Published online Jan 28, 2010. doi: 10.3748/wjg.v16.i4.496
Revised: December 11, 2009
Accepted: December 18, 2009
Published online: January 28, 2010
AIM: To identify the mucosal patterns of Helicobacter pylori (H. pylori)-related gastritis in the gastric corpus using standard endoscopy and to evaluate their reproducibility.
METHODS: A total of 112 consecutive patients underwent upper gastrointestinal endoscopy. The endoscopists classified the endoscopic findings into 4 patterns. In the second part of the study, 90 images were shown to 3 endoscopists in order to evaluate the inter-observer and intra-observer variability in image assessment.
RESULTS: The mucosal patterns of the gastric body were categorized into 4 types. Type 1 pattern was defined as cleft-like appearance, type 2 as regular arrangement of red dots, type 3 pattern as the mosaic mucosal pattern and type 4 pattern as the mosaic pattern with a focal area of hyperemia. Type 1 and type 2 mucosal patterns were statistically significant in predicting H. pylori-negative status as compared with other mucosal types (χ2 = 12.79 and 61.25 respectively, P < 0.01). Type 3 and type 4 mucosal patterns were statistically significant in predicting a H. pylori-positive status as compared with other mucosal types (χ2 = 21.22 and 11.02 respectively, P < 0.01). Furthermore, the sensitivity, specificity, positive and negative predictive values of type 3 plus type 4 patterns for predicting H. pylori-positive gastric mucosa were 100%, 86%, 94%, and 100%, respectively. The mean κ values for inter- and intra-observer agreement in assessing the various endoscopic patterns were 0.808 (95% CI, 0.678-0.938) and 0.826 (95% CI, 0.727-0.925) respectively.
CONCLUSION: Our study suggests that mucosal patterns in H. pylori-infected gastric mucosa without atrophy can be reliably identified using standard endoscopy in the gastric corpus.
-
Citation: Yan SL, Wu ST, Chen CH, Hung YH, Yang TH, Pang VS, Yeh YH. Mucosal patterns of
Helicobacter pylori -related gastritis without atrophy in the gastric corpus using standard endoscopy. World J Gastroenterol 2010; 16(4): 496-500 - URL: https://www.wjgnet.com/1007-9327/full/v16/i4/496.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i4.496
After the discovery of Helicobacter pylori (H. pylori) in 1983, strong evidence has indicated that H. pylori infection plays an important role in the pathogenesis of chronic gastritis, peptic ulcer and gastric carcinoma[1,2]. H. pylori infection can be diagnosed by invasive and noninvasive techniques; however, at least 2 different tests are necessary to make the diagnosis of infection according to the European guidelines[3]. Although endoscopic features of H. pylori have been reported in the literature, there is still some debate over whether H. pylori-related gastritis can be diagnosed via endoscopic features alone. Most studies concluded that it is not possible to diagnose H. pylori-related gastritis on the basis of endoscopic findings[4-7]. Recent publications suggest that high resolution magnification endoscopy has been proved to be useful in the identification of normal gastric mucosa and H. pylori-related gastritis[8-10]. However, practicing high resolution magnification endoscopy in daily endoscopy examinations seems not to be feasible, because it takes more examination time and needs more training and experience. If specific mucosal patterns of H. pylori-related gastritis can be identified using standard endoscopy, they may be applicable to targeted biopsy of suspected H. pylori infection in daily practice. Up to the present, there have been no reports regarding specific mucosal patterns of H. pylori-related gastritis in the gastric corpus using standard endoscopy.
The aim of this study was to classify the mucosal patterns of H. pylori-related gastritis in the gastric corpus using standard endoscopy and to evaluate their reproducibility.
A pilot phase of this study was conducted from May 2007 to July 2007, in which 2 experienced endoscopists observed mucosal morphology of the gastric body, and agreed on the classification of mucosal patterns. From August 2007 to February 2008, a total of 112 consecutive patients who underwent upper gastrointestinal endoscopy for the investigation of dyspeptic symptoms were enrolled in the study. The exclusion criteria were the following: anemia; history of cirrhosis; use of certain drugs, including non-steroidal antiinflammatory drugs, proton pump inhibitors, and H2-receptor antagonists; bleeding tendency; a history of gastric surgery; and a history of eradication of H. pylori. The study was performed in accordance with the principles of the Declaration of Helsinki, and approved by the audit department of our institution.
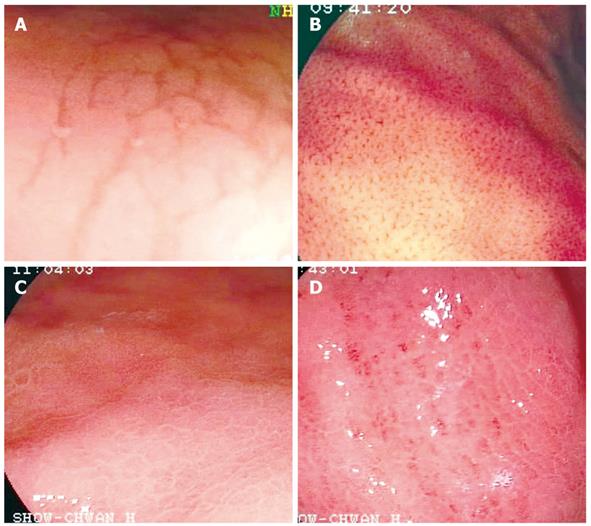
Premedication was the same as for conventional upper gastrointestinal (GI) endoscopic examination. The endoscopic procedures were performed using an upper GI videoendoscope (Fujinon Corporation, Saitama, Japan). The whole stomach was examined first with conventional endoscopy. The gastric corpus was chosen for observation according to previous studies using magnification endoscopy[8-10]. The observed mucosal morphology of the gastric body was classified into 4 patterns. Type 1 was defined as cleft-like appearance mainly extending along the longitudinal axis of gastric body (Figure 1A). Type 2 was defined as regular arrangement of red dots (Figure 1B). Type 3 was defined as the mosaic mucosal pattern without a focal area of hyperemia (Figure 1C). Type 4 was defined as the mosaic pattern with a focal area of hyperemia (Figure 1D). Four biopsy samples were taken directly from the observation sites as shown in Figure 1. Two samples were sent for histological analysis and 2 for a rapid urease test (Hpfast, GI Supply, Camp Hill, PA, USA). If a gastric ulcer or gastric atrophy was present, the nearby non-atrophic mucosa was chosen for observation and biopsy sampling. All endoscopies were performed by 2 experienced endoscopists (Yan SL and Chen CH), who were unaware of the results of histology and rapid urease tests before determining the mucosal types of examined patients. The sensitivity, specificity, positive and negative predictive values of various mucosal types were calculated.
Specimens for histological analysis were placed in 10% formalin solution and routinely processed. The hematoxylin and eosin stain and Giemsa stain were used for identification of H. pylori. The pathologist was blinded to the clinical and endoscopic findings but was aware of the region in the stomach where each biopsy specimen had been obtained.
A diagnosis of H. pylori infection was made if H. pylori were seen on histopathological examination and the rapid urease test was positive. Patients with negative results in both examinations were considered to be H. pylori-negative. According to the European guidelines for the diagnosis of H. pylori infection[3], patients were excluded from the study when they had only one positive result of the rapid urease test or histological examination.
All endoscopic examinations were digitally recorded and still images of observation site were captured for use in the reproducibility study. The selected images were transferred to a software program without distorting brightness, contrast or color balance. Three endoscopists (Hung YH, Yang TH and Pang VS) who had performed over 2000 upper endoscopies were invited to review these still images and were asked to classify them into type 1 to type 4 mucosal patterns as described above. All endoscopists were blinded to the result of H. pylori status and histology before reviewing the slides. A total of 110 images were selected and 20 of these were shown to endoscopists as a reference guide to the 4 types of mucosal pattern. The remaining 90 images (3 type 1, 19 type 2, 50 type 3, 18 type 4) were shown to each endoscopist independently. One week after initial assessment, all endoscopists had to reassess the same images in a random sequence. No time limit for reviewing the slide was imposed. The endoscopists recorded their results on a preprinted form. Data obtained were used for calculation of inter- and intra-observer variabilities.
Statistical analysis was performed using Minitab 14.1 (Minitab Incorporated, Pennsylvania, USA). Inter-group differences were evaluated by the χ2 test. A P value < 0.05 was considered to be statistically significant. The sensitivity, specificity, positive and negative predictive values of the various mucosal patterns were calculated. To examine the chance-adjusted agreement, the κ value was calculated for inter- and intra-observer variabilities. Inter-observer variation was calculated from the results of the first reading, with 3 pairs in all. Intra-observer variation was determined by comparing the first and second assessment for each endoscopist, with 3 pairs in all. κ values below 0.4 indicated poor agreement, values between 0.4 and 0.8 represent moderate agreement, values between 0.6 and 0.8 represented substantial agreement, and values greater than 0.8 corresponded to excellent agreement.
A total of 112 consecutive patients (59 men, 53 women; mean age 47.0 years, range 17-91 years) were enrolled in the study from August 2007 to February 2008. Of the 112 patients included, 7 patients showed type 1 pattern, 24 patients showed type 2, 59 patients showed type 3, and 22 patients showed type 4 (Table 1). H. pylori infection was demonstrated by a positive result of the rapid urease test and histological examination in 76 patients (68%).
| Pattern | H. pylori (+) | H. pylori (-) | Total |
| Type 1 | 0 | 7 | 7 |
| Type 2 | 0 | 24 | 24 |
| Type 3 | 54 | 5 | 59 |
| Type 4 | 22 | 0 | 22 |
| Total | 76 | 36 | 112 |
All 7 patients with a type 1 mucosal pattern corresponded to an H. pylori-negative stomach (type 1 vs other mucosal types, χ2 = 12.79, P < 0.01). The sensitivity, specificity, positive and negative predictive values of the type 1 pattern for predicting H. pylori-negative gastric mucosa were 20%, 100%, 100%, and 72%, respectively (Table 2).
| Pattern predicting | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
| H. pylori (-) | ||||
| Type 1 | 20 | 100 | 100 | 72 |
| Type 2 | 67 | 100 | 100 | 86 |
| Type 3 | 14 | 29 | 8 | 42 |
| H. pylori (+) | ||||
| Type 3 | 71 | 86 | 92 | 58 |
| Type 4 | 29 | 100 | 100 | 40 |
| Type 3+4 | 100 | 86 | 94 | 100 |
All 24 patients with a type 2 mucosal pattern corresponded to an H. pylori-negative stomach (type 2 vs other mucosal types, χ2 = 61.25, P < 0.01). The sensitivity, specificity, positive and negative predictive values of the type 2 pattern for predicting H. pylori-negative gastric mucosa were 67%, 100%, 100%, and 86%, respectively (Table 2).
Fifty four out of 59 patients with a type 3 mucosal pattern corresponded to an H. pylori-positive stomach (type 3 vs other mucosal types, χ2 = 21.22, P < 0.01). The sensitivity, specificity, positive and negative predictive values of type 3 pattern for predicting H. pylori-positive gastric mucosa were 71%, 86%, 92%, and 58%, respectively (Table 2).
All 22 patients with a type 4 pattern corresponded to an H. pylori-positive stomach (type 4 vs other mucosal types, χ2 = 11.02, P < 0.01). The sensitivity, specificity, positive and negative predictive values of type 4 pattern for predicting H. pylori-positive gastric mucosa were 29%, 100%, 100%, and 40%, respectively (Table 2).
Type 3 and type 4 patterns were combined for analysis because these 2 mucosal types were generally mosaic in appearance. A combination of these 2 mucosal types was statistically significant in predicting H. pylori-positive status as compared with other mucosal types (χ2 = 82.80, P < 0.01). The sensitivity, specificity, positive and negative predictive values of type 3 plus type 4 patterns for predicting H. pylori-positive gastric mucosa were 100%, 86%, 94%, and 100%, respectively (Table 2).
The mean κ values for inter- and intra-observer agreement in assessing the various endoscopic patterns were 0.808 (95% CI: 0.678-0.938) and 0.826 (95% CI: 0.727-0.925), respectively.
Few studies have addressed the endoscopic features of Helicobacter-related gastritis and most of these studies concluded that H. pylori infection cannot be diagnosed based on endoscopic findings alone[4-7]. Recently, Yagi et al[9] first described the characteristic magnification endoscopic findings of the H. pylori-negative stomach. Further, Anagnostopoulos et al[8] demonstrated the usefulness of magnifying endoscopy in the identification of H. pylori-associated gastritis in a Western population. However, practicing magnification endoscopy takes more examination time and needs more training and experience. It seems not to be feasible to practice magnification endoscopy in daily endoscopy examinations.
In our study, mucosal patterns of the gastric corpus were classified into 4 patterns using standard endoscopy. The type 1 pattern corresponded to H. pylori-negative gastric mucosa with a specificity of 100% but a rather low sensitivity of 20%. The type 2 pattern corresponded to H. pylori-negative gastric mucosa with a sensitivity of 67% and a specificity of 100%. Type 1 and type 2 mucosal patterns were both statistically significant in predicting H. pylori-negative status compared to other mucosal types (P < 0.01). The red-dot appearance of the type 2 mucosal pattern represented the regular arrangement of collecting venules (RAC) of the gastric corpus under magnification endoscopy. Yagi et al[9] first proposed this magnification endoscopic finding as the normal gastric mucosa. In their study, RAC had a sensitivity of 93.8% and a specificity of 96.2% as an indicator of the H. pylori-negative stomach. Nakagawa et al[11] further classified the morphology of collecting venules into 3 magnification endoscopic patterns: regular (R), irregular (I), and obscure (O). The R pattern corresponded to H. pylori-negative gastric mucosa with a sensitivity of 66.7% and a specificity of 100% in the greater curvature of the gastric body.
Type 3 and type 4 mucosal patterns were both statistically significant in predicting H. pylori-positive status as compared with other mucosal types (P < 0.01). Because type 3 and type 4 were generally mosaic in appearance, these 2 mucosal patterns were further combined for analysis, yielding a higher sensitivity of 100%, and a specificity of 86%. The positive and negative predictive values for predicting H. pylori-positive gastric mucosa were 94%, and 100%, respectively. The mosaic mucosa pattern in our study was similar to prominent areae gastricae, which has been shown to be a characteristic double contrast radiological finding in gastritis caused by H. pylori infection[12]. Furthermore, the prominent areae gastricae observed in our study is also similar to the mosaic mucosal pattern in patients with portal hypertension[13]. However, patients with cirrhosis were excluded in our study. Therefore, the mosaic mucosal pattern in our study seems to be a good indicator in predicting H. pylori-positive gastric mucosa in the gastric corpus.
The reproducibility of a classification system is very important in clinical practice. The κ values below 0.4 correspond to poor agreement, values between 0.4 and 0.8 indicate moderate agreement, values between 0.6 and 0.8 represent substantial agreement, and values greater than 0.8 correspond to excellent agreement. In our reproducibility study, the mean κ values for inter- and intra-observer agreement in assessing the various endoscopic patterns were 0.808 (95% CI: 0.678-0.938) and 0.826 (95% CI: 0.727-0.925) respectively. Therefore, our classification system is good to excellent.
In conclusion, our study suggests that mucosal patterns in H. pylori-infected gastric mucosa without atrophy can be reliably identified using standard endoscopy in the gastric corpus.
Although endoscopic features of Helicobacter pylori (H. pylori) infection have been reported in the literature, most studies concluded that it is not possible to diagnose H. pylori-related gastritis on the basis of endoscopic findings alone. Recent studies suggest that high resolution magnification endoscopy has been proved to be useful in the identifying of normal gastric mucosa and H. pylori-related gastritis. However, there have been no reports regarding specific mucosal patterns of H. pylori-related gastritis in the gastric corpus using standard endoscope.
Although high resolution magnification endoscopy has been proved to be useful in the identifying of normal gastric mucosa and H. pylori-related gastritis, practicing high resolution magnification endoscopy in daily endoscopy examinations takes more examination time and needs more training and experience. If specific mucosal patterns of H. pylori-related gastritis can be identified using standard endoscope, they may be applicable to targeted biopsy of suspected H. pylori infection in daily endoscopy examinations.
In this study, endoscopic findings in the gastric corpus without atrophy were classified into 4 patterns. Type 3 and type 4 patterns were generally mosaic in appearance. The sensitivity, specificity, positive and negative predictive values of type 3 plus type 4 patterns for predicting H. pylori-positive gastric mucosa were 100%, 86%, 94%, and 100%, respectively.
The result of this study suggests that the mosaic mucosal pattern in the gastric corpus seems to be a good indicator in predicting H. pylori-positive gastric mucosa and may guide endoscopists to targeted biopsy of suspected H. pylori infection.
This is an interesting paper that may be very beneficial for the gastroenterologist who performs significant numbers of endoscopy and treats patients with H. pylori infection.
Peer reviewer: Joseph J Cullen, MD, Professor, Department of Surgery, University of Iowa Carver College of Medicine, 4605 JCP, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52242, United States
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
| 1. | Komoto K, Haruma K, Kamada T, Tanaka S, Yoshihara M, Sumii K, Kajiyama G, Talley NJ. Helicobacter pylori infection and gastric neoplasia: correlations with histological gastritis and tumor histology. Am J Gastroenterol. 1998;93:1271-1276. |
| 2. | Mihara M, Haruma K, Kamada T, Komoto K, Yoshihara M, Sumii K, Kajiyama G. The role of endoscopic findings for the diagnosis of Helicobacter pylori infection: evaluation in a country with high prevalence of atrophic gastritis. Helicobacter. 1999;4:40-48. |
| 3. | Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167-180. |
| 4. | Bah A, Saraga E, Armstrong D, Vouillamoz D, Dorta G, Duroux P, Weber B, Froehlich F, Blum AL, Schnegg JF. Endoscopic features of Helicobacter pylori-related gastritis. Endoscopy. 1995;27:593-596. |
| 5. | Calabrese C, Di Febo G, Brandi G, Morselli-Labate AM, Areni A, Scialpi C, Biasco G, Miglioli M. Correlation between endoscopic features of gastric antrum, histology and Helicobacter pylori infection in adults. Ital J Gastroenterol Hepatol. 1999;31:359-365. |
| 6. | Loffeld RJ. Diagnostic value of endoscopic signs of gastritis: with special emphasis to nodular antritis. Neth J Med. 1999;54:96-100. |
| 7. | Redéen S, Petersson F, Jönsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946-950. |
| 8. | Anagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007;39:202-207. |
| 9. | Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:39-45. |
| 10. | Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002;34:376-381. |
| 11. | Nakagawa S, Kato M, Shimizu Y, Nakagawa M, Yamamoto J, Luis PA, Kodaira J, Kawarasaki M, Takeda H, Sugiyama T. Relationship between histopathologic gastritis and mucosal microvascularity: observations with magnifying endoscopy. Gastrointest Endosc. 2003;58:71-75. |
| 12. | Dheer S, Levine MS, Redfern RO, Metz DC, Rubesin SE, Laufer I. Radiographically diagnosed antral gastritis: findings in patients with and without Helicobacter pylori infection. Br J Radiol. 2002;75:805-811. |
| 13. | Misra SP, Dwivedi M, Misra V, Agarwal SK, Gupta R, Gupta SC, Mital VP. Endoscopic and histologic appearance of the gastric mucosa in patients with portal hypertension. Gastrointest Endosc. 1990;36:575-579. |