INTRODUCTION
Pancreatic metastasis from a non-pancreatic primary tumor is rare, accounting for approximately 2% of all pancreatic tumors[1,2]. The clinical occurrence of isolated metastasis to the pancreas is even less. There is currently very limited experience with surgical resection of isolated pancreatic metastasis[3]. In fact, pancreatic resection is associated with a high morbidity and mortality, and metastatic disease to the pancreas is considered a terminal-stage condition[4]. However, recent improvement in morbidity and mortality rates of such patients after pancreaticoduodenectomy has made the indication for this operation more acceptable[5]. The potential benefit of metastasectomy for such cases is documented because it can improve their quality of life and survival time[6,7]. Here, we present a rare male Chinese case of an isolated pancreatic metastatic melanoma, which was surgically treated at our hospital. The patient has survived 25 mo without any signs of local recurrence or other metastatic diseases after operation. The related literature was reviewed.
CASE REPORT
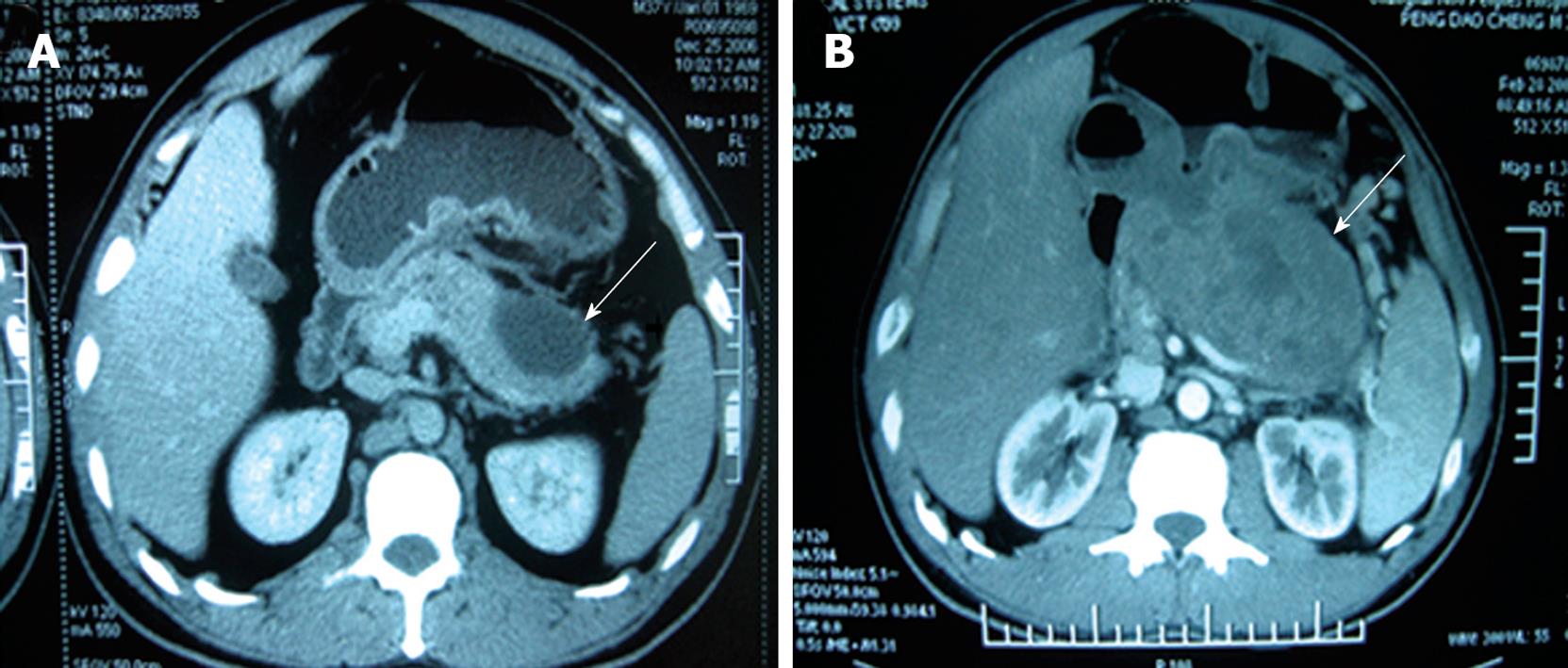
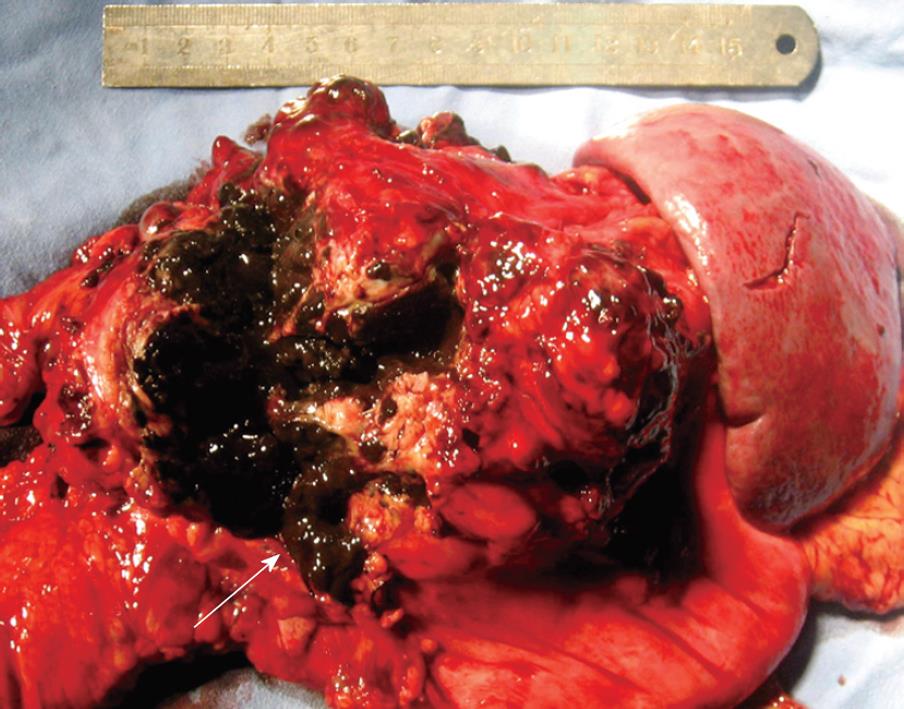
A 39-year-old male Chinese patient was admitted to the Department of General Surgery, Affiliated Changhai Hospital of Second Military Medical University in March 2008 because of a 3-year history of back pain and a cystic tumor in pancreatic tail. He underwent excision of a left eyeball melanoma in a local hospital 5 years ago (2003) with no regional and distant metastases observed. Pathological stage was pT4aN0M0, stage III. The patient did not receive any chemotherapy or radiotherapy after surgery. Subsequently, the patient had back pain in 2006 with a tumor found in pancreatic tail. Computed tomography (CT) showed a cystic tumor in distal pancreas (Figure 1A). The clinical and CT manifestations suggested a benign disease for years. In 2008, because the tumor grew bigger and seemed invasive, the patient was surgically treated at our hospital. Physical examination upon admission in March 2008 revealed the excision of the left eyeball and no local recurrence. Laboratory tests showed normal CEA and CA199 levels, and blood cell count. CT showed a pseudocyst in pancreatic tail with a diameter of 7 cm and poor demarcation from surrounding tissue (Figure 1B). Magnetic resonance imaging (MRI) of the pancreas showed similar results. Endoscopic retrograde pancreatography (ERCP) revealed a deviated main pancreatic duct with no branching in pancreatic tail. Endoscopic examination did not reveal a tumor in any portion of duodenum. In March 2008, a partial pancreatic excision was performed. A tumorous mass measuring 18 cm × 13 cm × 8 cm was found in the pancreatic tail (Figure 2), causing adherence to duodenum and the back of stomach. Frozen section biopsy showed a malignant melanoma which was completely resected with combined distal pancreatectomy and splenectomy, during which no other metastatic foci were found in abdomen.
Figure 1 Computed tomography showing a pseudocystic tumor (arrows) as a benign cyst (A) and a bigger pseudocystic tumor poorly demarcated from the surrounding tissue (B).
Figure 2 Specimen showing a metastatic melanoma as a black-brown mass (arrow) in pancreatic tail.
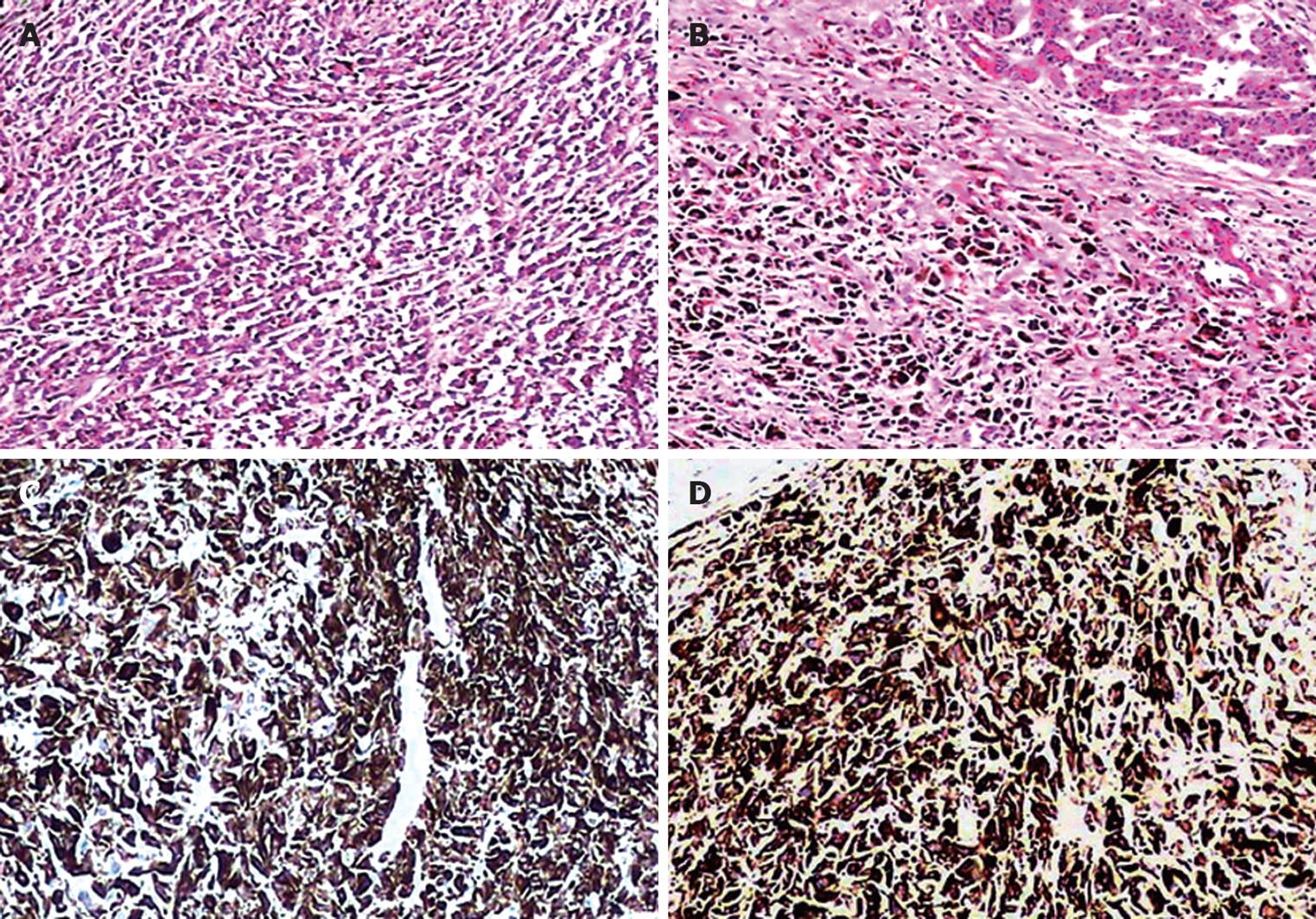
Histopathology revealed ill-defined epithelioid or polygon tumor cells infiltrating the pancreatic tail (Figure 3A) and dark brown granular intracytoplasmic pigmentation in most parts of the infiltration (Figure 3B). The histopathologic pattern of biopsy specimens was very similar to that of specimens examined postoperatively 5 years ago. Fontana-Masson histochemical staining confirmed a melanin pigment. Immunoreactivity was strongly positive for anti-HMB45 (Figure 3C) and anti-S100 protein (Figure 3D) and weakly positive for anti-melan A. Tumor cells were negative for CAM5.2, EMA, CK8/18, CA199 and CDX2. Ki-67 was over 80%. This malignant melanoma was proven to be a metastasis from the initial lesion of the eyeball. Solitary metastatic melanoma of the pancreas from the eyeball was thus diagnosed.
Figure 3 Microscopy showing epithelioid or polygon tumor cells infiltrating the pancreatic tail (A), dark brown granular intracytoplasmic pigmentation in most tumor cells (B), positive HMB-45 (C) and S-100 protein (D).
Original magnification × 200. A and B: HE staining; C and D: Immunostaining.
The patient underwent chemotherapy after surgery and was followed up for 2 years. He has survived 25 mo without any signs of local recurrence or other metastatic lesions after operation.
DISCUSSION
Only 5% of malignant melanomas have been found in the eye, and most ocular melanomas commonly metastasize to the viscera[8]. Ocular melanoma rarely metastasizes to lymph nodes due to lack of lymphatic vessels in the uveal tract[8,9]. Pancreatic metastases occur in less than 2% of patients with visceral melanoma metastases, but most pancreatic metastases disproportionately originate from primary ocular melanoma[9,10]. To date, few cases of pancreatic metastatic melanoma from ocular melanoma have been reported[9-14]. We present an unusual case of solitary pancreatic metastatic melanoma after a latency period of 5 years from melanoma of the eyeball. The patient had a 3-year history of back pain and a cystic mass in pancreatic tail. CT showed a pseudocyst in pancreatic tail with a diameter of 4-7 cm. The clinical and CT manifestations suggested a benign disease for years. Eventually, because the tumor grew bigger and seemed invasive, the patient was surgically treated at our hospital. The distal pancreas and spleen were carefully resected with no other metastatic foci observed in abdomen. Histopathology and immunohistochemistry showed that the tumor was a melanoma, which was considered a metastasis from the initial lesion of eyeball, and chemotherapy was arranged. The patient has survived 25 mo without any signs of local recurrence or other metastatic lesions after operation.
Few reports are available on secondary pancreatic malignancies from a malignant melanoma, and most of them did not explain the features of the tumors. The clinical manifestations of most pancreatic metastases include disturbance of exocrine and/or endocrine function, various gastrointestinal problems, progressive back pain, with or without obstructive jaundice, and pancreatitis[8,9,11-13]. However, in some individuals, pancreatic metastasis is completely asymptomatic[9]. It has been reported that patients with previously diagnosed eye or occult melanoma presenting as a pancreatic metastasis have upper quadrant abdominal pain, nausea, back pain or are asymptomatic[10-14]. In our patient, the melanoma metastasized to the pancreatic tail to form a solitary lesion, and the tumor in pancreatic tail extended to the wall of duodenum and the back of stomach. However, the patient recently experienced back pain. CT showed that the tumor was a cystic lesion as a benign cystic disease for years. ERCP revealed a deviated main pancreatic duct with no branching in pancreatic tail but no obstructive jaundice in the patient. Such invasiveness and CT performance are unique[10-14]. Cystic pancreatic masses seen on imaging and a prolonged history of upper abdominal pain or back pain are clinically suggestive of benign diseases such as inflammatory pseudocyst, true cyst or cystadenoma. The patient was diagnosed as a benign pancreatic cystic lesion. Eventually, because the tumor grew bigger and seemed invasive, he was surgically treated. In fact, the exact preoperative diagnosis of the tumor could not be established, primarily due to the tumor location in the distal pancreas where biopsy remains difficult. The most reliable method for verifying the diagnosis remains histological biopsy of the pancreas. In this case, intraoperative frozen biopsy is necessary and helpful.
It has been reported that the 5-year survival rate of patients with metastatic disease from cutaneous melanoma and those with distant metastasis of ocular melanoma is 18% and 39%, respectively after complete resection[15], while their survival rate is 0% when they are managed non-operatively[16]. It has been shown that median survival time of patients with pancreatic metastatic melanoma after complete and incomplete resection is 24 and 8 mo with a 5-year survival rate of 37% and 0%, respectively[16,17], indicating that complete resection can improve the survival rate of such patients. We think that, if resection is not too dangerous to perform, as in the present case, it is beneficial for prolonging the survival time of such patients. If a concurrent metastatic disease is found elsewhere, it is prudent to adopt a less aggressive approach[2,12-14,16].
The prognosis of patients with pancreatic metastatic malignant melanoma is variable and unpredictable. The interval time from the diagnosis of a primary tumor to pancreatic metastasis is 2-28 years[17,18]. It was reported that the disease-free interval time is not related the survival time[19,20] and that a prolonged interval time free of malignant melanoma does not imply a better prognosis once metastasis occurs[21,22]. Our patient with a solitary pancreatic metastatic melanoma after a latency period of 5 years from melanoma of the eyeball has survived 25 mo without any signs of local recurrence or other metastatic diseases after complete resection of the tumor. It is unclear whether the presence of solitary or multifocal lesions is a determinant for the survival time of patients with pancreatic metastatic malignant melanoma. The prognosis of patients reported in the literature is different[12-14,22,23]. The aim of this report is to add a new case of isolated pancreatic metastatic melanoma in a Chinese male, which was successfully treated with surgical operation, indicating that complete resection of a solitary pancreatic metastatic lesion can prolong the survival time of such patients[23].