Published online Oct 21, 2010. doi: 10.3748/wjg.v16.i39.4905
Revised: August 11, 2010
Accepted: August 18, 2010
Published online: October 21, 2010
Alcoholic hepatitis (AH) remains a common and life threatening cause of liver failure, especially when it is severe. Although the adjective “acute” is frequently used to describe this form of liver injury, it is usually subacute and has been developing for weeks to months before it becomes clinically apparent. Patients with this form of alcoholic liver disease usually have a history of drinking heavily for many years. While certain aspects of therapy, mainly nutritional support and abstinence are well established, significant debate has surrounded the pharmacologic treatment of AH, and many institutions practice widely varying treatment protocols. In recent years a significant amount of literature has helped focus on the details of treatment, and more data have accumulated regarding risks and benefits of pharmacologic treatment. In particular, the efficacy of pentoxifylline has become increasingly apparent, and when compared with the risks associated with prednisolone, has brought this drug to the forefront of therapy for severe AH. This review will focus on the clinical and laboratory diagnosis and pharmacologic therapies that should be applied during hospitalization and continued into outpatient management. We conclude that the routine use of glucocorticoids for severe AH poses significant risk with equivocal benefit, and that pentoxifylline is a better, safer and cheaper alternative. While the full details of nutritional support lie beyond the scope of this article, nutrition is a cornerstone of therapy and must be addressed in every patient diagnosed with AH. Finally, while traditional psychosocial techniques play a major role in post-hospitalization care of alcoholics, we hope to make the medical clinician realize his or her role in reducing recidivism rates with early and frequent outpatient visits and with the use of baclofen to reduce alcohol craving.
- Citation: Amini M, Runyon BA. Alcoholic hepatitis 2010: A clinician’s guide to diagnosis and therapy. World J Gastroenterol 2010; 16(39): 4905-4912
- URL: https://www.wjgnet.com/1007-9327/full/v16/i39/4905.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i39.4905
The term alcoholic hepatitis (AH) was first used by Beckett et al[1] in 1961, but reports of clinical jaundice after excessive ethanol consumption were not unusual in the early medical literature and they likely represented instances of AH[1,2]. Despite this longstanding observational relationship between alcohol consumption and liver disease, significant work has been required to determine how much alcohol must be consumed to cause liver disease, and which cohort of patients are at highest risk of developing significant liver injury.
Observational studies have shown an increased risk of cirrhosis with ingestion of greater than 10-20 g of alcohol per day in women and more than 20-40 g/d in men[3]. In addition to total duration of alcohol intake, a variety of genetic, environmental and gender-related factors appear to independently influence the development of alcoholic liver disease. Age, female gender, and excess body weight [body mass index (BMI) > 27 kg/m2 in men, BMI > 25 kg/m2 in women] have been identified as independent risk factors for development of liver disease including AH[3-6]. In addition to a smaller volume of distribution, women are at higher risk due to a relative deficiency of gastric alcohol dehydrogenase compared to men[7]. An oral dose of alcohol in a woman is more like an intravenous dose. We have recently seen several women who developed AH after gastric bypass, in which the amount of gastric mucosa available to metabolize alcohol was reduced. More severe forms of AH are associated with consumption of large amounts of alcohol or binge drinking, and concomitant malnutrition[8]. Not surprisingly, the presence of coexisting hepatitis C has also been linked to a poorer prognosis[9].
Diagnosing AH can be challenging as the disease has widely varying presentations and in severe cases can mimic a bacterial infection and/or biliary obstruction. A detailed and thorough history remains the cornerstone of diagnosis[10]. Obtaining such a history can be rather difficult if patients feel ashamed about their drinking habits. Often, lengthy discussions are required to reveal the full extent of alcohol intake.
Questions that are relevant to ask are detailed in Table 1. Patients regularly tell us that they “quit drinking” when in reality they simply reduced the amount or switched from hard liquor or fortified wine to beer. It is important to obtain the exact time sequence and volume and type of alcohol consumption. Many patients have the mistaken impression that beer is not alcohol. It is actually difficult to drink enough beer on a daily basis to develop AH-perhaps 48 beers per day. To develop AH, patients usually have to supplement beer with wine, fortified wine, and/or hard liquor.
| When did you first start to drink alcohol? |
| How many days per week do you usually drink? |
| How many years have you been drinking on a regular or daily basis? |
| How many times have you been arrested for driving under the influence of alcohol? |
| How many times have you been arrested for public intoxication? |
| What type of alcohol do you usually drink? Beer? Wine? Hard liquor? |
| How many drinks of each type of alcohol do you drink on an average day? |
| Do you usually drink at home? Bars? |
| Have you been through an alcohol rehabilitation program? What type-inpatient or outpatient? How many times? |
| Have there been prolonged times when you drank no alcohol? |
| When was your last drink? |
Alcoholism and alcohol-related health problems are common in patients seen in county or university healthcare systems. Detailed histories regarding alcohol are therefore common in these settings. In contrast, patients admitted or seen in private systems may not be questioned at all about alcohol or may be asked only a few superficial questions that the patient finds easy to respond negatively to.
In general, patients with AH have been drinking heavily for years and then report a dramatic increase in the amount of alcohol intake, usually relating to a major life stressor, such as death of a parent, loss of a job, divorce, etc. Also, patients have often stopped drinking alcohol days to weeks prior to presentation due to malaise, poor appetite, and/or the realization that their drinking finally “caught up” with them. Most commonly patients present with nonspecific complaints such as anorexia, nausea and vomiting, abdominal pain, and weight loss (Table 2)[10,11].
| % | |
| Common Presenting Symptoms of Alcoholic Hepatitis[10-13] | |
| Anorexia | 27-77 |
| Nausea and vomiting | 34-55 |
| Abdominal pain | 27-46 |
| Weight loss | 29-43 |
| Physical Examination Findings | |
| Hepatomegaly | 71-81 |
| Ascites | 35 |
| Encephalopathy (from asterixis to coma) | 18-23 |
| Gastrointestinal bleeding requiring transfusion | 23 |
| Jaundice | 37-100 |
| Malnutrition | 56-90 |
| Hepatic bruit | 59 |
In addition to the patient’s history, noteworthy physical findings that may help the clinician focus on AH as the diagnosis include hepatomegaly, ascites, encephalopathy (ranging from asterixis only to coma) and gastrointestinal bleeding requiring transfusion, especially if the cause is esophageal varices. Nonspecific findings of jaundice and malnutrition are also commonly seen[10-12]. With severe AH, jaundice is present in essentially 100% of patients. Notably, fever ranging from 100.4º to 104º, due to AH and not attributable to infection can be seen in over half of patients diagnosed with severe AH[11]. Fever is a common cause of confusion among physicians and can lead to extensive and relatively useless evaluations for fever of unknown origin, when AH should be the obvious explanation. The presence of a hepatic bruit is also very helpful in providing strong evidence for AH, if there is no malignant mass that could also cause a bruit. In one large series, 59% of patients with severe AH had a bruit[13].
Laboratory findings in AH are often nonspecific, but can on occasion provide clues to the diagnosis. These include mild to moderately elevated transaminases, usually with an aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio above 1.5 with AST greater than 45 U/L but usually < 300 U/L[10,14]. However, an unusual variant of AH, known as alcoholic foamy degeneration, can lead to an AST as high as 730 U/L[15]. A serum bilirubin > 2 mg/dL is often required to make a diagnosis, but clinical jaundice is usually present and the bilirubin is regularly greater than 10 mg/dL. Other nonspecific but established markers of alcohol intake include gamma-glutamyltransferase activity (GGT) and erythrocyte mean corpuscular volume[14]. As the severity of alcohol-related liver injury increases, the bilirubin can increase with a concomitant decrease in GGT[16]. Also, it has been our experience that total blood cholesterol levels < 100 mg/dL can predict poor outcome; the lower the cholesterol, the worse the prognosis. Finally, a mild to very elevated leukocytosis (up to 40 000/mm3) is very characteristic of AH. While less common, reports of severe leukemoid reactions are readily found with documented peripheral white blood cell counts > 130 000/mm3[17-20]. In general, a severe leukemoid reaction in AH portents a very poor prognosis[17,19].
Clinicians can be led astray by the presence of both fever and prominent leukocytosis, leading to concern for sepsis and overshadowing the possibility of AH, particularly if the patient’s history is unclear or unattainable due to altered mental status. As these patients may be profoundly nutrient deficient and commonly have comorbidities such as cirrhosis, they are generally at increased risk of infection and therefore an evaluation for bacterial infection should be performed[11]. This evaluation should, at a minimum, include a chest X-ray, blood cultures, abdominal paracentesis (ascites is frequently present), and urinalysis with urine culture. More specific testing should be pursued for localizing clinical signs of infection such as excessive sputum production or diarrhea. However, the clinician must also remember that profound leukocytosis can be seen without a concomitant infection, and extensive evaluation and prolonged use of broad spectrum antibiotics can lead to loss of time and resources, but more importantly, can carry specific risks (superinfection with resistant bacteria or fungi) and delay appropriate therapy[17-19].
In rare instances in which diagnosis is still unclear despite a thorough history, physical examination and laboratory evaluation, a technetium sulfur colloid liver spleen scan can confirm the diagnosis of AH noninvasively[21]. This is perhaps the last remaining indication for this very old imaging modality. However the single photon updated version, otherwise known as the perfused hepatic mass, may have utility in assessing prognosis in parenchymal liver disease.
The dramatic “colloid shift” to the bone marrow and spleen seen in the older version of this scan is characteristic of severe AH and is unusual in other diagnoses. The liver may be nearly invisible on a liver spleen scan and the bone marrow may be so visible that one can count ribs easily.
Liver biopsy is rarely needed outside of research protocols or perhaps when the patient and family fabricate a story of total alcohol abstinence when AH is clinically obvious. This conspiracy is common when the patient is pursuing liver transplantation. A histologic diagnosis of AH rules out the possibility of liver transplantation, at least in the Unites States. Because of the high risk of recidivism, organs can not be allocated to patients with a diagnosis of AH. Only patients who survive AH and continue to have liver failure after 6 mo of observed and documented abstinence can be listed for transplant. Patients usually improve so dramatically with several months of abstinence that a transplant is not needed.
The rare biopsy is usually performed transjugularly because of ascites and/or coagulopathy. The most common characteristic finding on pathology is macrovesicular steatosis which can also be seen in nonalcoholic fatty liver disease[22]. Intrahepatic cholestasis can also be seen and requires the clinician to rule out mechanical obstruction of the bile ducts, and evaluate the patient for other causes of cholestasis such as drug toxicity or viral hepatitis. Mallory bodies can be seen in up to 65% of patients with AH but can also be found in other causes of hepatocyte injury and has been described as indicative but not pathognomonic of AH[2]. Giant mitochondria, and in particular Type I megamitochondria, can also provide a diagnostic clue for AH and correlate with the presence and amount of daily alcohol consumption[23,24]. Ultimately however, biopsy must be correlated with the patient’s history and will rarely provide all the diagnostic information needed in the absence of other details.
Some inexperienced clinicians may assume that such a biopsy could represent nonalcoholic steatohepatitis (NASH). However, patients with NASH do not present with deep jaundice, ascites, coagulopathy, etc. NASH is a much less inflammatory condition. In fact, patients with NASH do not develop jaundice until their advanced cirrhosis is near terminal.
Once a diagnosis is made, treatment should be initiated that addresses all aspects of the disease, including alcohol cessation, correction of nutritional deficiencies and initiation of pharmacologic therapy when needed. In fact, a 3-pronged approach can help clinicians formulate a plan to guide therapy from the time of presentation through hospitalization and following into the outpatient setting to help reduce recidivism and prevent recurrence.
The first consideration for the hospitalized patient, after evaluation and treatment for any signs of alcohol withdrawal, should be nutrition and electrolyte repletion, because AH induces a profound catabolic state. In part because of malnutrition, AH carries a considerably high mortality rate, therefore nutrition remains a key aspect of therapy. Nutrition should be provided orally if the patient is able to eat or via nasojejunal feeding if nausea, vomiting or poor appetite prevent adequate intake of calories. Calorie counting is essential to assure adequate intake as patients require a higher than average caloric intake (approximately 1.2-1.4 times the normal resting intake)[25]. Furthermore, nighttime supplementation of nutrition (approximately 700 kcal/d) may prevent muscle wasting and improve lean muscle mass and should be considered in hospital and beyond if the patient has any evidence of cirrhosis[26]. Furthermore, patients with longstanding alcohol abuse usually require liberal multivitamin, folic acid and thiamine supplementation. Many of these patients are profoundly depleted of potassium due to high aldosterone levels and lack of intake of solid food for weeks to months. Many have been living on a total liquid alcohol diet. Serum potassium levels do not accurately reflect intracellular levels. It may take many days of potassium repletion to finally achieve normokalemia. As these nutritional considerations are being addressed, the next step for the clinician is deciding upon whether pharmacologic therapy and anticipating potential complications of AH.
Patients with pure severe AH in the absence of cirrhosis have relatively little problem with ascites. They eat so little that they do not take in enough sodium to retain much fluid. Maintenance intravenous fluids should be avoided to minimize fluid retention. When cirrhosis is also present, they may have more problematic fluid retention. In this case, if blood urea nitrogen and creatinine are normal, spironolactone can be given. This drug will increase urinary excretion of sodium and water, increase serum potassium, and decrease the need for potassium supplementation. Once serum potassium is normal without supplementation, oral furosemide can be added, if needed. If azotemia occurs, diuretics should be stopped and the patient should be evaluated for hepatorenal syndrome. The first step is to give 1 g of 25% albumin/kg body weight (100 g maximum) intravenously daily for 2 d and to monitor creatinine. If creatinine improves with albumin, the azotemia is probably diuretic-induced. If creatinine continues to rise, hepatorenal syndrome is probably present, as this commonly occurs in severe AH (see below).
Similar to the situation with ascites, the authors have observed that patients with pure severe AH in the absence of cirrhosis have relatively little problem with upper gut hemorrhage. The relatively short duration of AH usually does not lead to formation of varices that are large enough to bleed. However, patients with underlying cirrhosis can bleed from esophageal varices. Urgent endoscopy with banding of varices is warranted when this occurs. Patients with severe AH are very intolerant of hypotension and seldom survive shock superimposed on AH.
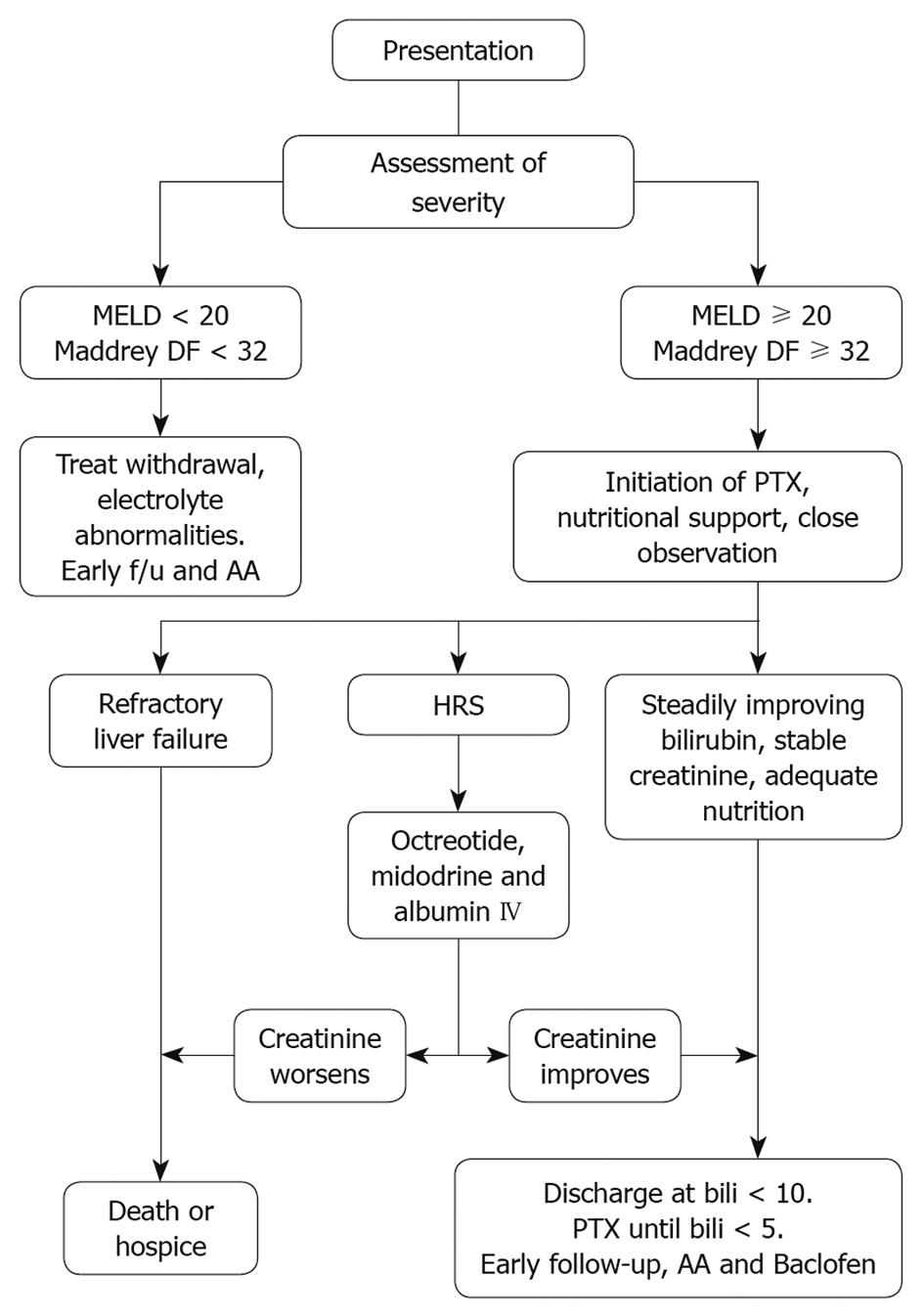
Deciding upon appropriate pharmacologic management of patients with AH relies heavily upon assessment of the severity of disease. Mild forms of AH may improve with abstinence and conservative management, while more severe disease is associated with significant mortality and should be treated more aggressively[27]. The Maddrey discriminant function (DF) [4.6 × (prothrombin time (PT) in seconds - control PT) + serum bilirubin (mg/dL)] was first introduced in 1978 in order to aid in the assessment of disease and guide therapy, which at that time relied mostly upon corticosteroid use[28]. A cutoff value of 32 was used to identify patients with a mortality rate above 50% without pharmacologic therapy. The shortcomings of the DF were outlined by Dunn et al[10], the most notable of which are the lack of standardized PT measurement techniques and values across different laboratories, and more importantly a relatively high risk of mortality (up to 17%) with DF values under 32. The model for end-stage liver disease (MELD) has been evaluated and compared with DF in predicting mortality and has helped guide initiation of pharmacologic therapy[10,29,30]. A MELD score of 11 has equal sensitivity but higher specificity then DF in predicting 30-d mortality[29]. A MELD score of 20 or higher at the time of admission has the highest sensitivity and specificity for predicting in-hospital mortality and outperformed both DF and Child-Pugh-Turcotte (CPT)[30]. We propose using a MELD score ≥ 20 or a DF of 32 or higher to prompt initiation of pharmacologic therapy (Figure 1).
There may be a component of malabsorption of vitamin K due to jaundice in addition to poor synthesis of coagulation components by the diseased liver. The International Normalized Ratio regularly decreases after 3 daily doses of 10 mg of vitamin K intravenously or subcutaneously. Oral dosing of vitamin K is not appropriate because of poor absorption in the setting of deep jaundice.
After diagnosing and assessing the severity of AH, the decision of which pharmacologic therapy to initiate has become a major point of debate among experts, but recent publications may help narrow the choices down considerably. A few small trials had suggested that glucocorticoids can improve short-term survival in patients with the severe AH (DF ≥ 32)[31]. Since then, however, significant work has challenged the efficacy of steroids and raised concerns regarding side effects. The first of these was a meta-analysis by Christensen et al[32] in 1995 which did not support the routine use of glucocorticosteroids in these patients. In addition, a 2008 Cochrane review of 15 randomized controlled trials with a total of 721 patients concluded that glucocorticosteroids did not statistically reduce mortality compared with placebo or no intervention. Only when a subset of patients with DF ≥ 32 or with encephalopathy were evaluated was a mortality benefit seen[33]. In addition to the limited efficacy found by these analyses, there is also a propensity for side effects with even relatively short-term use of steroids. The clinician must take these side effects into consideration. Approximately 16% of patients experience adverse effects, primarily in the form hyperglycemia or Cushing’s syndrome, but also with increased risk of infection as compared with only a 4% adverse event rate in control patients [relative risk (RR), 3.63; 95% confidence interval (CI): 1.95-6.76][33].
As there is a lack of strong evidence of a survival benefit and a propensity for adverse events, routine use of glucocorticosteroids is discouraged. In the ongoing obesity epidemic, many patients with severe AH now have frank diabetes or insulin resistance. Corticosteroids can make diabetes overt or convert non-ketosis-prone patients to a ketosis-prone state.
Another serious practical issue regarding corticosteroid treatment is discontinuation of this highly problematic drug. In some of the trials, the drug was stopped abruptly. In others the dose was tapered. Many physicians are reluctant to stop steroid treatment abruptly and the patients may remain on it too long. Many patients with severe AH are in county hospitals or other settings in which they see a different physician in the clinic than in the hospital. The patient may be homeless and not return to the clinic at all.
In the USA these patients are frequently taken to or transferred to an academic hospital but then discharged to a county hospital because of a lack of insurance that is required to return to the academic system. The new clinic physician may not have access to the patient’s medical information and may not even know why the patient is on steroids. It has been the authors’ experience that too often the drug is continued for more than 30 d because the clinic physician does not know what to do with the steroid dose. Too often it is just continued at the high dose, putting the patient into what the authors call “steroid auto-pilot”. The drug may be continued for months in this setting with the dose being adjusted up or down, for no apparent reason, unless someone finds out that it is supposed to be stopped. Then the issue of tapering the dose comes up.
Yet another issue is prednisone vs prednisolone for treatment. When steroids are avoided, such a debate is unlikely.
Unless a physician makes a commitment to see the same patient in the clinic and stop the drug when appropriate, it is the authors’ opinion that the physician should not start steroid treatment for this condition. The hospitalist movement in the USA has led to different physicians caring for the patient in the hospital vs in the clinic and has made continuity and follow-through on a plan of care very difficult.
More recently, trials and reviews of pentoxifylline (PTX) have shown a better risk benefit profile than that of steroids, and point to PTX as a better first-line agent in treatment of severe AH than glucocorticosteroids. The efficacy of PTX in severe AH was demonstrated by Akriviadis et al[13] in 2000 with a randomized, placebo-controlled trial showing significant benefit in both short-term survival and in preventing development of hepatorenal syndrome (HRS), a key cause of mortality in AH.
Since then, there has been debate regarding the magnitude of the effect PTX has on mortality, and recent publications have examined the results of the Akriviadis trial along with other available data to clarify this issue. A Cochrane analysis of PTX, published in 2009, performed a detailed analysis of all the combined randomized, controlled trials available at that time. Routine meta-analysis showed reduced mortality (RR, 0.64; 95% CI: 0.46-0.89) and reduced hepatic-related mortality due to HRS (RR, 0.40; 95% CI: 0.22-0.71), and trial sequential analysis found strong support for PTX in lowering serum creatinine, a surrogate marker of HRS. However, the group concluded that there was not a significant effect on mortality based primarily upon trial sequential analysis which included, among other data, an abstract by Lebrec et al[34] which was published fully in 2010. This abstract did not demonstrate the same mortality benefit as that seen in the Akriviadis trial and therefore led to doubt regarding overall efficacy. Since that time, however, the full manuscript by Lebrec has been published and reveals that a likely reason for the discrepancy is based upon the significantly different populations used in each study[13,34]. The recent article included only CPT class C patients with cirrhosis while the older study excluded such patients completely. This may explain the lack of a clear cut mortality benefit, as any advanced cirrhosis patient diagnosed with AH stands to have a predictably poorer outcome than his counterpart without cirrhosis. More important than the differences between the 2 trials, however, is the common finding that PTX therapy correlated significantly with a lower rate of liver-related complications (including HRS and hepatic encephalopathy).
A recent randomized trial comparing PTX to steroids in the setting of severe AH (DF ≥ 32) also showed significantly lower mortality with PTX (35.29% vs 14.71%, P = 0.04) and no incidence of HRS[35]. In terms of adverse events with the use of PTX, gastrointestinal upset including diarrhea, vomiting and or epigastric pain, are the primary complaints. Publications vary widely on the reported rate of adverse events, with gastrointestinal complaints ranging from 9.9% to 26.5%, and overall events including headache, skin rash, spontaneous bacterial peritonitis and urinary tract infection reported as 67.3% for PTX vs 28.2% in the control group. In our experience of treating many hundreds of patients with PTX, side effects rarely lead to discontinuation of this life-saving drug. These patients have so many digestive symptoms routinely that they do not notice the upset stomach that healthy patients may experience with PTX. No life threatening or severe reactions were reported with PTX and therefore a trial should be attempted. We have treated hundreds of patients with PTX with results similar to the Akriviadis trial.
We therefore recommend PTX as the routine first line treatment of severe AH at a dose of 400 mg orally 3 times daily for a period of at least 4 wk. We usually continue this safe, inexpensive drug until the bilirubin is < 5 mg/dL. Patients are usually discharged from hospital when the bilirubin is approximate 10 mg/dL (see below). The drug is usually stopped in the outpatient setting. This strategy may require up to several months of treatment with the latter component taking place in the clinic.
Another advantage of PTX over steroids is that it can be safely given for months, whereas steroids can not.
Due to the prevalence of HRS in AH patients, the clinician must also be prepared to diagnose and treat this disorder. If the serum creatinine is abnormal or the patient has only minimal fluid overload on admission, it is prudent to withhold diuretics. If diuretics are initiated, it can be confusing as to whether subsequent azotemia is diuretic-induced or HRS. A retrospective review of patients with advanced liver disease and renal failure found that misdiagnosis of HRS occurred in approximately 40% of cases[36]; therefore care must be taken to make an appropriate diagnosis before initiating therapy, and a table paraphrasing the diagnostic criteria set by the International Ascites Club is provided[37] (Table 3). Diagnosis should begin with a careful review of medications, cessation of diuretics and any potentially nephrotoxic drugs, urinalysis and urine electrolytes to give a rapid assessment of underlying nephropathy and acute tubular necrosis. This should be followed by a 24 h urine collection to rule out proteinuria, and Doppler ultrasound of the kidneys to rule out parenchymal disease and obstructive uropathy. After the appropriate evaluation is performed and HRS is diagnosed one should initiate therapy with intravenous albumin infusion of 1 g/kg per day (100 g maximum) for a total of 2 d and pharmacotherapy. A study of octreotide and midodrine used in combination showed significant reduction in mortality (43% vs 71%, P < 0.05) and sustained reduction in serum creatinine (40% vs 10%, P < 0.05)[38]. We recommend initiation of octreotide 50 μg/h continuous infusion and midodrine 5 mg orally given every 8 h followed by gradual increases in the dose of midodrine by 2.5 mg increments with each dosing. There is no reason to wait 24 h between dose increases. Time is of the essence in treating this life-threatening complication of severe AH. The goal is to achieve an increase in mean arterial pressure of 15 mmHg or until a systolic blood pressure of 140 mmHg is reached.
| Cirrhosis with ascites |
| Serum creatinine > 1.5 mg/dL (> 133 μmol/L) |
| No improvement in serum creatinine (< 1.5 mg/dL) after at least 2 d with diuretic withdrawal, and volume expansion with intravenous albumin. The recommended dose is 1 g/kg of body weight per day up to a maximum of 100 g/d |
| Absence of shock |
| No current or recent treatment with nephrotoxic drugs |
| Absence of parenchymal kidney disease as indicated by proteinuria > 500 mg/d, microhematuria (> 50 red blood cells per high power field) and/or abnormal renal ultrasonography |
Finally, consideration of the use of an anabolic steroid, in particular oxandrolone, can be made. In a study comparing oxandrolone to prednisolone and placebo, oxandrolone was found to improve conditional mortality rates beyond 30 d. This effect was especially pronounced in patients with moderate severity AH[39]. A recent review of oxandrolone use in AH, as well as other catabolic diseases associated with muscle wasting, showed evidence of clinical efficacy and few side effects[40]. Improvements in body composition, muscle strength and function, recovery from acute catabolic injury and nutritional status have been shown in various trials[40].
It has been the authors’ policy to add oxandrolone at a dose of 40 mg orally daily for 30 d maximum in the following circumstances: (1) Maddrey score ≥ 80 on admission, or (2) lack of improvement in Maddrey score or MELD after 10-14 d of PTX. Androgenic steroids could theoretically increase the risk of hepatocellular or prostate carcinoma. Because of this potential risk, physicians who have used oxandrolone extensively for severe AH do not prescribe it for more than 30 d. Oxandrolone seems to improve survival in patients with ultra-severe or refractory AH (Table 4).
| Dose | Oxandrolone 40 mg orally daily |
| Duration of therapy | 30 d maximum |
| Circumstances for use | Maddrey score ≥ 80 on admission |
| No improvement in Maddrey score or MELD after 10-14 d of pentoxifylline |
The final consideration, for those patients who survive the initial bout of AH, involves establishing a plan for increasing the likelihood of abstinence from alcohol. This usually involves a multidisciplinary approach involving self-help or 12-step programs, some level of psychiatric or behavioral therapy and also pharmacotherapy. The efficacy of self-help programs is well established and usually accessible if not always funded, and we recommend routine referral and strong encouragement of attendance[41].
The role of the clinician in this recovery program has been relatively undefined, primarily as most pharmacologic therapies have been less than efficacious. Furthermore, clinicians may feel that closely monitoring a patient’s abstinence may be difficult to confirm without measurable markers.
With regard to pharmacotherapy, baclofen has recently been evaluated in terms of safety and efficacy in the setting of alcoholic cirrhosis. Baclofen significantly reduced alcohol cravings and significantly lengthened time to relapse with no significant adverse effects noted after 12 wk of continuous use in a well run randomized, controlled trial[42]. Notably, patients with CPT class C cirrhosis had the most significant effect from treatment, and may reflect the importance of the patient’s commitment to the treatment program. Discharge is considered as the bilirubin level approaches 10 mg/dL, and the clinician can use this to help plan initiation of baclofen.
Many of the early randomized trials of treatment for AH were conducted on a dedicated approximate 100 bed Liver Unit at the University of Southern California. This unit was mostly populated by patients with AH. The founders of this unit, Drs Reynolds and Redeker, determined through 5 decades of experience in treating these patients, that a bilirubin approximate 10 mg/dL was a good marker of stability for discharge. When patients were sent out with higher bilirubin levels, they were regularly rapidly readmitted with further deterioration.
Currently, we are initiating baclofen in the final days of hospitalization, starting at 5 mg orally 3 times daily then increasing to 10 mg on day 3. The authors have had great success in eliminating alcohol craving and eliminating alcohol consumption with baclofen. No side effects have been recognized in this patient population. We are continuing it indefinitely. Some patients who have stopped it have then redeveloped an alcohol craving and requested that it be continued.
With respect to monitoring abstinence, close follow-up with interview may not be adequate and usually requires a committed family member or friend to corroborate abstinence and honestly report recidivism. In addition to, or in place of such an informant, the clinician may find the use of serologic markers useful for monitoring or diagnosing alcoholic recidivism. Carbohydrate-deficient transferrin (CDT) has been approved by the US Food and Drug Administration for identification of heavy alcohol use and is found in high prevalence in alcoholics. Furthermore, CDT is not detectable after approximately 2 wk of abstinence and may help confirm patient reports of abstinence[14]. A second helpful test is measurement of ethyl glucuronide (EtG), a non-volatile, water-soluble, direct metabolite of ethanol which tests positive shortly after consumption and remains positive for up to 80 h after complete alcohol excretion[43]. EtG may play a role in monitoring for recidivism or in drug and alcohol treatment programs that monitor patients more closely.
Peer reviewer: Bronislaw L Slomiany, PhD, Professor, Research Center, C-875, UMDNJ-NJ Dental School, 110 Bergen Street, PO Box 1709, Newark, NJ 07103-2400, United States
S- Editor Wang JL L- Editor Cant MR E- Editor Lin YP
| 2. | Jensen K, Gluud C. The Mallory body: morphological, clinical and experimental studies (Part 1 of a literature survey). Hepatology. 1994;20:1061-1077. |
| 3. | Becker U, Deis A, Sørensen TI, Grønbaek M, Borch-Johnsen K, Müller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025-1029. |
| 4. | Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Crocè L, Sasso F, Pozzato G, Cristianini G. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845-850. |
| 5. | Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108-111. |
| 6. | Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635-638. |
| 7. | Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95-99. |
| 8. | Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408-413. |
| 9. | Zhang T, Li Y, Lai JP, Douglas SD, Metzger DS, O'Brien CP, Ho WZ. Alcohol potentiates hepatitis C virus replicon expression. Hepatology. 2003;38:57-65. |
| 10. | Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353-358. |
| 11. | Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis. 1971;16:481-494. |
| 12. | Sass DA, Shaikh OS. Alcoholic hepatitis. Clin Liver Dis. 2006;10:219-237, vii. |
| 13. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. |
| 14. | Delanghe JR, De Buyzere ML. Carbohydrate deficient transferrin and forensic medicine. Clin Chim Acta. 2009;406:1-7. |
| 15. | Uchida T, Kao H, Quispe-Sjogren M, Peters RL. Alcoholic foamy degeneration--a pattern of acute alcoholic injury of the liver. Gastroenterology. 1983;84:683-692. |
| 16. | Poynard T, Zourabichvili O, Hilpert G, Naveau S, Poitrine A, Benatar C, Chaput JC. Prognostic value of total serum bilirubin/gamma-glutamyl transpeptidase ratio in cirrhotic patients. Hepatology. 1984;4:324-327. |
| 17. | Mitchell RG, Michael M 3rd, Sandidge D. High mortality among patients with the leukemoid reaction and alcoholic hepatitis. South Med J. 1991;84:281-282. |
| 18. | Juturi JV, Hopkins T, Farhangi M. Severe leukocytosis with neutrophilia (leukemoid reaction) in alcoholic steatohepatitis. Am J Gastroenterol. 1998;93:1013. |
| 19. | Morales AM, Hashimoto LA, Mokhtee D. Alcoholic hepatitis with leukemoid reaction after surgery. J Gastrointest Surg. 2006;10:83-85. |
| 20. | Antillon MR, Runyon BA. Effect of marked peripheral leukocytosis on the leukocyte count in ascites. Arch Intern Med. 1991;151:509-510. |
| 21. | Hoefs JC, Green G, Reynolds TB, Sakimura I. Mechanism for the abnormal liver scan in acute alcoholic liver injury. Am J Gastroenterol. 1984;79:950-958. |
| 22. | Ishak KG, Zimmerman HJ, Ray MB. Alcoholic liver disease: pathologic, pathogenetic and clinical aspects. Alcohol Clin Exp Res. 1991;15:45-66. |
| 23. | Bruguera M, Bertran A, Bombi JA, Rodes J. Giant mitochondria in hepatocytes: a diagnostic hint for alcoholic liver disease. Gastroenterology. 1977;73:1383-1387. |
| 24. | Uchida T, Kronborg I, Peters RL. Giant mitochondria in the alcoholic liver diseases--their identification, frequency and pathologic significance. Liver. 1984;4:29-38. |
| 25. | McCullough AJ, O'Connor JF. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2022-2036. |
| 26. | Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, McIlroy K, Donaghy AJ, McCall JL. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48:557-566. |
| 27. | Morgan TR. Treatment of alcoholic hepatitis. Semin Liver Dis. 1993;13:384-394. |
| 28. | Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193-199. |
| 29. | Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2002;2:2. |
| 30. | Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005;42:700-706. |
| 31. | Ramond MJ, Poynard T, Rueff B, Mathurin P, Théodore C, Chaput JC, Benhamou JP. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507-512. |
| 32. | Christensen E, Gluud C. Glucocorticoids are ineffective in alcoholic hepatitis: a meta-analysis adjusting for confounding variables. Gut. 1995;37:113-118. |
| 33. | Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis--a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther. 2008;27:1167-1178. |
| 34. | Lebrec D, Thabut D, Oberti F, Perarnau JM, Condat B, Barraud H, Saliba F, Carbonell N, Renard P, Ramond MJ. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138:1755-1762. |
| 35. | De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613-1619. |
| 36. | Watt K, Uhanova J, Minuk GY. Hepatorenal syndrome: diagnostic accuracy, clinical features, and outcome in a tertiary care center. Am J Gastroenterol. 2002;97:2046-2050. |
| 37. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. |
| 38. | Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci. 2007;52:742-748. |
| 39. | Mendenhall CL, Anderson S, Garcia-Pont P, Goldberg S, Kiernan T, Seeff LB, Sorrell M, Tamburro C, Weesner R, Zetterman R. Short-term and long-term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. N Engl J Med. 1984;311:1464-1470. |
| 40. | Orr R, Fiatarone Singh M. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs. 2004;64:725-750. |
| 41. | Humphreys K, Wing S, McCarty D, Chappel J, Gallant L, Haberle B, Horvath AT, Kaskutas LA, Kirk T, Kivlahan D. Self-help organizations for alcohol and drug problems: toward evidence-based practice and policy. J Subst Abuse Treat. 2004;26:151-158; discussion 159-165. |
| 42. | Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D'Angelo C, Caputo F, Zambon A. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915-1922. |