Published online Oct 28, 2010. doi: 10.3748/wjg.v16.i40.5016
Revised: April 27, 2010
Accepted: May 4, 2010
Published online: October 28, 2010
Endoscopic Imaging has progressed tremendously over the last few decades. Novel imaging technologies such as high-resolution and high-magnification white light endoscopy, narrow band imaging, optimal band imaging, autoflourescence imaging and optical coherence tomography not only aid the endoscopist in detecting malignant or pre-malignant lesions but also assist in predicting histology. Recently, the introduction of Endocytoscopy (EC) and Confocal Endomicroscopy has taken us into a new realm of diagnostic endoscopy. With the ability to magnify up to 1000 ×, cellular structures can be visualized in real-time. This advance in technology could potentially lead to a paradigm shift negating the need to obtain biopsies. EC is, however, still in the early stages of development and further research needs to be carried out before it can be accepted as standard practice. This review will focus on the diagnostic utility of the Endocytoscope.
- Citation: Singh R, Mei SLCY, Tam W, Raju D, Ruszkiewicz A. Real-time histology with the endocytoscope. World J Gastroenterol 2010; 16(40): 5016-5019
- URL: https://www.wjgnet.com/1007-9327/full/v16/i40/5016.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i40.5016
Recent advances in endoscopic technology have pushed the boundaries of diagnostic endoscopy further, enabling more accurate and better lesion recognition and characterization. Endocytoscopy (EC) is the latest innovation in the ever-expanding armamentarium of devices available to the endoscopist. This technology uses the principles of light microscopy, producing a clear and in-focus image of a thin layer of tissue located within a biologically thick sample. This is possible using a high-power fixed focus objective lens that provides ultra-high magnification images of surface morphology at cellular resolution.
The EC device manufactured by Olympus Medical Systems Co., Tokyo, Japan either comes as a probe-based system or one that can be incorporated into the endoscope. Both devices are prototypes and are currently not available commercially. The probe-based Endocytoscope consists of a catheter-type device 380 cm in length, measuring 3.2 mm in diameter and is available at 2 levels of magnification. The lower magnification, the XEC 300 Endocytoscope, has a magnification capability of 450 × and a field of view of 300 μm × 300 μm whilst the higher magnification, the XEC120 Endocytoscope, enables magnification up to 1125 × with a field of view of 120 μm × 120 μm; both based on a 19-inch-high resolution monitor. These instruments should be passed into the working channel of a therapeutic endoscope which has a channel diameter of 3.7 mm (Olympus GIF-IT240). Alternatively, the integrated-type device incorporates the Endocytoscope into the endoscope itself. This system also comes in 2 different configurations, the upper (103 cm), (XGIF-Q260EC1) endoscope and the lower (133 cm) (XCF-Q260EC1) endoscope. Both of these devices have a magnification capability of 580 × and a field of view of 400 μm × 400 μm. The depth of penetration of both the probe-based and integrated device is limited to 30 μm.
Similar to the endoscope, the Endocytoscope is connected to a light source and a video processor. Thus, for visualization of images in real-time, it is necessary to utilise 2 processors simultaneously. As in conventional histopathology, vital staining is used to further elucidate cellular detail. Prior to staining, the mucosal surface should be vigorously flushed with water and a mucolytic agent (such as 10% N-acetyl cysteine). To maintain stability of the Endocytoscope when it approximates an area of interest, a plastic cap is attached to the tip of the endoscope prior to commencement of the procedure. Various dyes have been used to stain the mucosa. In a recent study regarding the optimal dye and concentration needed, 60 s of exposure of the mucosa to 1% methylene blue for the esophagus and 0.25% toluidine blue for the stomach and the colon were found to be the best staining techniques[1]. Recently, another technique aptly called the double staining technique which utilizes 1% methylene blue (to stain the nucleus) and 0.1% crystal violet (to stain both the nucleus and the cytoplasm) has been used to approximate hematoxylin and eosin staining seen in conventional histology.
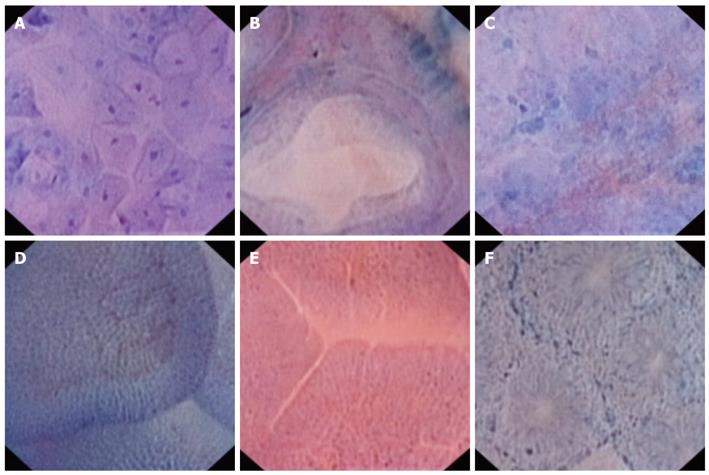
In the esophagus, staining is optimal with methylene blue. With the low power Endocytoscope (XEC 300), two to three layers of cells can be observed. The nuclei are regular in size and shape and exhibit a low nuclear to cytoplasmic ratio (Figure 1A). With the high power Endocytoscope (XEC 120), nucleoli can also be observed. In an ex-vivo pilot study of esophageal squamous cell carcinomas (SCC), EC images closely correlated with histologic results for both cancerous and normal esophageal squamous cells although the quality of some images was deemed inferior[2]. In another study on in-vivo diagnosis, EC demonstrated increased density of the cells with loss of cellular uniformity in esophageal SCC[3]. The nuclei in SCC were also of different shapes and sizes. Prominent nucleoli were noted on the higher magnification Endocytoscope and the nucleus to cytoplasm ratio appeared altered. In Barrett’s esophagus, the nuclei are uniform and the cell density is low. These cells are arranged radially forming a regular glandular and crypt structure (Figure 1B). In cancer, the cell density is high with loss of the glandular pattern whilst the crypts are destroyed and the nuclei may appear pleomorphic[4] (Figure 1C).
The gastric mucosa with its multiple folds is generally more difficult to observe with EC. Benign gastric mucosa exhibits regularly arranged tubules and nuclei. In gastric cancer, irregular branched and destroyed tubules are noted and cells show nuclear pleomorphism. EC has also been successful in ex-vivo demonstration of Helicobacter pylori (H. pylori)[5]. This study was performed after preparing cultures from gastric mucus obtained from patients with gastric ulcers. Live H. pylori could be observed directly using the Endocytoscope.
EC can clearly visualize the villi in the duodenum with the presence of regular capillaries and regularly placed nuclei (Figure 1D). In Celiac disease, the mucosa appears atrophic with complete absence of the villi. There is a “cracked mud”-like appearance and capillaries are distinctly absent (Figure 1E). EC at 450 × magnification has been shown to accurately identify mucosal histopathology in advanced Celiac disease[6]. The investigators identified four criteria which were significant predictors of Marsh III pathology: low number of villi per visual field (< 3), confluence of villi, irregular epithelial lining and inability to delineate loop capillaries. They were, however, unable to identify any salient features which were good predictors of early morphological changes in Celiac disease.
EC allows clear visualization of the mucosa and microvasculature of the colon[7]. The EC images of normal mucosa reveal uniform glands arranged neatly in a radial or hexagonal fashion around crypts with borders which are demarcated by microvasculature observed as red blood cells circulating through the arterioles (Figure 1F). In hyperplastic polyps, the glands are serrated with foamy changes in the cytoplasm and star-like pits. In low grade adenomatous polyps, homogenous tubular glands with regular fusiform nuclei which have uniform polarity can be visualized. High grade adenomas have nuclei on the luminal side of the gland with disordered polarity. The glands also tend to be irregularly branched. Sasajima et al[8] reported that it was possible to distinguish neoplastic from non-neoplastic lesions with EC. Conventional histology and EC had high concordance values in the diagnosis of colorectal neoplasia with accuracies of 93.3% and a κ score of 0.91, suggesting substantial agreement. EC also allows in-vivo real-time visualization of blood flow in rectal microvasculature[7] and clear visualization of the dysplasia in aberrant crypt foci (ACF) in the colon and rectum[9]. In dysplastic ACF, crypt contours are polygonal, the lumen is linear and the nuclei are elongated with pseudo-stratification toward the luminal half of the crypt. In this study, histology confirmed low grade dysplasia with a sensitivity of 91.4% and specificity of 100%.
Recent promising developments in wide field technologies such as Narrow Band Imaging, Optimal Band Imaging and High-Resolution High-Definition White Light Endoscopy have increased the capability of the endoscopist for visualizing and detecting pathology. EC and Confocal Endomicroscopy (CLE) are novel techniques which enable lesions which are already detected to be investigated further, thus allowing real-time visualization of cellular detail. Both of these are in essence ‘point biopsy’ techniques. Apart from using a totally different technology, CLE differs from EC in that it requires different staining agents (intravenous fluorescein and occasionally topical acriflavin). The resolution of the images is, however, superior although they do appear in black and white unlike those of the EC. CLE also enables the mucosa to be assessed up to a depth of 250 μm. Similar to the EC, it is available either as a probe-based system or integrated into the endoscope.
There are some key challenges which need to be addressed before EC can be accepted into routine practice. Extensive preparation is required prior to Endocytoscope assessment such as intensive washing of the mucosa, as well as staining which can be very labour intensive. The area to be assessed by the Endocytoscope is very small and the time spent studying it has not yet been looked into. Peristalsis, respiratory and cardiac movement artefacts can also make the assessment of lesions technically challenging. Being a new technique, standardized criteria describing the various morphological appearances of the different lesions encountered should be set in place. Systematic training for the novice endoscopist in recognition of these new patterns should then follow.
The future, though, does appear to be bright for the Endocytoscope. Whilst histopathology remains the gold standard for diagnosis of lesions, it involves a multistep process which may take a few days before the final conclusion(s) can be reached. EC simulates histology in real-time and has potential to ultimately replace conventional histopathology. In the shorter term however, this technology may assist the endoscopist in targeting biopsies based on some of the characteristic features described above. It may have the capability of having a very high negative predictive value which could be clinically relevant, especially in patients undergoing surveillance for Barrett’s esophagus or ulcerative colitis where random biopsies normally have a very poor yield. Real-time histologic diagnosis may ultimately allow endoscopists to make decisions in a one stop approach, where lesions can be assessed and management decisions made depending on findings all in a single session. This may ultimately lead to an overall reduction in cost.
In conclusion, EC is a promising novel technology which enables in vivo assessment of cellular detail. Further large scale multicentre randomized controlled trials are needed to confirm the true utility of this technology. Issues such as training, reproducibility as well as cost-effectiveness need to be addressed before EC can be accepted into routine clinical practice. Numerous studies are presently underway assessing this exciting new technology and results with regard to its true clinical benefit are eagerly awaited.
Peer reviewers: Peter L Moses, MD, FACG, AGAF, Professor, University of Vermont College of Medicine Section of Gastroenterology and Hepatology, 111 Colchester Avenue, Smith 237B, MCHV, Burlington, VT 05401, United States; Atsushi Nakajima, Professor, Division of Gastroenterology, Yokohama City University Graduate School of Medicine, 3-9 Fuku-ura, Kanazawa-ku, Yokohama 236-0004, Japan
S- Editor Wang YR L- Editor Logan S E- Editor Lin YP
| 1. | Kodashima S, Fujishiro M, Takubo K, Kammori M, Nomura S, Kakushima N, Muraki Y, Tateishi A, Kaminishi M, Omata M. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy. 2006;38:1115-1121. |
| 2. | Fujishiro M, Takubo K, Sato Y, Kaise M, Niwa Y, Kato M, Muto M. Potential and present limitation of endocytoscopy in the diagnosis of esophageal squamous-cell carcinoma: a multicenter ex vivo pilot study. Gastrointest Endosc. 2007;66:551-555. |
| 3. | Kumagai Y, Monma K, Kawada K. Magnifying chromoendoscopy of the esophagus: in-vivo pathological diagnosis using an endocytoscopy system. Endoscopy. 2004;36:590-594. |
| 4. | Kumagai Y, Iida M, Yamazaki S. Magnifying endoscopic observation of the upper gastrointestinal tract. Digestive Endoscopy. 2006;18:165-172. |
| 5. | Kimura S, Inoue H, Sato Y, Aoyama Y, Shimojima M, Masuyama T, Kudo SE. Ex vivo visualization of Helicobacter pylori using an endocytoscopic probe. Biomed Res. 2006;27:255-257. |
| 6. | Pohl H, Rösch T, Tanczos BT, Rudolph B, Schlüns K, Baumgart DC. Endocytoscopy for the detection of microstructural features in adult patients with celiac sprue: a prospective, blinded endocytoscopy-conventional histology correlation study. Gastrointest Endosc. 2009;70:933-941. |
| 7. | Yan BM, Van Dam J. In vivo real-time endocytoscopic visualization of blood flow in rectal microvasculature. Endoscopy. 2008;40:534-536. |
| 8. | Sasajima K, Kudo SE, Inoue H, Takeuchi T, Kashida H, Hidaka E, Kawachi H, Sakashita M, Tanaka J, Shiokawa A. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc. 2006;63:1010-1017. |