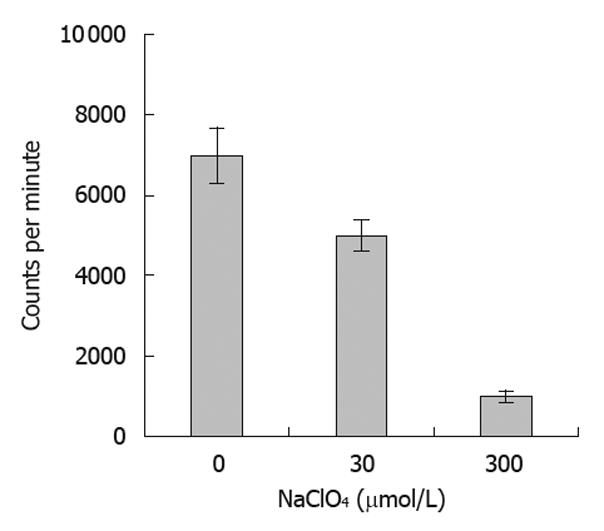
NIS-mediated iodine uptake in thyroid tissues can be specifically inhibited by ClO4-. In the present study, we tested the ability of ClO4- at various concentrations to inhibit the iodine uptake by tumor cells expressing the NIS protein. Our results showed that the radioiodine uptake of SW1116 cells was significantly reduced with escalating doses of ClO4- (Figure 6), indicating that the function of the expressed NIS protein in the infected tumor cells could also be inhibited by the presence of NaClO4.
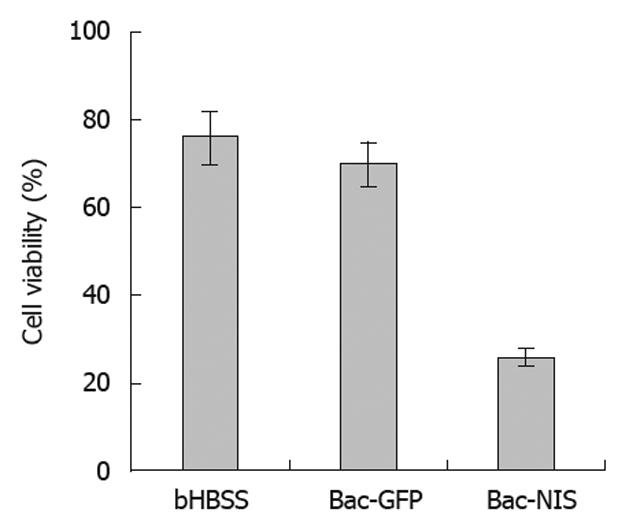
In order to evaluate the killing effect of 131I in infected tumor cells, a cell colony formation test was performed with cells infected by Bac-GFP as the control. The results showed that the cell viability of SW1116 cells infected with Bac-NIS was reduced to about 20%, and that of SW1116 cells infected with Bac-GFP (70%) was similar to that of the non-infected group (76%) (Figure 7). These observations suggested that NIS-mediated tumor cells could be effectively and specifically killed by 131I.
Since 1996 when Dai et al[9] and Smanik et al[10] cloned the rat and human NIS genes, respectively, researches on the function of these genes have progressed with a breakthrough in the understanding of their role in iodine uptake of thyroid cells. NIS can mediate the active transport of iodine ions. In addition to thyroid tissues, NIS is expressed at low levels in salivary glands, gastric mucosa tissues, breast tissues in secretory phase and other tissues[11]. The 131I therapy for differentiated thyroid carcinoma is performed by expressing the NIS gene in clinical applications.
There have been significant advances in the field of radiotherapy by NIS gene transfer using either non-viral or viral vectors in recent years. Boland et al[12] investigated the use of targeted radiotherapy in several tumor cell lines (including cervix cancer, breast cancer and prostate carcinoma) by adenovirus-mediated NIS gene transfer and showed uptake of iodine in tumor cells. Sieger et al[13] screened stable cell lines expressing the NIS gene from a retrovirus-infected hepatoma cell line (MH3924A) and showed significant radioiodine uptake as well. While Haberkon et al[14] found a low absorbance of radioactive iodine in infected rat prostate carcinoma cells by a retrovirus expressing the NIS gene due to the rapid clearance of iodine in transplanted tumors, Dingli et al [15] achieved considerable therapeutic effects by lentivirus-mediated NIS gene transfer in myeloma cells. All of these studies suggest that NIS gene is a promising candidate for targeted tumor radiotherapy.
Presently, viral vectors most commonly used for gene transfer are based on adenoviruses, retroviruses and lentiviruses. Adenoviruses can induce strong host immune responses[16], and antibodies against adenovirus has been found in 97% of the population[17]. These pre-existing antibodies against the viral vector can inhibit the efficiency of gene transfer. Furthermore, transgenes carried by lentiviruses and retroviruses can randomly insert into the genomic DNA of the host cells and potentially activate oncogenes in normal tissues.
Therefore, it is critical to choose a proper vector system to deliver the gene of interest in gene therapy. The baculovirus vector system has a high transduction efficiency and protein expression in mammalian cells[18]. It is a replication competent vector that displays less cytotoxicity to mammalian cells when compared with other viral vectors. Because the baculovirus DNA does not integrate into the host genome, baculovirus vectors have an extremely high biological safety profile. Since baculoviruses also have very species-specific tropisms among the invertebrates, there would be virtually no pre-existing neutralizing antibody and specific T cells in mammals to prevent the application of these vectors in vivo. Meanwhile, the packaging capacity of baculovirus is about 38 kb, allowing insertion and expression of large genes, and high titer virus stocks can easily be produced[19]. All these advantages show that the baculovirus is a promising vector for use in gene transfer applications.
In this study, we successfully cloned and purified baculovirus with the NIS gene driven by the cytomegalovirus immediate-early gene promoter. Some studies have shown that the transduction efficiency of recombinant baculovirus can be increased by adding sodium butyrate in SW1116 cells. Infection of Bac-NIS in SW1116 cells showed that it can uptake iodine, and that this process can be specifically inhibited by sodium perchlorate. This suggested that the NIS protein expressed by baculoviruses functioned similarly to the natural NIS protein. The proliferation of SW1116 cell was significantly lower in cells infected with Bac-NIS than those infected with Bac-GFP, indicating that tumor cells infected with Bac-NIS could specifically uptake 131I, and as a result the emitted β-rays could kill the target cells effectively. These experiments provided solid evidence for the feasibility of radionuclide therapy for colon cancer.
The expression level of an exogenous gene positively correlates with the amount of stable transgenes in host cells, and the regulation of the transgene in mammalian cells is the key to its expression. Modifications of the transgenes such as acetylation, methylation and/or the chromatin compactness may play important roles in the expression of the transgenes carried by the baculovirus. Sodium butyrate is a four-carbon short-chain fatty acid derived from dietary fiber in the intestinal tract via bacterial fermentation. The concentration of sodium butyrate is very high in the intestinal tract of healthy individuals especially those with a high-fiber diet. Sodium butyrate is a histone deacetylase inhibitor that has been shown to relax chromatin by inducing the acetylation of chromosomes which can in turn facilitate gene expression. Furthermore, it can also significantly improve the transduction efficiency of baculovirus and the expression level of exogenous genes[19]. In this study, we found that sodium butyrate could significantly improve the transduction efficiency of baculovirus and the expression of the NIS transgene in SW1116 cells which led to a significant killing effect (viability = 20%) of SW1116 tumor cells. Therefore, baculovirus-mediated gene transfer of the NIS gene allows targeted radiotherapy for intestinal tumors.
The therapeutic effect of radioiodine therapy depends on the absorbance of target tissues which are affected by the effective half-life of 131I in tumors, the total mass of tumor and the total absorbed dose of 131I. However, iodine uptake in SW1116 cells mediated by NIS gene transfer is rapidly cleared from the body. This is different from the observation in thyroid cells due to the absence of thyroid peroxidase (TPO) in SW1116 cells. Therefore, the organification of inorganic iodine taken up by SW1116 cells to produce thyroid hormone for storage cannot take place, ultimately affecting the therapeutic effect for tumors that can be achieved with 131I. Thus, an important issue in NIS gene-mediated tumor therapy is the improvement of the retention of 131I in tumor cells. Huang et al[20] transfected NIS and the TPO gene together into non-small cell lung cancer cell lines and found that the rate of clearance of iodine was reduced. However, it is still controversial whether or not the organification of iodine mediated by TPO would increase the retention of iodine[21].
Various approaches to improve radiotherapy have been reported. It has been shown that NIS-mediated 188Re (Rhenium) radiating β-rays [22] and 211At (Astatine) radiating α-rays[23] in various tissues resulted in better radiotherapeutic effects than with 131I due to higher radiation energy deposition. Additionally, as the serum complement system is the primary inhibitory factor of baculovirus-mediated gene transfer, some researchers have created certain modifications in baculovirus to prevent inactivation by complement in serum. For example, human decay-accelerating factor expressed on the envelope of the virion could render recombinant baculovirus resistant to the complement system, and the expression of transgene could be detected when the modified baculovirus was injected into murine hepatic parenchyma[24]. The inhibition of serum inactivation can also be achieved by injecting complement inhibitors such as cobra-venom factor prior to baculovirus vector delivery[25]. Thus, baculovirus vectors will provide a new approach for the gene therapy of malignant tumors especially when the related techniques are improved.
Sodium/iodide symporter (NIS) is a transmembrane glycoprotein located in the basal membrane of thyroid follicular cells. It can effectively co-transport two Na+ and one I- into cells, and this transportation of iodine is an active process against the chemical gradient. The uptake of radioactive 131I can be achieved by the expression of the NIS protein in tumor cells via vector-mediated gene transfer, and the radiotherapeutic destruction of the tumor occurs by emission of β rays from 131I.
It is a new approach to use NIS gene in radiotherapeutic destruction of the tumor. The authors adopted Bac-NIS to transduce colon cancer cell line, SW1116, to investigate the feasibility of NIS as a therapeutic gene in 131I mediated cell killing. There is currently no report on this.
This is the first report about the baculovirus vector-mediated radiotherapy for colon cancer cell line. NIS was used as target gene, and the results showed that the transduction of baculovirus in cancer cells increased in the presence of sodium butyrate and cancer cells infected with Bac-NIS could be rendered 131I uptake and specifically killed by β-rays from 131I.
Baculovirus vectors will provide a new approach for the gene therapy of malignant tumors especially when the related techniques are improved.
NIS is a transmembrane glycoprotein located in the basal membrane of thyroid follicular cells.
131I uptake in cancer cell mediated by NIS gene transfer is rapidly cleared. The therapeutic effects of radiotherapy will be improved with the further research in this field.
Peer reviewer: Kevin Cheng-Wen Hsiao, MD, Assistant Professor, Colon and Rectal Surgery, Tri-Service General Hospital, No. 325, Sec. 2, Cheng-Kung Rd, Nei-Hu District, Taipei 114, Taiwan, China
S- Editor Cheng JX L- Editor Ma JY E- Editor Lin YP
| 1. | Dohán O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48-77. |
| 2. | Kim SH, Chung HK, Kang JH, Kim KI, Jeon YH, Jin YN, Yun CO, Chung JK. Tumor-targeted radionuclide imaging and therapy based on human sodium iodide symporter gene driven by a modified telomerase reverse transcriptase promoter. Hum Gene Ther. 2008;19:951-957. |
| 3. | Dwyer RM, Bergert ER, O'connor MK, Gendler SJ, Morris JC. In vivo radioiodide imaging and treatment of breast cancer xenografts after MUC1-driven expression of the sodium iodide symporter. Clin Cancer Res. 2005;11:1483-1489. |
| 4. | Liu RS, Hsieh YJ, Ke CC, Chen FD, Hwu L, Wang FH, Hwang JJ, Chi CW, Lee CH, Yeh SH. Specific activation of sodium iodide symporter gene in hepatoma using alpha-fetoprotein promoter combined with hepatitis B virus enhancer (EIIAPA). Anticancer Res. 2009;29:211-221. |
| 5. | Zhou X, Li B, Wang J, Yin H, Zhang Y. The feasibility of using a baculovirus vector to deliver the sodium-iodide symporter gene as a reporter. Nucl Med Biol. 2010;37:299-308. |
| 6. | Hu YC. Baculoviral vectors for gene delivery: a review. Curr Gene Ther. 2008;8:54-65. |
| 7. | Weiss SJ, Philp NJ, Grollman EF. Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology. 1984;114:1090-1098. |
| 8. | Kogai T, Schultz JJ, Johnson LS, Huang M, Brent GA. Retinoic acid induces sodium/iodide symporter gene expression and radioiodide uptake in the MCF-7 breast cancer cell line. Proc Natl Acad Sci USA. 2000;97:8519-8524. |
| 9. | Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458-460. |
| 10. | Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, Jhiang SM. Cloning of the human sodium lodide symporter. Biochem Biophys Res Commun. 1996;226:339-345. |
| 11. | Spitzweg C, Harrington KJ, Pinke LA, Vile RG, Morris JC. Clinical review 132: The sodium iodide symporter and its potential role in cancer therapy. J Clin Endocrinol Metab. 2001;86:3327-3335. |
| 12. | Boland A, Ricard M, Opolon P, Bidart JM, Yeh P, Filetti S, Schlumberger M, Perricaudet M. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000;60:3484-3492. |
| 13. | Sieger S, Jiang S, Schönsiegel F, Eskerski H, Kübler W, Altmann A, Haberkorn U. Tumour-specific activation of the sodium/iodide symporter gene under control of the glucose transporter gene 1 promoter (GTI-1.3). Eur J Nucl Med Mol Imaging. 2003;30:748-756. |
| 14. | Haberkorn U, Kinscherf R, Kissel M, Kübler W, Mahmut M, Sieger S, Eisenhut M, Peschke P, Altmann A. Enhanced iodide transport after transfer of the human sodium iodide symporter gene is associated with lack of retention and low absorbed dose. Gene Ther. 2003;10:774-780. |
| 15. | Dingli D, Diaz RM, Bergert ER, O'Connor MK, Morris JC, Russell SJ. Genetically targeted radiotherapy for multiple myeloma. Blood. 2003;102:489-496. |
| 16. | Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6:215-226. |
| 17. | Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11 Suppl 1:S10-S17. |
| 18. | Liang CY, Wang HZ, Li TX, Hu ZH, Chen XW. High efficiency gene transfer into mammalian kidney cells using baculovirus vectors. Arch Virol. 2004;149:51-60. |
| 19. | Hu YC, Tsai CT, Chang YJ, Huang JH. Enhancement and prolongation of baculovirus-mediated expression in mammalian cells: focuses on strategic infection and feeding. Biotechnol Prog. 2003;19:373-379. |
| 20. | Huang M, Batra RK, Kogai T, Lin YQ, Hershman JM, Lichtenstein A, Sharma S, Zhu LX, Brent GA, Dubinett SM. Ectopic expression of the thyroperoxidase gene augments radioiodide uptake and retention mediated by the sodium iodide symporter in non-small cell lung cancer. Cancer Gene Ther. 2001;8:612-618. |
| 21. | Boland A, Magnon C, Filetti S, Bidart JM, Schlumberger M, Yeh P, Perricaudet M. Transposition of the thyroid iodide uptake and organification system in nonthyroid tumor cells by adenoviral vector-mediated gene transfers. Thyroid. 2002;12:19-26. |
| 22. | Shen DH, Marsee DK, Schaap J, Yang W, Cho JY, Hinkle G, Nagaraja HN, Kloos RT, Barth RF, Jhiang SM. Effects of dose, intervention time, and radionuclide on sodium iodide symporter (NIS)-targeted radionuclide therapy. Gene Ther. 2004;11:161-169. |
| 23. | Willhauck MJ, Samani BR, Wolf I, Senekowitsch-Schmidtke R, Stark HJ, Meyer GJ, Knapp WH, Göke B, Morris JC, Spitzweg C. The potential of 211Astatine for NIS-mediated radionuclide therapy in prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:1272-1281. |
| 24. | Hüser A, Rudolph M, Hofmann C. Incorporation of decay-accelerating factor into the baculovirus envelope generates complement-resistant gene transfer vectors. Nat Biotechnol. 2001;19:451-455. |