Published online Nov 14, 2010. doi: 10.3748/wjg.v16.i42.5334
Revised: June 7, 2010
Accepted: June 14, 2010
Published online: November 14, 2010
AIM: To investigate the proliferative effect of advanced glycation end-products (AGEs) and the role of their cellular receptor (RAGE) on hepatocellular carcinoma (HCC) cells, and the inhibitory effects of MK615, an extract from Japanese apricot, against AGEs were also evaluated.
METHODS: Two HCC cell lines, HuH7 and HepG2, were used. Expression of RAGE was investigated by polymerase chain reaction, Western blotting, and flow cytemetry (FACS). The effect of MK615 on RAGE expression was also evaluated by FACS. The proliferative effects of a control (unglycated bovine serum albumin), glucose-derived AGEs (Glc-AGE), and glyceraldehyde-derived AGEs (Glycer-AGE), and the anti-proliferative effect of MK615 against AGEs, were evaluated using MTT assays.
RESULTS: Expression of RAGE was confirmed at both the mRNA and protein levels in both HuH7 and HepG2. FACS revealed that the level of RAGE expression was higher in HuH7 than in HepG2. Treatment with 0.1 μg/mL MK615 decreased the expression level of RAGE from 24.3% to 3.7% in HuH7 and from 6.2% to 4.8% in HepG2. The growth indices for the control, Glc-AGE, and Glycer-AGE were 1.06 ± 0.08, 0.99 ± 0.04, and 1.38 ± 0.05, respectively, in HuH7 (P = 0.037), and were 1.03 ± 0.04, 1.04 ± 0.03, and 1.07 ± 0.05, respectively, in HepG2 (P > 0.05). When the cells were cultured simultaneously with Glycer-AGE and MK615, MK615 abrogated the proliferative effect of Glycer-AGE in HuH7.
CONCLUSION: Only Glycer-AGE has a proliferative effect on HuH7, which expresses a higher level of RAGE. MK615 suppresses the proliferative effect of Glycer-AGE on HuH7 by decreasing the expression of RAGE.
- Citation: Sakuraoka Y, Sawada T, Okada T, Shiraki T, Miura Y, Hiraishi K, Ohsawa T, Adachi M, Takino JI, Takeuchi M, Kubota K. MK615 decreases RAGE expression and inhibits TAGE-induced proliferation in hepatocellular carcinoma cells. World J Gastroenterol 2010; 16(42): 5334-5341
- URL: https://www.wjgnet.com/1007-9327/full/v16/i42/5334.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i42.5334
MK615, an extract from the Japanese apricot, Prunus mume Sieb. et Zucc (ume in Japanese), contains several triterpenoids and other unknown components. We have previously demonstrated the anti-cancer effects of MK615, which induces apoptosis[1] and autophagy[2] in cancer cells by modulation of the cell cycle through inhibition of aurora kinases A and B[3,4]. It has also been shown that MK615 exerts its anti-inflammatory effects by inhibiting the release of high mobility group box 1 protein (HMGB1) through activation of the transcription factor, Nrf2[5]. The nuclear protein HMGB1 is released into the extracellular space during necrosis and apoptosis, and causes inflammation[6]. The cellular receptor of HMGB1 is a receptor of advanced glycation end-products (AGEs), and is known as receptor of AGE (RAGE). Thus, HMGB1 and AGEs share the same cellular receptor, RAGE.
AGEs are products of non-enzymatic, irreversible glycation of proteins, and are formed under conditions of sustained hyperglycemia[7]. Binding of AGEs to RAGE initiates a potent inflammation response and induces various pathological conditions[8], such as diabetic complications[9], Alzheimer’s disease[10], non-alcoholic steatohepatitis[11], and cancers[12].
Although the cellular events that operate in the AGEs/RAGE system are not fully understood, it has been shown that binding of AGEs to RAGE produces reactive oxygen species[13] and increases the transcription of vascular endothelial growth factor (VEGF), followed by angiogenesis[14]. Also, the AGEs/RAGE system activates p38/MAP kinase[15] and nuclear factor (NF)-κB[16]. These cellular events induce the production of proinflammatory cytokines[6].
Because AGEs constitute a heterozygous group, some molecules that are categorized as AGEs are not particularly toxic, including N-(carboxymethyl) lysine (CML) and pyrroline. Accumulated evidence indicates that glyceraldehyde-derived AGEs (Glycer-AGE) have a predominantly toxic structure, and these are referred to as toxic AGEs (TAGE)[10,17]. Although a number of studies have investigated the roles of various AGEs, including CML, pyrroline, and glucose-derived AGEs (Glc-AGE), and also the function of RAGE in cancers[18,19], the role of Glycer-AGE in tumorigenesis still remains unclear, especially in hepatocellular carcinoma (HCC).
Recently, it was shown that MK615 inhibits the activation of ERK1/2, p38MAPK, and NF-κB by the AGEs/RAGE system, and suppresses the release of proinflammatory cytokines[20].
The purposes of the present study were, using HCC cell lines, to determine (1) the type of effect that MK615 exerts on HCC; (2) whether Glycer-AGE has a proliferative effect on HCC; and (3) whether MK615 attenuates the effect of Glycer-AGE on HCC.
Two human hepatocellular carcinoma cell lines, HuH7 and HepG2, were used. HuH7 was purchased from the Cell Resource Center of the Biomedical Research Institute of Development, Aging and Cancer, Tohoku University (Miyagi, Japan), and HepG2 was purchased from DS Pharma Biomedical Co., Ltd. (Osaka, Japan). The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal calf serum (FCS) in an incubator with a 5% CO2 atmosphere.
AGEs: Unglycated bovine serum albumin (BSA) (control), glucose-derived AGEs (Glc-AGE:AGE1), and glyceraldehyde-derived AGEs (Glycer-AGE:AGE2) were produced by one of the co-authors, M. Takeuchi, as described previously[21,22]. Briefly, BSA at 25 mg/mL was incubated under sterile conditions with 0.5 mol/L D-glucose for 8 wk (Glc-AGE), or with 0.1 mol/L D-glyceraldehyde for 7 d (Glycer-AGE) at 37°C. Then, low-molecular-weight reactants and sugars were removed using PD-10 column (GE Healthcare UK Ltd., Buckinghamshire, England) chromatography and dialysis against phosphate-buffered saline (PBS). Control unglycated BSA was incubated under the same conditions except for an absence of reducing sugar. Preparations were tested for endotoxin using an Endospecy ES-20S system (Seikagaku Co., Tokyo, Japan). For all experiments, the control, Glc-AGE, and Glycer-AGE were used at a concentration of 0.1 mg/mL in the culture medium.
MK615: MK615 was provided by AdaBio Co., Ltd. (Gunma, Japan). MK615 is derived from Japanese apricot fruit[1-4]. Briefly, the preparation procedure involves extraction of the apricot juice using a press, and this is then heated and concentrated. The concentrate is dissolved in water, and then adjusted to pH 7.0 with NaOH. The solution is finally sterilized in an autoclave. MK615 was used at a concentration of 0.1 μg/mL[5], except for the experiment shown in Figure 1.
Cells were plated at 1 × 105/well in 24-well plates and cultured for 24 h. Then, MK615 (0.1 μg/mL) was added to the medium and culture was continued for another 24 h. Total RNA from each cell line was isolated using a Total RNA Isolation kit (MACHEREY-NAGEL, Düren, Germany). Reverse transcription reactions were performed using a Rever Tra Ace α-First Strand cDNA Synthesis Kit (TOYOBO, Osaka, Japan). Briefly, 1 μg of total RNA, oligo dT-primer, and dNTPs were incubated at 65°C for 5 min, then 10 μL of cDNA synthesis mixture was added and the mixture was incubated at 50°C for 50 min. The reaction was terminated by adding 1 μL of RNaseH and incubating the mixture at 37°C for 20 min.
The sequences of the primers were as follows: β-actin: sense-primer 5′-GGACTTCGAGCAAGAGATGG-3′, anti-sense 5′-AGCACTGTGTTGGCGTACAG-3′; RAGE: sense-primer 5′-CACACTGCAGTCGGAGCTAA-3′, anti-sense 5′-GCTACTGCTCCACCTTCTGG-3′. Conventional polymerase chain reaction (PCR) was performed with Hottaq mix (x,x). The PCR conditions consisted of 95°C for 5 min, 40 cycles at 95°C for 30 s, 62°C for 30 s, and 60°C for 30 s. The PCR products were separated on 1% agarose gel.
Real-time PCR was performed with an ABI Prism 7700 sequence detector (Applied Biosystems, Warrington, UK). The PCR reaction was carried out in a final volume of 25 μL, which included 2 μL cDNA, 12.5 μL 2 × SYBR Green (Applied Biosystems), 0.5 μL of 25 nmol/L sense and antisense primers, and H2O. The PCR conditions consisted of 40 cycles at 95°C for 30 s and 60°C for 30 s. Samples were assayed in triplicate. Means and standard deviations were calculated from the data obtained. For each sample, at least three assays were performed. The t value was calculated as the mean of three different assays. The level of expression was calculated using the formula: Relative expression (t-value) = (Copy number of target molecule/Copy number of β-actin) × 1000[3,4].
Anti-RAGE antibodies (#ab54741) were purchased from Abcam Inc. (Cambridge, MA). After cells had been collected, they were washed twice with cold PBS, lysed with 200 μL of 0.5% (w/v) SDS, and centrifuged at 10 000 r/min. The supernatants were adjusted by dilution so as to contain equal amounts of protein, as tested using a BCA Protein Assay Kit (Pierce, Rockford, IL). Samples (20 μg protein) were run on 12.5% (w/v) SDS-PAGE with 10% gel and electroblotted onto PVDF membranes. The blots were blocked for 1 h with 5% (w/v) non-fat milk powder and 0.1% (v/v) Tween 20 in Tris-NaCl, then exposed to the primary antibody at a 1000-fold dilution overnight at 4°C. After extensive washing, the blots were incubated with the secondary horseradish-peroxidase-conjugated antibody (1:2000) for 2 h at room temperature. Immunoreactive bands were visualized using an enhanced chemiluminescence detection system (Amersham Life Sciences, Arlington Heights, IL)[2-4].
Anti-RAGE antibody was the same as that used in Western blotting. An isotype-matched antibody was purchased from Abcom. The cells were collected and stained with control or anti-RAGE monoclonal antibody for 1 h, washed twice with PBS, and then stained with FITC-conjugated anti-mouse IgG monoclonal antibody for 30 min. Then cells were again washed twice with PBS and analyzed using a FACSCalibur (Becton-Dickinson). The percentage shift was calculated by subtracting the histogram obtained with anti-RAGE monoclonal antibody from that obtained with the control antibody[23].
For the MTT assay, 15 μL MTT (5 g/L) was added to each well, and the cells were incubated for 4 h. Then, 100 μL solubilization solution/stop mix was added, in accordance with the manufacturer’s recommendation, and the plates were left to stand for 60 min. The absorbance at 570 and 630 nm was then measured with an ELISA reader. The actual counts were calculated by subtracting the absorbance at 570 nm from that at 630 nm. Each assay was performed in triplicate and the average absorbance was calculated[2-4].
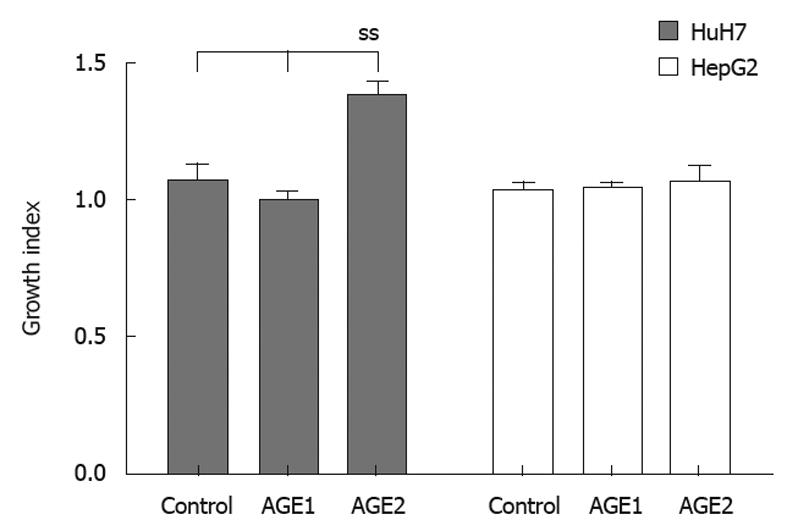
Growth index (Figure 2) was calculated as follows. Cells were plated out at 5 × 103 cells/well in 96-well plates and cultured for 24 h, then AGEs were added to the culture medium (Medium) at 0.1 mg/mL. After 24 h, the MTT assay was performed, and experiments were repeated 3 times. The mean values of the counts were calculated for each experiment. Growth index was calculated by dividing the mean for the control, Glc-AGE, or Glycer-AGE by the mean for the Medium. Standard deviation was calculated for all three experiments.
The percentage inhibition was calculated as follows. Two culture sets were set up, the cells being plated out at 5 × 103/well in 96-well plates, and cultured for 24 h. The cells were then cultured with Medium, control, Glc-AGE, or Glycer-AGE, and each well was cultured in quadruplicate. One plate was cultured without MK615 and the other with MK615 0.1 μg/mL. The percentage inhibition was calculated as: % inhibition = (count of cells with MK615 - count of cells without MK615) × 100/count without MK615. The experiments were repeated three times, and the means and standard deviations were calculated.
Cells were cultured under control conditions, or with Glc-AGE, Glycer-AGE, or MK615 for 24 h, then harvested by trypsinization and washed twice with PBS. The annexin V binding assay was performed using an Annexin V-FITC Apoptosis Detection kit (Becton-Dickinson) in accordance with the supplier’s instructions. At least 1 × 106 cells were incubated with FITC-conjugated annexin V at room temperature for 15 min, and the cells were then analyzed on a FACscan (Becton-Dickinson).
One-factor analysis of variance (ANOVA) with post-hoc test was used for comparison of the three subgroups by GraphPad Prism 5.0 (Graphpad Software, La Jolla, CA). The results obtained by flow cytometry were analyzed statistically with the Kolmogorov-Smirnov test using CellQuest software (Becton-Dickinson)[23]. Differences at P < 0.05 were accepted as significant. Statistical analysis of percentage growth inhibition was performed using the unpaired, two-tailed t test.
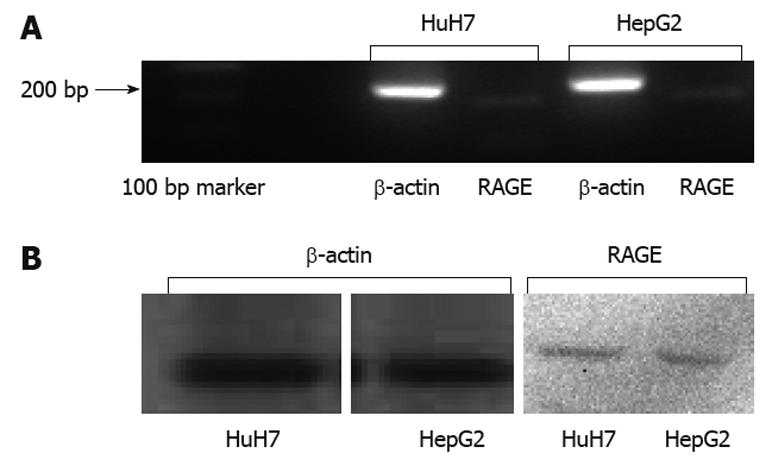
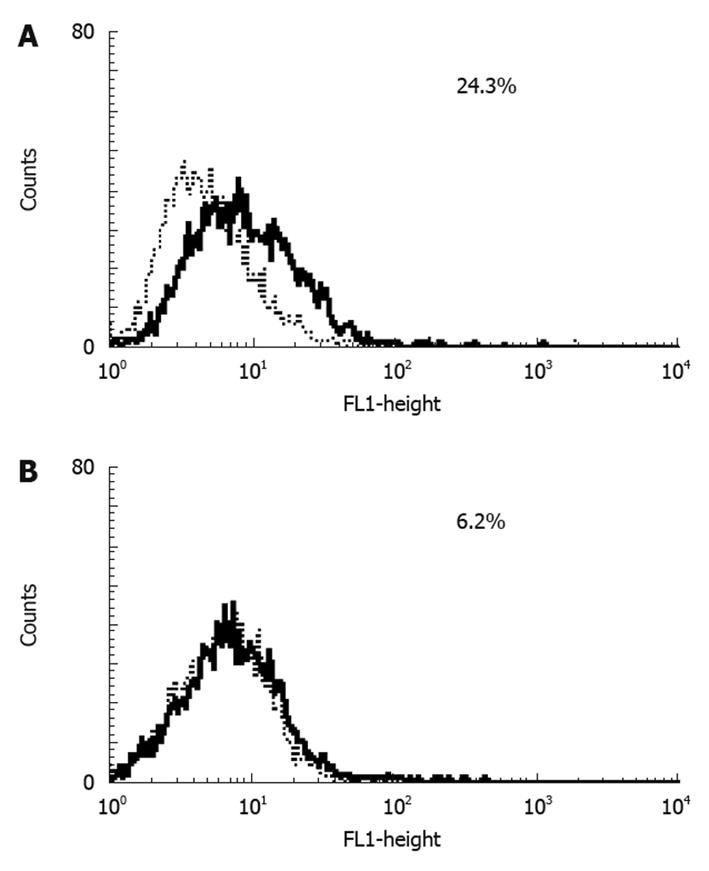
Results of RT-PCR (Figure 3A) and Western blotting (Figure 3B) showed that HuH7 and HepG2 expressed RAGE endogenously. Flow cytometry with anti-RAGE antibody staining demonstrated the expression of membrane-bound RAGE in both HuH7 and HepG2 cells at 24.3% and 6.2%, respectively (Figure 4A and B).
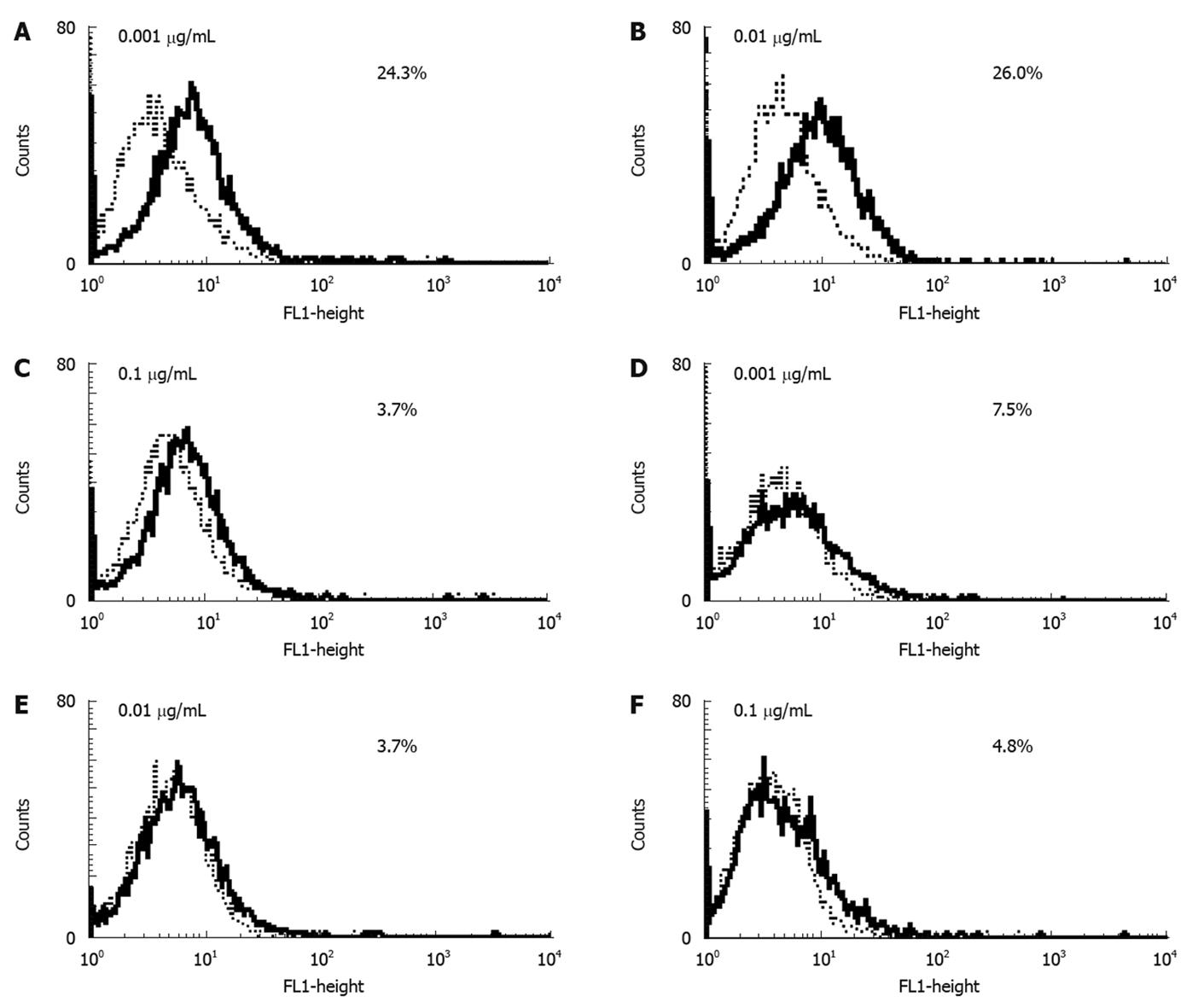
Next, we investigated the effect of MK615 on the cell surface expression of RAGE in HuH7 and HepG2 cells. Cells were incubated with different concentrations of MK615. In HuH7, the expression levels of RAGE at MK615 concentrations of 0.001, 0.01, and 0.1 μg/mL were 24.3%, 26.0%, and 3.7%, respectively (Figure 1A-C), whereas the corresponding levels in HepG2 were 7.5%, 3.7%, and 4.8%, respectively (Figure 1D-F).
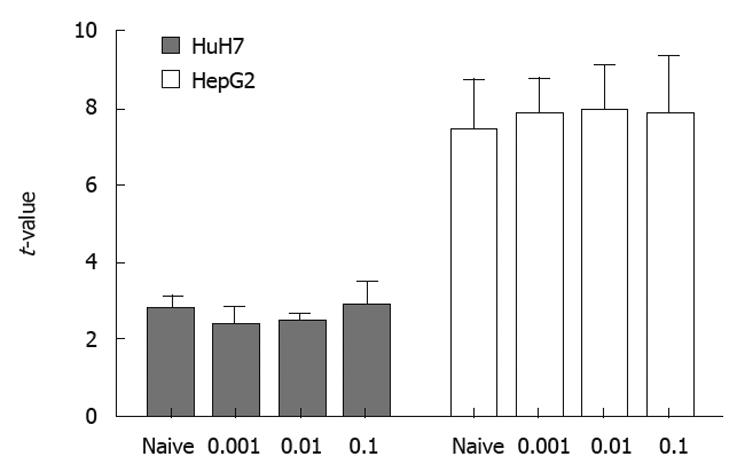
The results of real-time PCR using samples of RNA obtained under the same conditions as those for flow cytomtery showed that transcription of RAGE mRNA did not differ between MK615-treated and non-treated cells (Figure 5).
To evaluate the stimulation of growth by AGEs, HuH7 and HepG2 cells were incubated with AGEs (Figure 5). The growth indices of the unglycated BSA control, Glc-AGE, and Glycer-AGE were 1.06 ± 0.08, 0.99 ± 0.04, and 1.38 ± 0.05 in HuH7, and 1.03 ± 0.04, 1.04 ± 0.03, and 1.07 ± 0.05 in HepG2, respectively. In HuH7, the growth index of Glycer-AGE was significantly higher than those of the control and Glc-AGE (P = 0.037). In HepG2, there were no significant differences in growth index among the three treatments (P = 0.905).
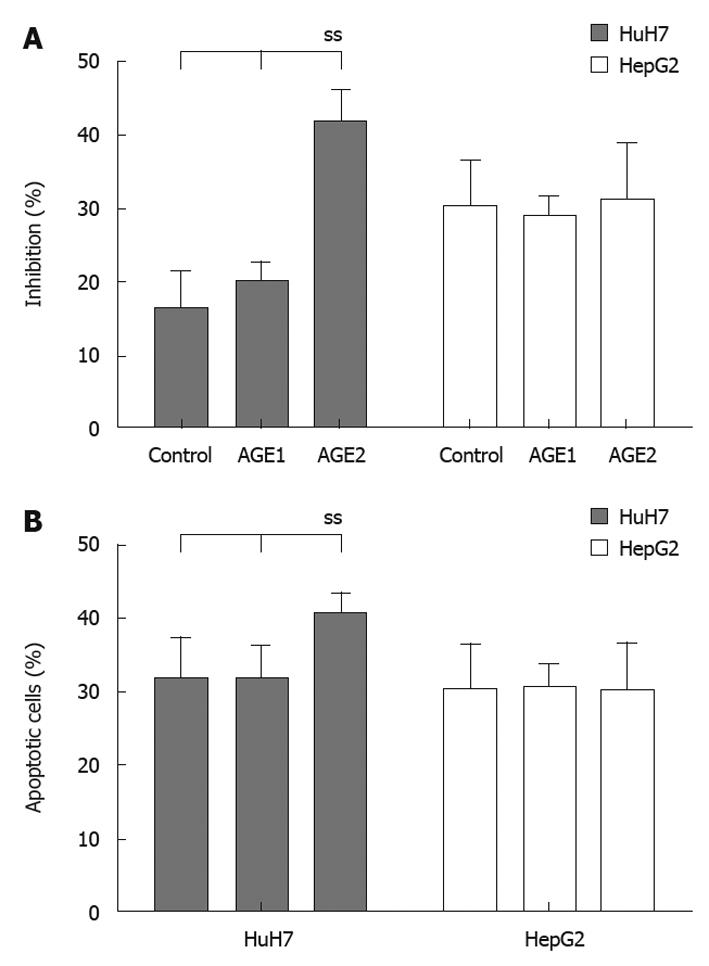
Figure 6A shows the suppression of AGEs growth stimulation by MK615. The percentages of inhibition for the Medium, control, Glc-AGE, and Glycer-AGE in HuH7 cells were 29.0% ± 3.5%, 16.2% ± 5.1%, 20.1% ± 2.7%, and 41.7% ± 4.1%, respectively. The percentage inhibition of Glycer-AGE-induced growth stimulation was significantly higher than that for Medium, Control, or AGE1 (P = 0.02 by one-way ANOVA). In HepG2 cells, the percentages of inhibition for the Medium, control, Glc-AGE, and Glycer-AGE were 28.9% ± 8.1%, 30.1% ± 6.3%, 29.0% ± 2.7% and 31.1% ± 7.9%, respectively. There were no significant differences among the groups (P = 0.18 by one-way ANOVA). For HuH7, the frequencies of apoptotic cells for the control, Glc-AGE, and Glycer-AGE were 31.5% ± 5.7%, 31.3% ± 4.9%, and 40.3% ± 3.1%, respectively (P = 0.04 by one-way ANOVA), and those for HepG2 were 30.2% ± 6.2%, 30.5% ± 3.1%, and 30.1% ± 6.3%, respectively (P = 0.12 by one-way ANOVA) (Figure 6B).
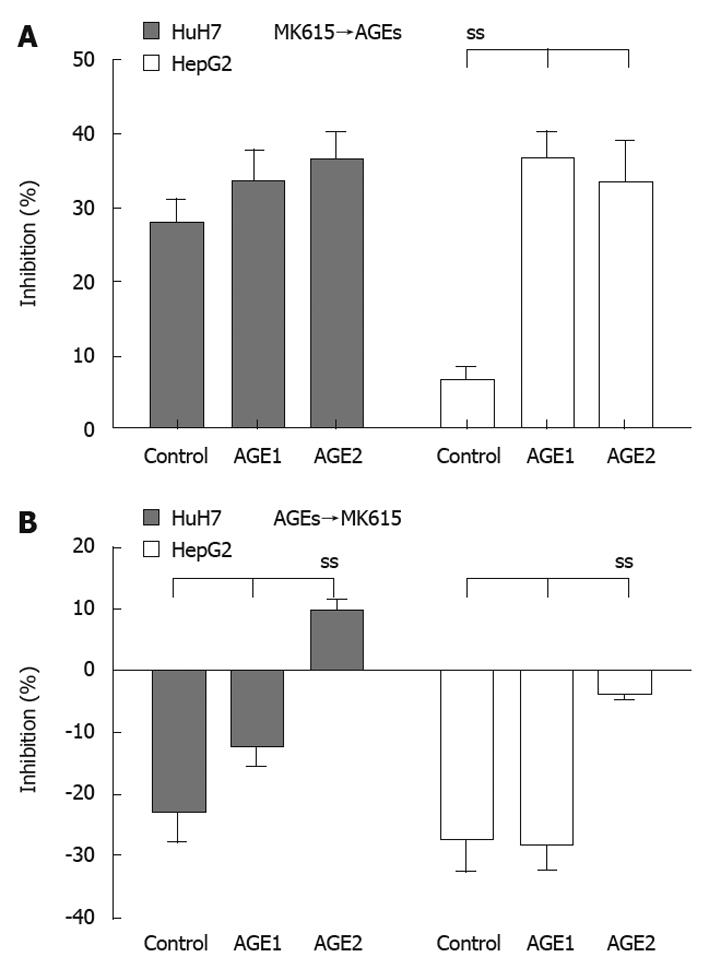
Figure 7A shows the percentages of inhibition when the cells were cultured with AGEs following 24 h of culture with MK615. In HuH7 cells, the percentages of inhibition for the control, Glc-AGE, and Glycer-AGE were 27.9% ± 3.2%, 33.4% ± 4.5%, and 36.3% ± 4.1%, respectively (P = 0.08 by one-way ANOVA), and the corresponding values in HepG2 were 6.7% ± 1.9%, 36.6% ± 3.7% and 30.1% ± 6.3%, respectively (P = 0.03 by one-way ANOVA). Figure 7B shows the percentages of inhibition when the cells were cultured with MK615 following 24 h of culture with AGEs. In HuH7 cells, the percentages of inhibition for the control, Glc-AGE, and Glycer-AGE were -22.3% ± 5.1%, -12.3% ± 3.2%, and 9.6% ± 2.2%, respectively (P = 0.02 by one-way ANOVA), and the corresponding values for HepG2 were -27.5% ± 5.9%, -27.8% ± 4.7%, and -3.9% ± 0.8%, respectively (P = 0.02 by one-way ANOVA).
HuH7 and HepG2 cells showed surface expression of RAGE, but the level of expression differed between them. A previous study reported that HepG2 did not express RAGE[24], although the present study confirmed weak expression of RAGE in HepG2 at both the mRNA and protein level. Flow cytometry revealed that HuH7 cells expressed RAGE at a higher level than did HepG2. On the other hand, results of real-time PCR (Figure 5) showed higher t-values for HepG2 than for HuH7. Because we calculated the expression of RAGE mRNA relative to β-actin mRNA, the relative expression of RAGE mRNA would have been influenced by the expression level of β-actin mRNA. However, the reason for the discrepancy in the expression of RAGE mRNA between flow cytometry and real-time PCR was unclear. Although both cell lines were derived from well differentiated HCC, HuH7 was established from a Japanese patient, whereas HepG2 was from a Caucasian. Hiwatashi et al[24] reported that expression of RAGE was lower in normal liver and hepatitis, and higher in HCC. Although the mechanism responsible for the difference in RAGE expression is not clear, and the level at which RAGE expression stimulates the growth of HCC was not determined, the difference would have been related to the response of the cells to AGEs, as described below.
HuH7 showed a statistically significant growth response to Glycer-AGE, but not to control or Glc-AGE. HepG2, which expressed a low level of RAGE, did not show any significant growth response to control, Glc-AGE, or Glycer-AGE (Figure 2). Our results indicated that Glycer-AGE stimulated the growth of HuH7 cells, which show higher surface expression of RAGE, whereas the degree of stimulation was not marked in HepG2 cells, which show low surface expression of RAGE. Furthermore, the control and Glc-AGE exerted no proliferative effect on either of the cell lines. It has been difficult to study the biological effects of AGEs because of their heterogeneity. As CML, pyrroline, and Glc-AGE are not as toxic as Glycer-AGE, it is necessary to obtain data using a representative TAGE, of which Glycer-AGE is one of the more toxic forms. Glycer-AGE, but not Glc-AGE, CML, and pyrroline, has strong binding affinity for RAGE and subsequently elicits oxidative stress and vascular inflammation, being implicated in the accelerated atherosclerosis seen in patients with diabetes mellitus[25,26]. Recently, we have also demonstrated that Glycer-AGE plays an important role in the pathogenesis of angiopathy in diabetic patients[27,28]. Moreover, there is a growing body of evidence to suggest that the interaction of TAGE with RAGE elicits oxidative stress in numerous types of cells, thereby possibly contributing to the pathological changes that characterize the vascular complications of diabetes. Glycer-AGE-RAGE interaction triggers the generation of NADPH oxidase-mediated reactive oxygen species (ROS), and subsequently activates p38/MAP kinase and NF-κB, resulting in cell proliferation[27,28].
As shown in Figure 1, MK615 decreased the expression of RAGE on HuH7 cells at a concentration of 0.1 μg/mL, whereas the effect on HepG2 cells was less marked. This decrease in the expression of RAGE was not due to suppression of transcription, because the results of real-time PCR revealed that the transcripts of RAGE mRNA did not differ between naïve and MK615-treated HuH7 and HepG2 (Figure 5). It remains to be clarified whether the decrease in the cell-surface expression of RAGE is due to solubilization of RAGE, degradation, or other mechanisms.
When cells were cultured with AGEs and MK615 simultaneously, MK615 was found to inhibit the growth effect of Glycer-AGE (Figure 6), and when the cells were cultured with AGEs following exposure to MK615, the growth effect elicited by Glycer-AGE was abrogated (Figure 7A). Furthermore, when the cells were cultured with AGEs followed by MK615, the growth effect elicited by Glycer-AGE was still evident, but was reduced (Figure 7B). MK615 inhibits the activation of MAPK/NF-κB[15,16], which is thought to be a major pathway of the AGEs/RAGE system. Because MK615 contains several triterpenoids and other unknown substances, the mechanism responsible for abrogation of the proliferative effect of Glycer-AGE on HCC cells might be multifactorial. However, the decrease of RAGE expression elicited by MK615 may play a crucial role in suppressing the Glycer-AGE-induced proliferation of HCC cells.
In conclusion, Glycer-AGE (a representative TAGE) exerts a potent proliferative effect on HuH7 cells, which express a high level of RAGE on their surface. The effect of RAGE may be partly dependent on the degree of cell-surface RAGE expression. MK615 inhibits Glycer-AGE-induced proliferation of HuH7 cells by decreasing their cell surface expression of RAGE. Our present results suggest that further studies are warranted to explore the role of Glycer-AGE in HCC, and to examine the therapeutic effect of MK615.
This study focused on two topics: advanced glycation-end products (AGEs), and MK615, an extract from the Japanese apricot, Prunus mume Sieb. et Zucc (ume in Japanese). AGEs are the products of non-enzymatic, irreversible glycation of proteins, and the causative agents of various disorders, including inflammation and cancers. AGEs constitute a heterozygous group, and recent studies have revealed that most of them are not really toxic, although glyceraldehyde-derived AGE (Glycer-AGE) is a toxic AGE (TAGE). In the present study, we used purified Glycer-AGE. AGEs bind to the cell-surface receptor of AGE (RAGE) to exert biological effects. It has been shown that MK615 exerts anti-inflammatory affects by modifying the expression of RAGE. Thus, AGEs and MK615 are connected via RAGE.
Glycer-AGE is a real TAGE, and a limited number of studies have been conducted to clarify the role of Glycer-AGE in cancer. As mentioned before, AGEs are a heterogenous group of products, and future studies should focus on AGEs using Glycer-AGE. As shown in the present study, Glycer-AGE exerts stronger effects than glucose-derived AGE. The biological effects of Glycer-AGE remain to be elucidated.
Glycer-AGE potently stimulated the growth of hepatocellular carcinoma (HCC) cells, and the effect was inhibited by MK615. This suggests that Glycer-AGE (TAGE) stimulates the proliferation of HCC, and that MK615 may have a therapeutic effect on this activity.
Clinically, MK615 ameliorates hepatitis due to hepatitis C virus. Although the mechanism remains unclear, MK615 may exert its effect by downregulating the expression of RAGE. Although further studies will be needed, MK615 may exert a therapeutic effect on HCC by inhibiting Glycer-AGE-dependent cancer growth.
MK615 is an extract from the Japanese apricot, Prunus mume Sieb. et Zucc (ume in Japanese). AGEs are products of the non-enzymatic, irreversible glycation of proteins. RAGE is a cell-surface receptor of AGEs. TAGE is a toxic AGE that is derived from glyceraldehyde (Glycer-AGE).
The study is focused on the interesting topic of RAGE-dependent cellular proliferation. The authors demonstrate semi-functional data that RAGE could be of relevance in hepatocellular tumour growth.
Peer reviewer: Nikolaus Gassler, Professor, Institute of Pathology, University Hospital RWTH Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH
| 1. | Nakagawa A, Sawada T, Okada T, Ohsawa T, Adachi M, Kubota K. New antineoplastic agent, MK615, from UME (a Variety of) Japanese apricot inhibits growth of breast cancer cells in vitro. Breast J. 2007;13:44-49. |
| 2. | Mori S, Sawada T, Okada T, Ohsawa T, Adachi M, Keiichi K. New anti-proliferative agent, MK615, from Japanese apricot "Prunus mume" induces striking autophagy in colon cancer cells in vitro. World J Gastroenterol. 2007;13:6512-6517. |
| 3. | Okada T, Sawada T, Osawa T, Adachi M, Kubota K. A novel anti-cancer substance, MK615, from ume, a variety of Japanese apricot, inhibits growth of hepatocellular carcinoma cells by suppressing Aurora A kinase activity. Hepatogastroenterology. 2007;54:1770-1774. |
| 4. | Okada T, Sawada T, Osawa T, Adachi M, Kubota K. MK615 inhibits pancreatic cancer cell growth by dual inhibition of Aurora A and B kinases. World J Gastroenterol. 2008;14:1378-1382. |
| 5. | Kawahara K, Hashiguchi T, Masuda K, Saniabadi AR, Kikuchi K, Tancharoen S, Ito T, Miura N, Morimoto Y, Biswas KK. Mechanism of HMGB1 release inhibition from RAW264.7 cells by oleanolic acid in Prunus mume Sieb. et Zucc. Int J Mol Med. 2009;23:615-620. |
| 6. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. |
| 7. | Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315-1321. |
| 8. | Yamagishi S, Takeuchi M, Inagaki Y, Nakamura K, Imaizumi T. Role of advanced glycation end products (AGEs) and their receptor (RAGE) in the pathogenesis of diabetic microangiopathy. Int J Clin Pharmacol Res. 2003;23:129-134. |
| 9. | Hudson BI, Schmidt AM. RAGE: a novel target for drug intervention in diabetic vascular disease. Pharm Res. 2004;21:1079-1086. |
| 10. | Sato T, Shimogaito N, Wu X, Kikuchi S, Yamagishi S, Takeuchi M. Toxic advanced glycation end products (TAGE) theory in Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2006;21:197-208. |
| 11. | Hyogo H, Yamagishi S, Iwamoto K, Arihiro K, Takeuchi M, Sato T, Ochi H, Nonaka M, Nabeshima Y, Inoue M. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:1112-1119. |
| 12. | Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354-360. |
| 13. | Yamagishi S, Amano S, Inagaki Y, Okamoto T, Takeuchi M, Makita Z. Beraprost sodium, a prostaglandin I2 analogue, protects against advanced gycation end products-induced injury in cultured retinal pericytes. Mol Med. 2002;8:546-550. |
| 14. | Yamagishi S, Amano S, Inagaki Y, Okamoto T, Koga K, Sasaki N, Yamamoto H, Takeuchi M, Makita Z. Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem Biophys Res Commun. 2002;290:973-978. |
| 15. | Yeh CH, Sturgis L, Haidacher J, Zhang XN, Sherwood SJ, Bjercke RJ, Juhasz O, Crow MT, Tilton RG, Denner L. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001;50:1495-1504. |
| 16. | Okamoto T, Yamagishi S, Inagaki Y, Amano S, Koga K, Abe R, Takeuchi M, Ohno S, Yoshimura A, Makita Z. Angiogenesis induced by advanced glycation end products and its prevention by cerivastatin. FASEB J. 2002;16:1928-1930. |
| 17. | Takeuchi M, Bucala R, Suzuki T, Ohkubo T, Yamazaki M, Koike T, Kameda Y, Makita Z. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J Neuropathol Exp Neurol. 2000;59:1094-1105. |
| 18. | Abe R, Shimizu T, Sugawara H, Watanabe H, Nakamura H, Choei H, Sasaki N, Yamagishi S, Takeuchi M, Shimizu H. Regulation of human melanoma growth and metastasis by AGE-AGE receptor interactions. J Invest Dermatol. 2004;122:461-467. |
| 19. | Abe R, Yamagishi S. AGE-RAGE system and carcinogenesis. Curr Pharm Des. 2008;14:940-945. |
| 20. | Morimoto Y, Kikuchi K, Ito T, Tokuda M, Matsuyama T, Noma S, Hashiguchi T, Torii M, Maruyama I, Kawahara K. MK615 attenuates Porphyromonas gingivalis lipopolysaccharide-induced pro-inflammatory cytokine release via MAPK inactivation in murine macrophage-like RAW264.7 cells. Biochem Biophys Res Commun. 2009;389:90-94. |
| 21. | Takeuchi M, Makita Z, Bucala R, Suzuki T, Koike T, Kameda Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol Med. 2000;6:114-125. |
| 22. | Takeuchi M, Yanase Y, Matsuura N, Yamagishi Si S, Kameda Y, Bucala R, Makita Z. Immunological detection of a novel advanced glycation end-product. Mol Med. 2001;7:783-791. |
| 23. | Okada T, Sawada T, Kubota K. Deferoxamine enhances anti-proliferative effect of interferon-gamma against hepatocellular carcinoma cells. Cancer Lett. 2007;248:24-31. |
| 24. | Hiwatashi K, Ueno S, Abeyama K, Kubo F, Sakoda M, Maruyama I, Hamanoue M, Natsugoe S, Aikou T. A novel function of the receptor for advanced glycation end-products (RAGE) in association with tumorigenesis and tumor differentiation of HCC. Ann Surg Oncol. 2008;15:923-933. |
| 25. | Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097-1109. |
| 26. | Yamamoto Y, Yonekura H, Watanabe T, Sakurai S, Li H, Harashima A, Myint KM, Osawa M, Takeuchi A, Takeuchi M. Short-chain aldehyde-derived ligands for RAGE and their actions on endothelial cells. Diabetes Res Clin Pract. 2007;77 Suppl 1:S30-S40. |
| 27. | Sato T, Iwaki M, Shimogaito N, Wu X, Yamagishi S, Takeuchi M. TAGE (toxic AGEs) theory in diabetic complications. Curr Mol Med. 2006;6:351-358. |
| 28. | Takeuchi M, Yamagishi S. Involvement of toxic AGEs (TAGE) in the pathogenesis of diabetic vascular complications and Alzheimer’s disease. J Alzheimers Dis. 2009;16:845-858. |