Published online Oct 28, 2010. doi: 10.3748/wjg.v16.i40.5011
Revised: July 30, 2010
Accepted: August 6, 2010
Published online: October 28, 2010
Small-for-size syndrome (SFSS) in adult-to-adult living-related donor liver transplantation (LRLT) remains the greatest limiting factor for the expansion of segmental liver transplantation from either cadaveric or living donors. Portal hyperperfusion, venous pathology, and the arterial buffer response significantly contribute to clinical and histopathological manifestations of SFSS. Here, we review the technical aspects of surgical and radiological procedures developed to treat SFSS in LRLT, along with the pathophysiology of this condition.
- Citation: Gruttadauria S, Pagano D, Luca A, Gridelli B. Small-for-size syndrome in adult-to-adult living-related liver transplantation. World J Gastroenterol 2010; 16(40): 5011-5015
- URL: https://www.wjgnet.com/1007-9327/full/v16/i40/5011.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i40.5011
Segmental liver transplantation based on cadaveric splitting or living-related donation has been developed as a valuable treatment for patients with end-stage liver disease. It is also a means of overcoming the shortage of organs, and mortality on the waiting list. However, small-for-size syndrome (SFSS) remains the greatest limiting factor for the expansion of segmental liver transplantation from either cadaveric or living donors[1]. If the volume of the engrafted liver is significantly less than standard liver weight in patients with end-stage liver disease who are undergoing partial liver transplantation, the excessive portal venous inflow might cause early portal hypertension[2,3] and increased morbidity and mortality due to SFSS[4]. Previous data have suggested that, in recipients of adult-to-adult living-related liver transplantation (LRLT), one of the most challenging tasks is to match a good size graft, balancing the clinical condition of the sick recipient and the safety of the healthy donor. More recently, emphasis has been placed not only on the evaluation of the ratio between donor and recipient liver volume, but also on the degree of portal hypertension and the stage of the liver disease in the recipient.
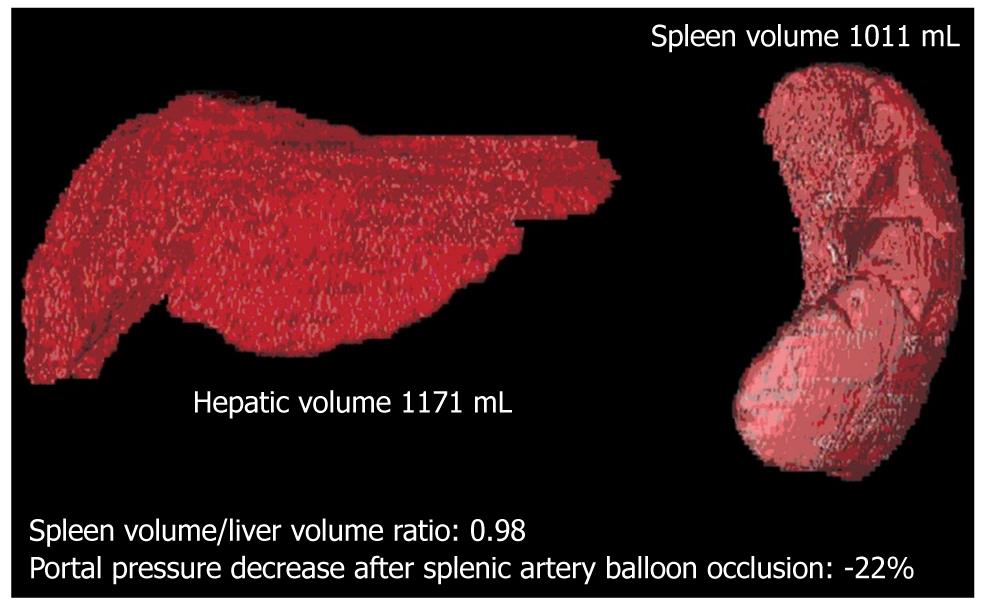
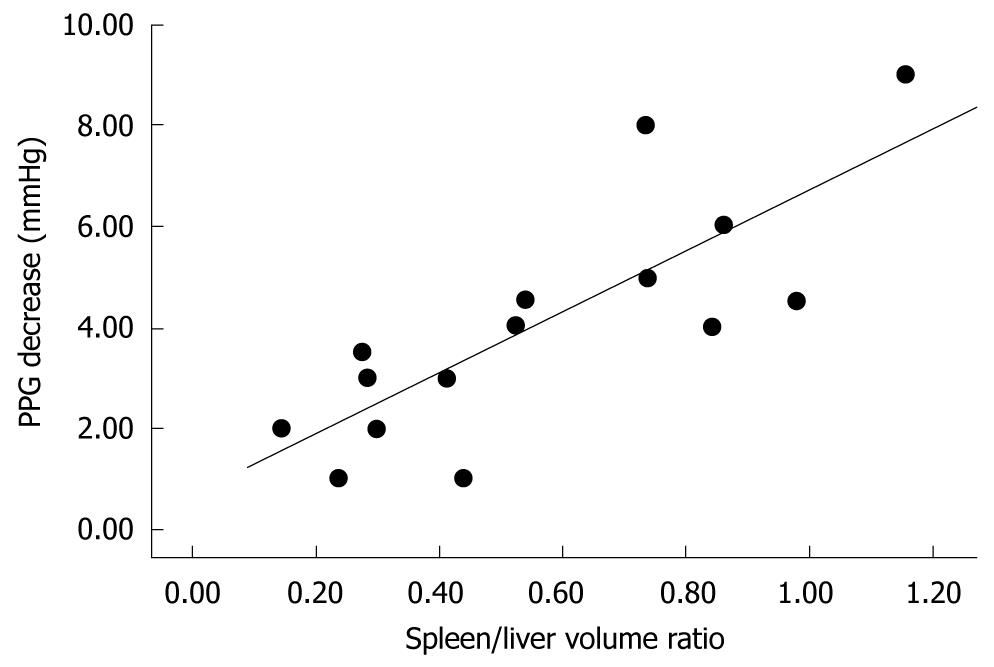
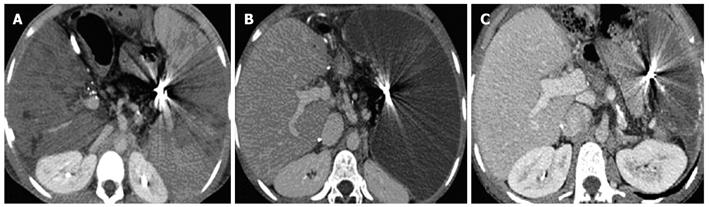
In the initial experience with the use of left graft for adult liver transplantation, recipients of small-for-size grafts developed SFSS, characterized by prolonged cholestasis with elevated serum bilirubin, coagulopathy, rises in cytolytic enzymes, ascites, and in severe cases, gastrointestinal bleeding, which occurred within the first week after transplantation[5-7]. In the most severe cases, this syndrome can progress to acidosis, hypoglycemia, encephalopathy, renal failure and septic shock unless retransplantation is promptly considered. Although recipients with low, or absent, portal hypertension could require less hepatic mass from the living donor, underestimation of the useful size-match between recipient and living donor can generate poor results. The pathogenesis of SFSS has yet to be fully clarified. Earlier data have suggested that a graft-to-recipient weight ratio (GRWR) of < 0.8% or a liver volume < 30% of standard estimated volume is a risk factor for the development of SFSS. Recent data, however, have suggested that the exposure of a small graft to persisting hyperdynamic circulation and high portal blood inflow induces impairment of liver regeneration, and hepatic dysfunction[2-4,8-10]. Furthermore, the high portal blood inflow causes a compensatory decrease in arterial blood flow. This phenomenon, known as the “buffering response”, is due to a reciprocal compensatory regulation between portal vein and hepatic artery inflow, and might contribute to a worsening of the graft injury. Marcos et al[11] has reported that balanced portal venous and hepatic arterial inflow, together with adequate venous blood drainage through hepatic veins, are crucial factors in a successful adult-to-adult LRLT. In 2006, for the first time, our group demonstrated that, in patients with cirrhosis and severe portal hypertension, occlusion of the splenic artery causes a significant reduction in portal pressure, which is directly related to spleen volume and indirectly to liver volume[10]. We have found that reduction of portal pressure after splenic artery occlusion is strictly related to the spleen liver volume ratio (SLVR) that is calculated by multidetector computed tomography (MDCT), which suggests that splenic artery embolization (SAE) can be proposed as a mini-invasive technique to treat or prevent SFSS, by decreasing portal graft perfusion in patients with an SLVR > 0.5 (Figures 1 and 2). This concept is at the center of our strategy for performing early SAE for the treatment of SFSS after LRLT. In light of our data, we believe that regardless of the presence of GRWR > 0.8, the presence of an elevated SLVR could be a risk factor for the development of SFSS, and that early treatment is crucial to preventing a cascade of events that might lead to graft failure or need for retransplantation. A liver graft-to-recipient spleen size ratio has been confirmed as a novel predictor of portal hyperperfusion syndrome in living donor liver transplantation[12,13]. A previous study has suggested that abnormalities in the intragraft gene expression, including endothelin-1 overexpression and plasma nitric oxide level reduction, could negatively affect portal hemodynamics after reperfusion and promote SFSS.
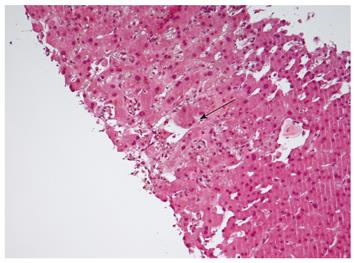
In summary, SFSS has a multifactorial genesis, in which the combination of donor factors (graft volume and quality of the graft, presence of hepatic artery steal syndrome) and recipient factors (portal hyperperfusion, SLVR and stage of liver disease) lead to allograft dysfunction after partial liver transplantation. Recently, Demetris et al[14] have shown that SFSS is characterized by early findings, including portal vein sinusoidal endothelial denudation and focal hemorrhage in the portal tract connective tissue, and poor hepatic arterial flow and vasospasm, which in severe cases, leads to functional de-arterialization, ischemic cholangitis, and parenchymal infarction. Late sequelae in grafts that survive the initial events include small portal vein branch thrombosis with occasional luminal obliteration or recanalization, nodular regenerative hyperplasia, and biliary strictures. In light of these findings, it has been suggested that portal hyperperfusion, venous pathology, and the arterial buffer response significantly contribute to clinical and histopathological manifestations of SFSS (Figure 3)[14].
Several surgical strategies for reducing portal blood inflow and portal pressure after partial liver transplantation or extended liver resection have been proposed. Historically, a decrease in portal hypertension has been obtained by mesocaval or portocaval shunts[15], portal vein banding, splenic artery ligation[3,4,8,9] and splenectomy[8,9]. All these procedures, however, have been used without a firm hemodynamic basis and without a clear documentation of efficacy.
Early diagnosis of SFSS by recognition in the immediate post-transplant period, and the beneficial effects of decreasing splenic venous inflow on portal hypertension have prompted our group to investigate the efficacy of performing early SAE in patients with SFSS. While other groups have routinely performed late SAE[16], we have found the early approach safer and more satisfactory.
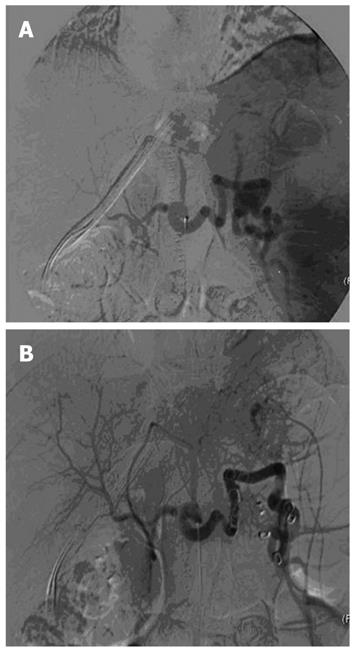
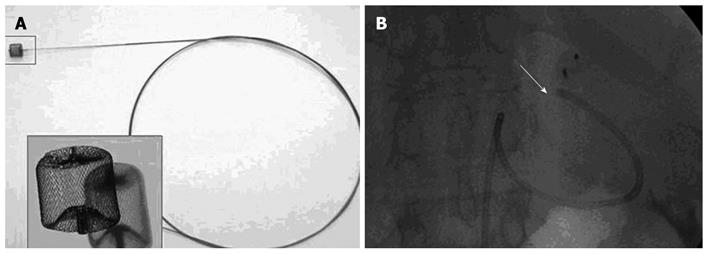
In our experience, as previously published[17], Doppler ultrasound and 64-slice MDCT (VCT; GE Medical Systems) have been performed before the procedure, to confirm the absence of vascular complications and to measure liver and spleen volumes. Antibiotic prophylaxis was administered to every patient. The procedure was performed under conscious sedation and local anesthesia with a right femoral artery approach (Figure 4). No major complications occurred, except in one patient who developed a massive splenic colliquation (which was twice drained percutaneously because of persistent fever and abdominal pain), and who then underwent abdominal wash out. In this case, distal embolization was performed using Gelfoam (Pfizer, Belgium) sponge and particles of Contour (Boston Scientific, Cork, Ireland) (Figure 5). In all the other cases, embolization was performed at a proximal level using metallic coils and/or an Amplatzer Vascular Plug (Figure 6).
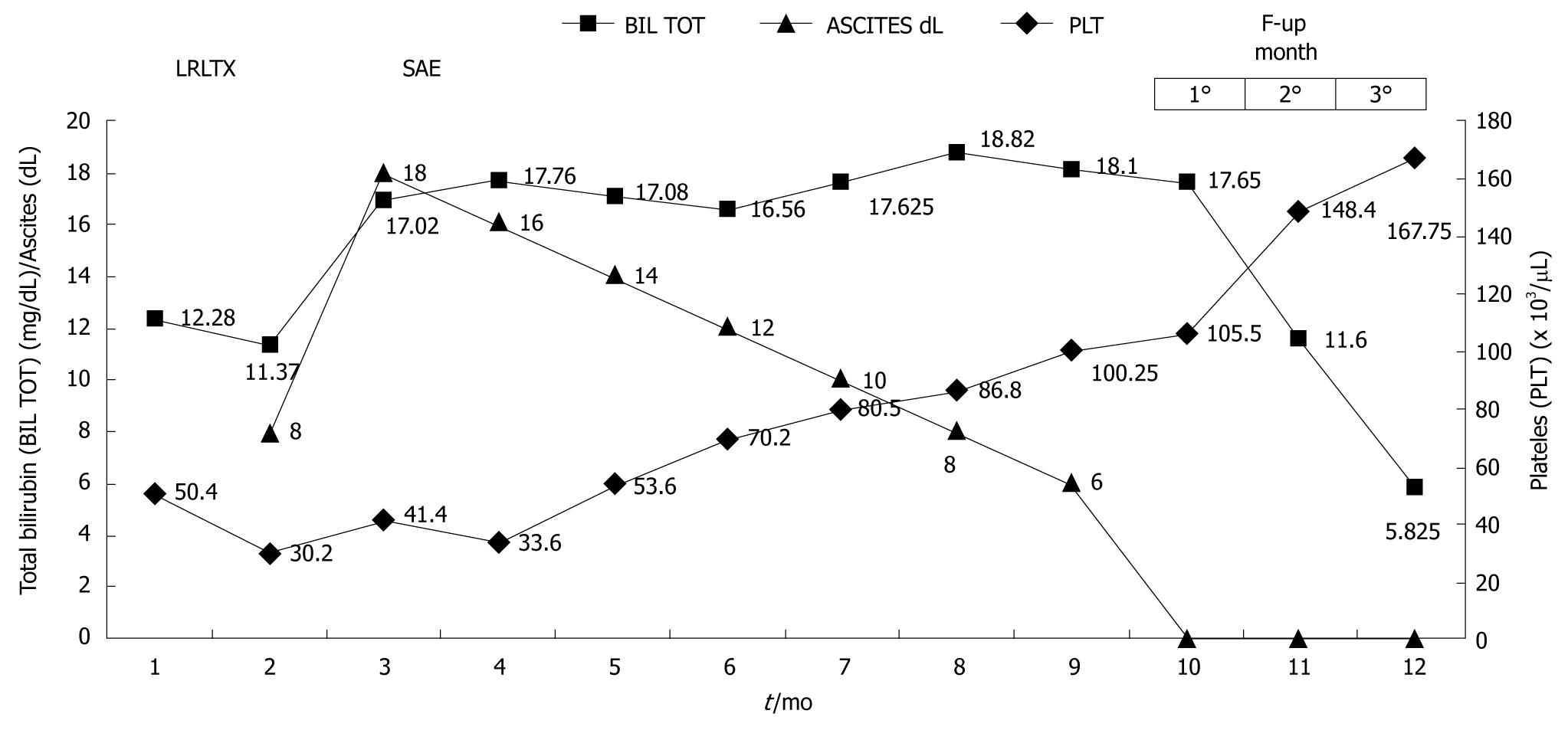
In this series, we confirmed what was evident in our previous study on cirrhotic patients undergoing occlusion of the splenic artery. In fact, there is clearly a contribution of splenic flow to portal hyperperfusion, therefore, early SAE seems to relieve the partial graft from the deleterious effect of this portal overflow. These data, however, need to be confirmed in controlled studies. SAE also has beneficial effects on post-transplant pancytopenia (Figure 7). It has been recently suggested that, in high-risk patients, preoperative SAE could potentially contribute to improved outcome.
Various attempts have been made with drugs to control the excessive portal blood inflow to a small graft. This earlier stage of impairment is treated with the use of continuous intravenous infusion of octeotride or somatostatin and oral consumption of selective β-blockers. The efficacy of these treatments needs to be confirmed in randomized studies.
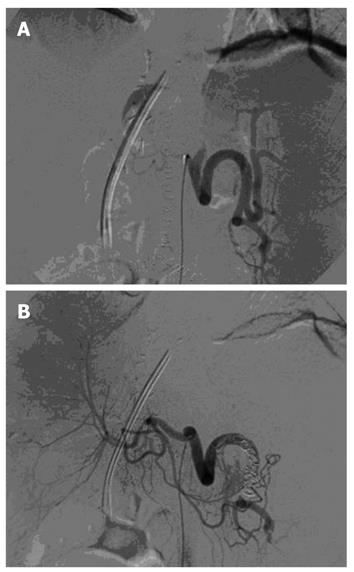
Splenic artery steal syndrome (SASS) is a relatively uncommon cause of graft arterial hypoperfusion; a condition that results from a diversion of celiac blood flow into the splenic artery. SASS usually occurs in patients who, before liver transplantation, have severe portal hypertension, hyperdynamic circulation, splenomegaly and enlarged splenic artery. An increase in intrahepatic resistance, due to rejection or hepatitis recurrence, can worsen SASS, which favors the diversion of blood flow away from the hepatic artery. The left gastric artery or gastroduodenal artery might also contribute to the steal phenomenon. SASS either occurs early or as late as several weeks after transplantation. Patients might have impaired liver function tests, cholestasis, ischemic damage of bile ducts or acute graft failure. The diagnosis of SASS is confirmed by selective angiography of the celiac trunk. Angiographic findings include enlarged splenic artery in comparison to the diameter of the hepatic artery, and early filling of splenic artery associated with delayed and dim filling of the hepatic artery (Figure 8). A variety of surgical procedures are performed to increase hepatic artery perfusion, including ligation or banding of the splenic artery, splenectomy, and insertion of a vascular graft from the abdominal aorta into the hepatic artery. SAE is a non-surgical therapeutic option for SASS.
The transplant community is actually focused on the possibility of detecting predictive factors based on simple biochemical and imaging assessments that could allow physicians to treat those patients at risk of early graft dysfunction immediately after surgery. Although prevention of SFSS should be the goal, how to best manage this once it is established is unclear. In the meantime, as illustrated in Figure 7, we suggest that SAE is a safe and prompt treatment option for patients in whom SFSS is recognized after adult-to-adult LRLT.
Peer reviewer: Dean Y Kim, MD, Surgical Director, Kidney and Pancreas Transplantation, Division of Hepatobiliary and Transplant Surgery, Henry Ford Hospital, 2799 W. Grand Blvd. Detroit, MI 48202, United States
S- Editor Shi ZF L- Editor Kerr C E- Editor Ma WH
| 1. | Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605-2610. |
| 2. | Man K, Fan ST, Lo CM, Liu CL, Fung PC, Liang TB, Lee TK, Tsui SH, Ng IO, Zhang ZW. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg. 2003;237:256-264. |
| 3. | Kiuchi T, Tanaka K, Ito T, Oike F, Ogura Y, Fujimoto Y, Ogawa K. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29-S35. |
| 4. | Ito T, Kiuchi T, Yamamoto H, Oike F, Ogura Y, Fujimoto Y, Hirohashi K, Tanaka AK. Changes in portal venous pressure in the early phase after living donor liver transplantation: pathogenesis and clinical implications. Transplantation. 2003;75:1313-1317. |
| 5. | Ben-Haim M, Emre S, Fishbein TM, Sheiner PA, Bodian CA, Kim-Schluger L, Schwartz ME, Miller CM. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient’s disease. Liver Transpl. 2001;7:948-953. |
| 6. | Emond JC, Renz JF, Ferrell LD, Rosenthal P, Lim RC, Roberts JP, Lake JR, Ascher NL. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Ann Surg. 1996;224:544-552; discussion 552-554. |
| 7. | Tucker ON, Heaton N. The ‘small for size’ liver syndrome. Curr Opin Crit Care. 2005;11:150-155. |
| 8. | Sato Y, Kobayashi T, Nakatsuka H, Yamamoto S, Oya H, Watanabe T, Hatakeyama K. Splenic arterial ligation prevents liver injury after a major hepatectomy by a reduction of surplus portal hypertension in hepatocellular carcinoma patients with cirrhosis. Hepatogastroenterology. 2001;48:831-835. |
| 9. | Mitsumoto Y, Dhar DK, Yu L, Soda Y, Kinugasa S, Kohno H, Nagasue N. FK506 with portal decompression exerts beneficial effects following extended hepatectomy in dogs. Eur Surg Res. 1999;31:48-56. |
| 10. | Luca A, Miraglia R, Caruso S, Milazzo M, Gidelli B, Bosch J. Effects of splenic artery occlusion on portal pressure in patients with cirrhosis and portal hypertension. Liver Transpl. 2006;12:1237-1243. |
| 11. | Marcos A, Olzinski AT, Ham JM, Fisher RA, Posner MP. The interrelationship between portal and arterial blood flow after adult to adult living donor liver transplantation. Transplantation. 2000;70:1697-1703. |
| 12. | Cheng YF, Huang TL, Chen TY, Concejero A, Tsang LL, Wang CC, Wang SH, Sun CK, Lin CC, Liu YW. Liver graft-to-recipient spleen size ratio as a novel predictor of portal hyperperfusion syndrome in living donor liver transplantation. Am J Transplant. 2006;6:2994-2999. |
| 13. | Gruttadauria S, di Francesco F, Vizzini GB, Luca A, Spada M, Cintorino D, Li Petri S, Pietrosi G, Pagano D, Gridelli B. Early graft dysfunction following adult-to-adult living-related liver transplantation: predictive factors and outcomes. World J Gastroenterol. 2009;15:4556-4560. |
| 14. | Demetris AJ, Kelly DM, Eghtesad B, Fontes P, Wallis Marsh J, Tom K, Tan HP, Shaw-Stiffel T, Boig L, Novelli P. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol. 2006;30:986-993. |
| 15. | Takada Y, Ueda M, Ishikawa Y, Fujimoto Y, Miyauchi H, Ogura Y, Ochiai T, Tanaka K. End-to-side portocaval shunting for a small-for-size graft in living donor liver transplantation. Liver Transpl. 2004;10:807-810. |
| 16. | Humar A, Beissel J, Crotteau S, Cohen M, Lake J, Payne WD. Delayed splenic artery occlusion for treatment of established small-for-size syndrome after partial liver transplantation. Liver Transpl. 2009;15:163-168. |
| 17. | Gruttadauria S, Mandala’ L, Miraglia R, Caruso S, Minervini MI, Biondo D, Volpes R, Vizzini G, Marsh JW, Luca A. Successful treatment of small-for-size syndrome in adult-to-adult living-related liver transplantation: single center series. Clin Transplant. 2007;21:761-766. |