INTRODUCTION
Gastric adenocarcinoma can be divided into intestinal and diffuse types using the Lauren classification system[1], or as differentiated and undifferentiated using the Nakamura classification system[2]. In general, intestinal-type adenocarcinoma is considered to be essentially equivalent to differentiated adenocarcinoma, as is diffuse-type and undifferentiated adenocarcinoma. These classifications are based on morphological characteristics centered largely on gland formation and histogenetic background.
These histologically different types of gastric tumors exhibit distinct biological behaviors. Intestinal metaplasia, which occurs as a result of Helicobacter pylori infection and consequent atrophic gastritis[3], is common in the human stomach and is associated with an increased risk of gastric cancer[4,5]. Generally, intestinal-type adenocarcinoma is preceded by metaplastic changes, whereas diffuse-type adenocarcinoma is thought to arise in normal gastric mucosa[2]. However, some cases of intestinal-type adenocarcinoma also arise from the gastric mucosa without intestinal metaplasia. Although these histological types of tumors can be distinguished using standard hematoxylin and eosin staining, recent advances in mucin histochemical and immunohistochemical methods using gastric and small intestinal cell markers have enabled the classification of gastric cancer into different phenotypes[6].
CLASSIFICATION ACCORDING TO MUCIN EXPRESSION
Mucins are heavily glycosylated glycoproteins that constitute most of the viscous gel that lines the gastrointestinal epithelium. Tumor phenotypes are generally classified on the basis of the expression various markers, including CD10 as a marker for the brush border on the luminal surface of small intestinal absorptive cells (enterocytes), mucin 2 (MUC2) as a marker of intestinal goblet cells, MUC5AC or human gastric mucin (HGM) as a marker of surface gastric epithelium (foveolar cells), and MUC6 as a marker for pyloric glands (pyloric gland cells, mucous neck cells, pseudopyloric gland cells). Several additional markers specific for the mucin core proteins encoded by the MUC genes are now also available[7].
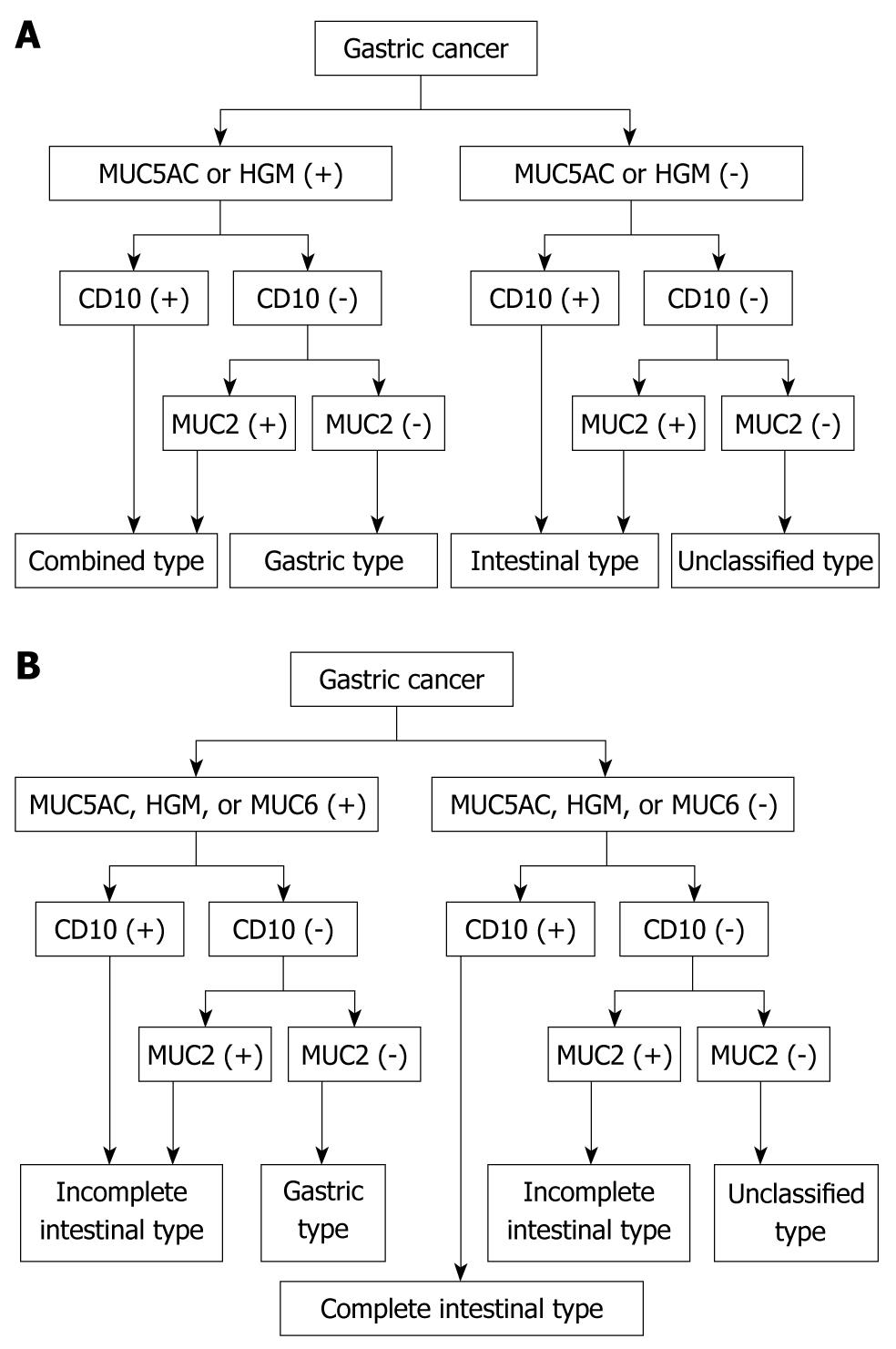
Accordingly, CD10 and MUC2 are considered useful diagnostic markers of the intestinal phenotype, whereas MUC5AC, HGM, and MUC6 can be used to differentiate the gastric phenotype. Although goblet cells are present in the small and large intestine, enterocytes are limited to the small intestine. Gastric cancer phenotypes can be classified quite simply into four groups depending on the combinations of the expression of these markers as intestinal type, gastric type, combined type, and unclassified type (Figure 1A).
Figure 1 Classification of gastric cancer based on mucous expression.
A: Classification of gastric cancer according to the phenotypic combination of mucin expression; B: Classification of gastric cancer by mucin-expression phenotype according to the type of intestinal metaplasia. HGM: Human gastric mucin.
Intestinal metaplasia of the stomach can be divided on the basis of morphology into incomplete, characterized by goblet cells in the gastric gland, and complete, which has small intestinal absorptive cells in addition to goblet cells[8]. These types differ in cell components and in the role they play in gastric carcinogenesis. The incomplete type of intestinal metaplasia is closely associated with carcinoma, whereas complete-type intestinal metaplasia is not considered a precancerous lesion[9,10].
Based on the type of intestinal metaplasia, gastric cancer phenotypes can be classified into four groups depending on the marker combinations as complete intestinal type, incomplete intestinal type, gastric type, and unclassified type (Figure 1B). The complete intestinal type is positive for CD10 and MUC2, and negative for MUC5AC (Figure 2). The incomplete intestinal phenotype is positive for CD10 and MUC5AC simultaneously, or positive for MUC2 alone. The gastric type is positive for MUC5AC, and negative for CD10 and MUC2 (Figure 3). Unclassified phenotypes are negative for CD10, MUC2, MUC5AC, and MUC6. Classification of the mucin phenotype based on the type of intestinal metaplasia is useful for understanding the biological behavior of carcinomas and when considering various therapeutic strategies.
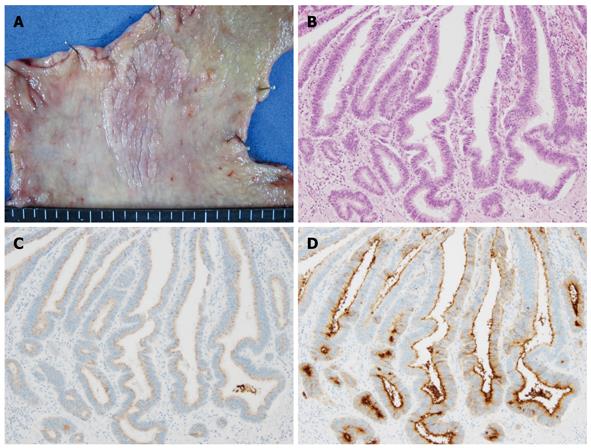
Figure 2 Complete intestinal-type differentiated adenocarcinoma.
A: Macroscopic appearance showing a slightly elevated granular lesion with a distinct margin; B: Well-differentiated tubular adenocarcinoma (HE staining); C: No staining of MUC5AC was found in the carcinomatous gland; D: Positive staining of CD10 was apparent on the luminal side of the carcinomatous gland.
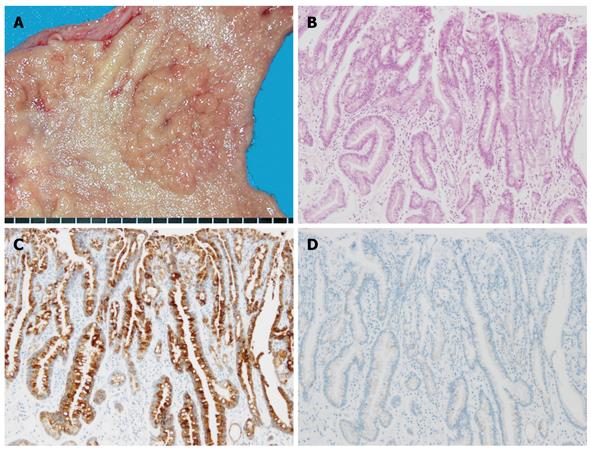
Figure 3 Gastric-type differentiated adenocarcinoma.
A: Macroscopic appearance, showing a fine-granule aggregated lesion; B: Well-differentiated tubular adenocarcinoma (HE staining); C: Diffuse positive staining of MUC5AC was apparent in the carcinomatous gland; D: No staining of MUC2 was evident.
DIAGNOSTIC DIFFICULTIES FOR GASTRIC-TYPE DIFFERENTIATED ADENOCARCINOMA
Mucins specific to the gastric mucosa are defined as gastric-type mucins, although differentiated adenocarcinomas of the stomach change their mucin phenotype as they increase in size and depth of invasion. Based on recent reports, the incidence of gastric-type differentiated adenocarcinoma in early gastric cancer (EGC) appears to be 7.9%-23.9%[11-13].
Early gastric-type differentiated adenocarcinomas tend to be significantly larger tumors and exhibit higher rates of submucosal invasion than intestinal-type EGC[12]. An investigation into the macroscopic features of gastric-type differentiated adenocarcinoma by Higuchi et al[14] has revealed that the incidence of a discolored surface and non-wavy tumor margins is significantly higher than in cases of intestinal-type differentiated adenocarcinoma. In another study, gastric-type differentiated adenocarcinomas showed indistinct margins and an even coloring across the mucosal layer, whereas intestinal-type differentiated adenocarcinomas had an elevated, distinct margin and a red mucosa[15]. These findings may also reflect the difficulty in correctly diagnosing gastric-type differentiated adenocarcinoma at an early stage.
Esophagogastroduodenoscopy is highly reliable in defining the area of cancer infiltration in EGC, although it remains difficult to diagnose gastric cancer accurately in some cases if biopsy specimens do not reveal the presence of adenocarcinoma. Oda et al[16] have reported that, in terms of endoscopic features, unclear margins and an even color tone are more common in the mucosal layer of gastric-type differentiated adenocarcinoma compared with the intestinal type. Taking these difficulties in endoscopic diagnosis into account[16,17], it is clear that a method enabling the precise diagnosis of gastric-type differentiated adenocarcinoma is needed.
Microscopically, it is often difficult to distinguish gastric-type differentiated lesions from regenerative or inflammatory changes to the intestinal epithelium. The high degree of differentiation and mild cellular atypia of gastric-type differentiated adenocarcinoma frequently causes diagnostic difficulties, especially with regard to presurgical biopsy specimens[17,18]. Despite the fact that these tumors are potentially highly malignant and associated with a high incidence of lymphatic or venous invasion and lymph node metastasis, the clinical and pathological diagnosis of this disease remains a challenge.
BIOLOGICAL BEHAVIOR OF GASTRIC-TYPE DIFFERENTIATED ADENOCARCINOMA
Gastric- and intestinal-types of differentiated adenocarcinoma exhibit some differences in terms of their biological behavior. Specifically, gastric-type tumors show scirrhous infiltration, whereas intestinal-type lesions show solid growth in the gastric wall[16,19]. In addition, although gastric-type differentiated adenocarcinomas commonly arise as well or moderately differentiated cancer, they often change histologically into a signet ring-cell carcinoma or poorly differentiated adenocarcinoma[11,20]. Tajima et al[21] have reported that, of patients with advanced gastric carcinoma, those with gastric-type tumors have a significantly poorer prognosis than those with intestinal-type tumors. Thus, gastric-type differentiated adenocarcinomas can be distinguished from other types of differentiated adenocarcinomas on the basis of their increased malignant potential in the incipient phase of invasion and metastasis[11].
Recent advances in limited surgery, including endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), or minimal surgical therapy such as laparoscopic surgery, now offer a better quality of life to patients with EGC. However, even in the early stages of gastric-type differentiated adenocarcinoma, it would seem that the decision to proceed with EMR, ESD, or minimal surgical procedures as a curative treatment should be made with care. Koseki et al[11] have reported a significantly higher incidence of lymphatic invasion, venous invasion, and lymph node metastasis in gastric-type compared with intestinal-type adenocarcinoma, whereas Kabashima et al[13] have found no significant difference between the different subtypes based on lymphatic or venous invasion and lymph node metastases. Accordingly, the malignant potential of gastric-type adenocarcinoma confined to the mucosa may not be obvious compared with tumors that invade deeper than the submucosa.
Cases of undifferentiated gastric adenocarcinoma show no clinicopathological differences between gastric and intestinal types[22]. However, gastric-type undifferentiated tumors display different growth patterns compared with tumors that have an intestinal phenotype[22]. Specifically, gastric-type undifferentiated adenocarcinomas tend to spread through the middle layer of the mucosa more frequently than intestinal-type lesions, and because the carcinoma cells do not appear on the surface of the mucosa in the gastric-type lesions, the margins of carcinoma are considered to be unclear.
GENETIC BACKGROUNDS ASSOCIATED WITH MUCIN PHENOTYPES
Recent studies have revealed that the genetic background of patients with differentiated adenocarcinoma differs among mucin phenotypes[12,23-25]. The p53 gene is a tumor suppressor. Overexpression of p53 protein is widespread in differentiated adenocarcinoma regardless of the mucin phenotype, but is rare in undifferentiated adenocarcinoma[12,23]. The microsatellite instability (MSI) status of a tumor represents mutations of short tandem repeat sequences distributed throughout the genome. Endoh et al [25] have demonstrated that MSI is closely related to expression of the gastric phenotype. Yamazaki et al[26] also have reported that MSI is significantly associated with gastric phenotype and, furthermore, that it is inversely associated with CD10 expression, whereas APC mutations are significantly associated with CD10 expression and the intestinal phenotype and inversely associated with expressions of HGM and MUC6. These results suggest that differentiation to gastric gland cells is related to MSI, whereas differentiation to intestinal epithelial cells reflects mutations in APC[27].
The c-erbB-2 oncogene encodes the receptor for an epidermal growth factor-like growth factor with tyrosine kinase activity. Overexpression of c-erbB-2 protein indicates a poor prognosis for patients with gastric cancer[28]. Overexpression of c-erbB-2 has been reported in some differentiated adenocarcinomas, but not in gastric-type differentiated adenocarcinoma[12]. Furthermore, Sugai et al[23] have suggested that the mucin phenotype of a differentiated adenocarcinoma of the stomach is dependent on distinct genetic profiling based on chromosomal allelic losses, MSI, and overexpression of the p53 protein[23]. Thus, classification according to phenotypic expression may provide us with an understanding of the genetic basis that underlies the carcinogenesis of differentiated adenocarcinomas.
RELATIONSHIP WITH BACKGROUND MUCOSA
The development of differentiated adenocarcinomas could be closely related to intestinal metaplasia. In general, the phenotype of gastric cancers tends to imitate the surrounding mucosa[29], with gastric-type gastric cancers developing in areas expressing gastric-type or mixed-type mucins. However, Matsuoka et al[12] have indicated that the mucin phenotype of an early-stage differentiated adenocarcinoma may not reflect the mucin type in the surrounding gastric mucosa, because pure intestinal metaplasia was observed in 11.8% of gastric-type differentiated adenocarcinomas[12].
Kabashima et al[29] have reported a higher incidence of gastric-type carcinomas and incomplete intestinal-type background mucosa among cases of multiple-type EGCs than among cases with a solitary type of cancer. Intestinal metaplasia surrounding differentiated adenocarcinoma with a gastric phenotype is proposed to be immature and incomplete compared with differentiated adenocarcinoma with a gastric-intestinal or intestinal phenotype[30]. The instability of differentiated adenocarcinomas and the background mucosa in multiple-type early gastric carcinomas have been implicated in the high neoplastic potential and multiple occurrences of these carcinomas[29].
CONCLUSION
The biological behavior of gastric cancer and the relationship with background mucosa reveal different characteristics depending on mucin phenotype expression. Namely, the disease entity of gastric-type differentiated adenocarcinoma has unique clinicopathological characteristics that distinguish it from the intestinal type, including malignant biological behavior and diagnostic difficulties. It is important to consider this type of carcinoma in diagnoses of gastric cancer, and mucin immunohistochemical staining should be conducted in addition to traditional macroscopic examinations to ensure a precise diagnosis. Furthermore, different genetic backgrounds associated with different mucin phenotypes may affect the pathway of gastric carcinogenesis.
Peer reviewers: Qin Su, Professor, Department of Pathology, Cancer Hospital and Cancer Institute, Chinese Academy of Medical Sciences and Peking Medical College, PO Box 2258, Beijing 100021, China; Mitsunori Yamakawa, Professor, Department of Pathological Diagnostics, Yamagata University, Faculty of Medicine, 2-2-2 Iida-Nishi, Yamagata 990-9585, Japan
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP