Published online Sep 21, 2010. doi: 10.3748/wjg.v16.i35.4436
Revised: May 24, 2010
Accepted: May 31, 2010
Published online: September 21, 2010
AIM: To determine the feasibility and safety of high dose rate intraluminal brachytherapy (HDR-ILBT) boost during preoperative chemoradiation for rectal cancer.
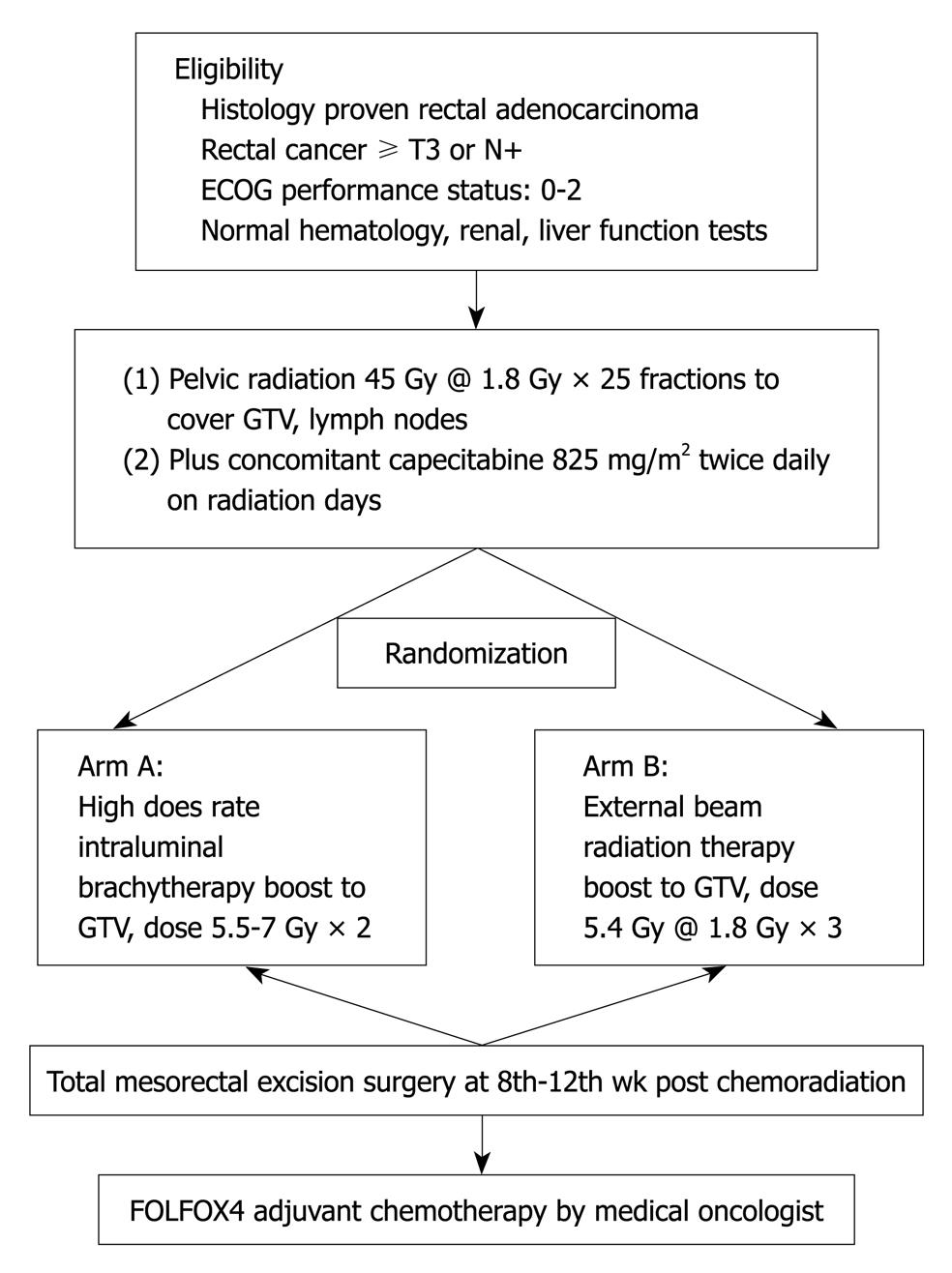
METHODS: Between 2008 and 2009, thirty-six patients with locally advanced rectal cancer (≥ T3 or N+), were treated initially with concurrent capecitabine (825 mg/m2 oral twice daily) and pelvic external beam radiotherapy (EBRT) (45 Gy in 25 fractions), then were randomized to group A; HDR-ILBT group (n = 17) to receive 5.5-7 Gy × 2 to gross tumor volume (GTV) and group B; EBRT group (n = 19) to receive 5.4 Gy × 3 fractions to GTV with EBRT. All patients underwent total mesorectal excision.
RESULTS: Grade 3 acute toxicities were registered in 12 patients (70.6%) in group A and in 8 (42.1%) in group B. Complete pathologic response of T stage (ypT0) in group A was registered in 10 patients (58.8%) and in group B, 3 patients (15.8%) had ypT0 (P < 0.0001). Sphincter preservation was reported in 6/9 patients (66.7%) in group A and in 5/10 patients (50%) in group B (P < 0.01). Overall radiological response was 68.15% and 66.04% in Group A and B, respectively. During a median follow up of 18 mo, late grade 1 and 2 sequelae were registered in 3 patients (17.6%) and 4 patients (21.1%) in the groups A and B, respectively.
CONCLUSION: HDR-ILBT was found to be effective dose escalation technique in preoperative chemoradiation for rectal cancers, with higher response rates, downstaging and with manageable acute toxicities.
- Citation: Tunio MA, Rafi M, Hashmi A, Mohsin R, Qayyum A, Hasan M, Sattar A, Mubarak M. High-dose-rate intraluminal brachytherapy during preoperative chemoradiation for locally advanced rectal cancers. World J Gastroenterol 2010; 16(35): 4436-4442
- URL: https://www.wjgnet.com/1007-9327/full/v16/i35/4436.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i35.4436
The incidence of rectal cancer in Pakistan is similar to those in other Asian countries, but much lower than in the developed countries. At present, the risk is equal in both sexes. However a 41% rise in incidence was noted in Pakistani males during the period 1995-1999, which may indicate a higher risk in males in the future[1]. Most rectal cancers present at advanced stages, and are not amenable to upfront curative surgery. Recent prospective randomized studies with large sample sizes and long-term follow up have reported that preoperative chemoradiation was superior to postoperative chemoradiation in terms of local control, feasibility and toxicity in patients with locally advanced rectal cancer[2,3].
For patients with ≥ T2 or N+ disease, preoperative chemoradiation is given in two phases; initially external beam radiation therapy (EBRT) is given to cover the pelvic nodes along with the primary tumor followed by a dose escalated boost which is given to the gross tumor volume (GTV) with either EBRT, contact X ray therapy or rarely high-dose-rate intraluminal brachytherapy (HDR-ILBT)[4-6].
HDR-ILBT due to its advantage of rapid fall-off of radiation dose allows the delivery of a high tumor dose while sparing normal structures such as normal rectal mucosa and small bowel[7,8]. Additionally HDR-ILBT may reduce overall radiation treatment time and the waiting period for radiation, especially in busy radiation centers. However, there is limited data available regarding the non-inferiority of HDR-ILBT with EBRT as compared to EBRT alone[9,10]. Kaufman et al[9] suggested HDR-ILBT as a boost along with external beam radiation after treating 27 patients with persistant and recurrent rectal, sigmoid and anal cancers.
We attempted to evaluate the feasibility and safety of HDR-ILBT as a boost during preoperative chemoradiation for locally advanced rectal cancer, and to determine whether HDR-ILBT has an advantage in terms of achieving higher pathologic response rates and sphincter preservation.
Patients referred to our department between November 2008 and October 2009 were selected when they met the following eligibility criteria: (1) histologically proven rectal adenocarcinoma; (2) distal margin of tumor located within 10 cm of the anal verge on endoscopy; (3) T stage ≥ T3 or nodes positive on preoperative imaging [Computed tomography (CT), magnetic resonance imaging (MRI)] and M0; (4) Eastern Cooperative Oncology Group performance status 0-2; and (5) normal hematological parameters (hemoglobin ≥ 10 g/dL, white blood cell ≥ 4000/mm3, absolute neutrophil count ≥ 1500/mm3 and platelets ≥ 100 000/mm3), normal hepatic parameters (serum bilirubin level ≤ 1.5 mg/dL and liver transaminase levels ≤ 3 times upper normal limit) and normal renal function (serum creatinine level ≤ 1.5 mg/dL). Patients who had received prior chemotherapy or pelvic radiotherapy or with poor functional status and severe comorbidities were excluded. The treatment protocol is represented in flow chart in Figure 1.
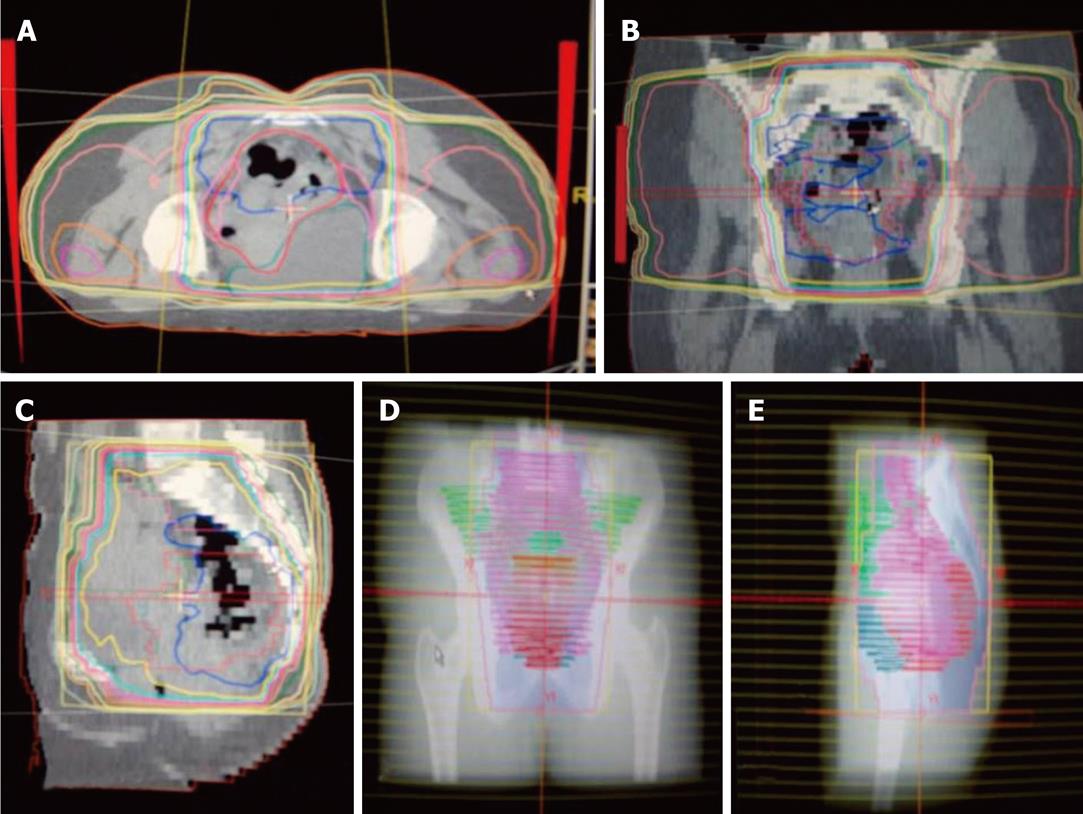
Radiotherapy: Preoperative radiotherapy was delivered using a high-energy multi-leaf collimator linear accelerator (15 MV). All patients were virtually simulated in the prone position using a Siemens® SOMATOM emotions 6 CT scanner, with a device to displace the small bowel (belly board). The whole pelvis was treated with the three field technique and up to 45 Gy in 25 fractions over 5 wk; The superior border was at the L5-S1 interspace, and the lower border was kept at least 3 cm below the tumor. The lateral borders of the anteroposterior-posteroanterior fields were defined 1 cm away from lymph nodes using vessels as surrogate markers (Figure 2). Lateral portals covered the full sacrum and coccyx with a margin; anteriorly they were 3 cm from the sacral promontory.
After the nature of HDR-ILBT was fully explained, patients were classified into two groups: group A; boost to GTV with HDR-ILB and group B; boost to GTV with EBRT as the control group, according to their own choice as to whether to receive the therapy. The study was performed with the approval of the institutional ethics committee. Written informed consent was obtained from all patients before treatment.
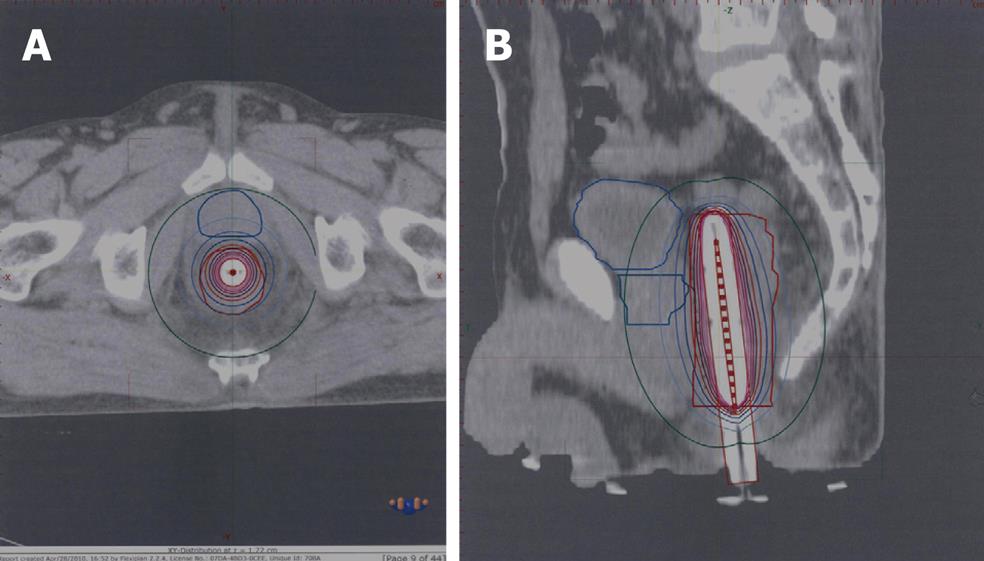
HDR-ILB technique: HDR brachytherapy was given in two sessions at 20 and 40 Gy of EBRT. In a dedicated room, where the patients were kept in the lithotomy position under analgesia, the oncologist inserted an in-house built rectal catheter after per rectal and endoscopic localization of the gross tumor. Catheter placement was confirmed with plain radiographs of the pelvis taken with a 12 inch C-arm unit (OEC 9800Plus; GE Medical Systems). After the procedure was completed, patients were transported to the CT simulator. 3 mm slice thickness images were obtained and were transferred via digital Imaging and Communications in Medicine to the Flexi-plan brachytherapy planning system version 2.2. All organs (GTV, normal rectum, bladder, prostate, uterus, and vagina) were contoured. The apparatus used for HDR-ILBT was a Flexitron® remote afterloading unit with an iridium-192 (Ir192) stepping source. Dwell positions were activated at 2.5 mm along each needle. Dwell times were optimized using a reverse planning optimization algorithm. The prescribed dose was 5.5-7 Gy at 1 cm of the rectal catheter in each session (Figure 3).
Chemotherapy: Oral capecitabine was given at 825 mg/m2 bid for the duration of radiotherapy with the initial dose starting 1 h before radiotherapy. The drug was given during radiation days only (5 d/wk). Dose modifications were given if a patient experienced Grade 2 hematologic toxicities, and capecitabine was stopped until these toxicities resolved. For Grade 2 or greater non-hematologic toxicities, the drug was reduced to 50% of the initial dose. If toxicities recurred, capecitabine was stopped until they resolved. Radiotherapy side effects were managed as per departmental protocols.
Surgery: After the completion of chemoradiation, patients underwent assessment (repeat CT/MRI, endoscopy, ± exploratory laparoscopy) for surgery at the 8th and 10th wk. The choice of procedure (low anterior resection or abdominoperineal resection) was at the discretion of the surgeon.
Postoperative chemotherapy: The choice of chemotherapy was at the discretion of the medical oncologist.
Radiologic and pathologic response rates: At the 8th and 10th wk after chemoradiation, the radiologic response was measured by comparing pre- and post-chemoradiation imaging (CT or MRI). The volume reduction rate was calculated by the following formula:
Tumor volume reduction rate = (pre-chemoradiation tumor volume - post-chemoradiation tumor volume) × 100/pre-chemoradiation tumor volume[11].
Following surgery, pathologic tumor staging was determined according to the TNM classification system by the International Union against Cancer and the American Joint Committee on Cancer. Downstaging was applied for T stage and was defined as “yp”, where “y” was after chemoradiation and “p” for postoperative pathologic examination. All resected specimens were evaluated for pathologic response with careful inspection of tumor, mesorectal fat and circumferential margins. The pathologic complete response of T stage (ypT0) was defined as the absence of cancer cells in resected specimens.
Toxicity profile: Adverse events were graded according to National Cancer Institute Common Toxicity Criteria version 2.0 and were recorded weekly during follow up. Hematology and serum chemistry was checked on a weekly basis and after completion of chemoradiation at 4 and 8 wk.
Pathologic complete response of T stage (yp T0) was considered a binary variable and was scored as 0 or 1 based on the presence of tumor cells. This study design was planned using Simon’s optimal two-stage design[12]. According to this design, in the first stage to document ≥ 2 ypT0, 19 patients were required, otherwise the study would be closed prematurely. The descriptive data (mean, median, range and frequency) were calculated using SPSS version 16.0. The response rates and toxicities were summarized with 95% CI. Comparisons between the two groups were performed with paired-sample t tests for metric data and chi-square tests for frequencies. For all tests, a P value less than 0.05 was considered statistically significant.
A total of 36 patients were considered eligible for the study. Patient characteristics in each treatment group are described in Table 1. There were no differences between the two groups with regard to mean age, gender, baseline TNM stage, site of primary tumor and performance scale. The study population was predominantly young males and the majority of patients had tumor grade T3N+.
| Variable | Group A (intraluminal brachytherapy) (n = 17) | Group B (control arm) (n = 19) |
| Median age (yr) | 34.7 (range 17-55) | 34.7 (range 17-55) |
| Gender | ||
| Male | 13 (76.5) | 14 (73.7) |
| Women | 4 (23.5) | 5 (26.3) |
| Site of primary tumor | ||
| Upper rectum | 8 (47.1) | 9 (47.4) |
| Lower rectum | 9 (52.9) | 10 (52.6) |
| Clinical/radiological stage | ||
| T2 N+ | 1 (5.9) | 1 (5.3) |
| T3 N0 | 2 (11.8) | 3 (15.8) |
| T3 N+ | 7 (41.1) | 8 (42.1) |
| T4 N0 | 5 (29.4) | 6 (31.5) |
| T4 N+ | 2 (11.8) | 1 (5.3) |
| Performance status (ECOG) | ||
| 0 | 13 (76.5) | 12 (63.2) |
| 1 | 4 (23.5) | 7 (36.8) |
A total of 34 procedures were successfully performed, with a technical success rate of 98.0%. The total dose in these 17 patients was 11-14 Gy (dose given each session was 5.5-7 Gy). The procedure was well tolerated with the exception of one patient who developed per rectal bleeding within 2 h of the procedure. The bleeding was stopped after securing hemostasis without the need for blood transfusion.
No treatment related deaths or life-threatening events were seen. Twelve patients (70.6%) had grade 3 rectal pain. Other grade 3 acute toxicities were diarrhea in 7 patients (41.2%), and nausea and vomiting in 3 patients (17.6%) which were more frequent when compared to group B patients (Table 2). Late toxicities in the HDR-ILBT group were mild grade 1 and 2; no grade 3 late toxicities were seen.
| Type of toxicity | Group A (HDR-ILBT) | Group B (EBRT boost) | P value |
| Hematologic | 0.3 | ||
| Leucopenia | 2 (11.7) | 2 (10.5) | |
| Neutropenia | 2 (11.7) | 2 (10.5) | |
| Thrombocytopenia | 1 (5.9) | 1 (5.3) | |
| Non-hematologic | |||
| Hand-foot syndrome | 1 (5.9) | 1 (5.3) | |
| Nausea/vomiting | 3 (17.6) | 5 (26.3) | 0.02 |
| Diarrhea | 7 (41.2) | 5 (26.3) | 0.001 |
| Rectal pain | 12 (70.6) | 4 (21.1) | 0.001 |
| Wound complications | 2 (11.8) | 3 (15.8) | |
| Cystitis | 2 (11.8) | 3 (15.8) |
All 19 patients tolerated EBRT very well with a success rate of 100%. Grade 3 nausea and vomiting was seen in 5 patients (26.3%), which was significantly higher than the HDR-ILBT group (17.6%) with a P value of 0.02. However, grade 3 diarrhea and rectal pain were less common when compared with the HDR-ILBT arm. Wound complications were slightly higher (15.8%) in this group when compared with the HDR-ILBR group (11.8%).
A CT and MRI volumetry evaluation was carried out in 36 patients. The mean pre-chemoradiation tumor volume was 21.9 cm3 (18.30-30, SD 3.7) in group A and 22.3 cm3 (19.3-30, SD 3.4) in group B. The mean post-chemoradiation tumor volume was 6.78 cm3 (5.3-10.3, SD 1.7) in group A and 7.5 cm3 (5.9-10.5, SD 1.6) in group B. The median tumor volume reduction rate was 68.15% and 66.04% in group A and B, respectively (P value not significant).
Pathologic response data were available for all 36 patients who underwent surgery. The pathologic response results are shown in Table 3. Complete pathologic response of T stage (ypT0) in group A was registered in 10 patients (58.8%) and in group B, 3 patients (15.8%) had ypT0 (P < 0.0001). Sphincter preservation was reported in 12 patients (70.6%) in group A and in 11 patients (57.9%) in group B (P = 0.04). When we compared the pathologic stage after chemoradiation and the clinical stage at the time of chemoradiation, overall downstaging for T stages was found in 55.5% and in 55% for N+ stages.
| Pathologic stage | Group A (HDR-ILBT) (n = 17) | Group B (control arm) (n = 19) | P value |
| yp T stage | |||
| ypT0 | 10 (58.8) | 3 (15.8) | 0.0001 |
| ypT1 | 3 (17.6) | 6 (31.6) | |
| ypT2 | 1 (5.9) | 4 (21) | |
| ypT3 | 2 (11.8) | 5 (26.3) | |
| ypT4 | 1 (5.9) | 1 (5.3) | |
| yp N stage | |||
| ypN0 | 6 (60) | 5 (50) | 0.02 |
| ypN+ | 4 (30) | 5 (50) |
High dose rate intraluminal brachytherapy is a well-known treatment modality for gynecologic, esophagus, lung, biliary tract, and nasopharyngeal malignancies[13-17]. The major advantage of HDR-ILBT over external beam irradiation is its rapid fall-off; a high dose can be delivered to the tumor without significantly affecting the adjacent normal tissues.
HDR-ILBT for advanced or inoperable tumors of the rectum has been used both palliatively and to dose escalate after chemoradiation for curative treatment[18]. For palliative relief, recurrent or inoperable rectal cancers, HDR-ILBT has been used worldwide[19-21], however, there is limited data available regarding the use of HDR-ILBT as a boost to GTV along with EBRT during preoperative chemoradiation for rectal cancers (Table 4). Our study showed higher sphincter preservation rates than in all the trials published so far and comparable ypT0 rates. Better pathological response following HDR-ILBT can easily be explained by higher radiation doses to tumor and shorter distances between the radiation source and tumor. One criticism which may arise is that no difference in radiological response was observed between the two groups. Looking at the differences in pathological response, we believe that radiation-induced trauma and fibrosis might be responsible for no radiological response being observed. In the HDR-ILBT arm, a higher incidence of grade 3 rectal toxicity was seen, especially rectal pain followed by diarrhea. We saw only one case with per rectal bleeding which was successfully managed. However, late toxicities were mild and manageable. In addition, more nausea and vomiting and wound complications in the control arm (EBRT) can be explained by greater irradiated bowel, skin and subcutaneous volumes by external beam irradiation.
| Study, period of study (type of study) | No. of patients | Follow up (mo) | Treatment, protocol | Sphincter preservation rates | ypT0 | Local recurrence rate | Distant metastasis | Acute toxicity (ileitis, proctitis), all grades | Disease free survival |
| Yanagi et al[6], 1986-1995 (retrospective) | Arm A: 96 | 60 | Arm A: MDR-ILBT→surgery | Arm A: 72% | Arm A: 8% | Arm A: 23% | 38% | Arm A: 72% | |
| Arm B: 19 | ArmB: HDR-ILBT→surgery | Arm B: 63% | - | Arm B: 5% | Arm B: 16% | 74% | Arm B: 68% | ||
| Arm C: 115 | Arm C: surgery alone | Arm C: 42% | Arm C: 21% | Arm C: 17% | Arm C: 65% | ||||
| Kusunoki et al[10], 1986-1995 (case series) | Perforation | ||||||||
| Arm A: 59 | 5-108 | Arm A: MDR-ILBT→surgery | Arm A: 74% | Arm A: 0% | |||||
| Arm B: 65 | Arm B: HDR-ILBT→surgery | Arm B: 63% | - | - | - | Arm B: 11% | - | ||
| Vuong et al[22], 1998-2001 (case series) | 49 | 29 (16-48) | HDR-ILBT→surgery→ chemoradiation | - | 64% | 2% | 4.10% | Grade 2 proctitis 100% | - |
| Grade 4 dermatitis 4% | |||||||||
| Ishikawa et al[23], 1988-1997 (case series) | 41 | 79.2 | EBRT 30 Gy→HDR-ILBT 10 Gy × 4→surgery | - | - | 15% | 10% | 61% | 71.80% |
| Jakobsen et al[24] (case series) | 50 | Not mentioned | CRT 45 Gy→HDR-ILBT boost→surgery | - | 27% | - | - | 30% | - |
| Present study | 36 | 18 (5-22) | Arm A: CRT 45 Gy→ILBT boost 5.5-7 Gy × 2→surgery | Arm A: 66.7% | Arm A: 58.8% | N/A | Arm A: 70.6% | - | |
| Arm B: CRT 45 Gy→EBRT boost 5.4 Gy→surgery | Arm B: 50% | Arm B: 15.80% | N/A | Arm B: 42.1% |
Our study had a few limitations; first, the primary endpoint was limited to pathologic response rate and the toxicity profile. This study did not determine the survival benefit at the time of analysis, however, further follow up of these patients will show the impact of HDR-ILBT on disease outcome. Our sample size was small, with more advanced unresectable stages and capecitabine was given as a radiosensitizer only during radiation days, rather than continuously[25]. The smaller sample size is justified by poor referral to tertiary care centers, lack of multidisciplinary approaches and lack of patient education especially in developing countries[26]. In our study, we used a single channel catheter with interchangeable shields, which gave better dose homogeneity in the tumor volume and minimal dose to normal rectal mucosa. This type of catheter has been proved to achieve better dose homogeneity as compared to multi-channel catheters[27]. Another finding was that the study population was composed of predominantly young males. One potential cause of the higher incidence of rectal cancer in the Indo-Pak region could be the number of inter-cousin marriages in this region. However, further research by epidemiologists, oncologists and geneticists is required.
In conclusion, the results of our single center experience were similar or better than previously published studies, albeit with higher but manageable gastrointestinal toxicities. High dose rate intraluminal brachytherapy was found to be more convenient, had satisfactory response rates and can be safely used as a tool to boost the gross tumor volume during preoperative chemoradiation. However, a multicenter randomized trial is warranted to evaluate long-term local control and survival benefit.
Preoperative chemoradiation is now the standard of care for locally advanced rectal cancers. Radiotherapy in these patients is given by external beam radiation therapy through the shrinking field technique, initially targeting the primary tumor and lymph nodes followed by a boost to the gross residual tumor. High dose rate intraluminal brachytherapy (HDR-ILBT) has been incorporated at various centers to treat recurrent and persistant rectal cancer. This is the first randomized controlled study in which intraluminal brachytherapy was given as a boost along with external beam radiation.
Tunio et al used high dose rate intraluminal brachytherapy during preoperative chemoradiation as a boost to the gross residual tumor.
This study showed a complete pathologic response rate of 58.8% in patients who were treated with high dose rate intraluminal brachytherapy along with external beam radiation compared with a complete pathologic response rate of 15.8% in patients who were treated with external beam radiation alone. The intraluminal brachytherapy group had more acute side effects (rectal pain and diarrhea) but these were successfully managed. Chronic side effects were minimal and mild.
The incidence of rectal cancer is increasing worldwide. High dose rate intraluminal brachytherapy during curative chemoradiation can provide better treatment outcomes in terms of downstaging and sphincter preservation, preventing many patients having colostomy bags.
Intraluminal (intra = inside, luminal = lumen), brachytherapy (brachium = hand, therapy = treatment) is a type of radiation therapy in which the radiation is given by introducing a radioactive source into the lumen, e.g. cervix, bowels, trachea, biliary tract or rectum.
The aim of the present study was to determine whether HDR-ILBT as boost during preoperative chemoradiation for advanced rectal cancer is feasible, safe as well as offer higher pathologic response rates and sphincter preservation.
Peer reviewer: Cuong D Tran, PhD, Research Fellow, Affiliate Lecturer, University of Adelaide, Gastroenterology Unit, Children, Youth and Women’s Health Service, 72 King William Rd, North Adelaide, SA 5006, Australia
S- Editor Wang YR L- Editor Webster JR E- Editor Lin YP
| 1. | Bhurgri Y, Bhurgri A, Nishter S, Ahmed A, Usman A, Pervez S, Ahmed R, Kayani N, Riaz A, Bhurgri H. Pakistan--country profile of cancer and cancer control 1995-2004. J Pak Med Assoc. 2006;56:124-130. |
| 2. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. |
| 3. | Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-4625. |
| 4. | Kim JC, Kim TW, Kim JH, Yu CS, Kim HC, Chang HM, Ryu MH, Park JH, Ahn SD, Lee SW. Preoperative concurrent radiotherapy with capecitabine before total mesorectal excision in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2005;63:346-353. |
| 5. | Marijnen CA. External beam radiotherapy and high dose rate brachytherapy for medically unfit and elderly patients. Clin Oncol (R Coll Radiol). 2007;19:706-710. |
| 6. | Yanagi H, Kusunoki M, Kamikonya N, Yamamura T, Utsunomiya J. Results of preoperative intraluminal brachytherapy combined with radical surgery for middle and lower rectal carcinomas. J Surg Oncol. 1997;65:76-81. |
| 7. | Kamikonya N, Hishikawa Y, Kurisu K, Taniguchi M, Miura T. Primary rectal cancer treated with high-dose-rate intraluminal brachytherapy following external radiotherapy. Radiat Med. 1991;9:85-87. |
| 8. | Jain N, Vashistha R, Kaur P, Aggarwal LM, Passi K, Arora R, Jain S. Carcinoma anorectum-preliminary experience with external radiotherapy combined with intraluminal brachytherapy. J Med Phys. 2000;25:72-74. |
| 9. | Kaufman N, Nori D, Shank B, Linares L, Harrison L, Fass D, Enker W. Remote afterloading intraluminal brachytherapy in the treatment of rectal, rectosigmoid, and anal cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 1989;17:663-668. |
| 10. | Kusunoki M, Shoji Y, Yanagi H, Kamikonya N, Sakanoue Y, Hishikawa Y, Utsunomiya J. Anorectal function after preoperative intraluminal brachytherapy and colonic J pouch-anal anastomosis for rectal carcinoma. Br J Surg. 1993;80:933-935. |
| 11. | Kim NK, Baik SH, Min BS, Pyo HR, Choi YJ, Kim H, Seong J, Keum KC, Rha SY, Chung HC. A comparative study of volumetric analysis, histopathologic downstaging, and tumor regression grade in evaluating tumor response in locally advanced rectal cancer following preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2007;67:204-210. |
| 12. | Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1-10. |
| 13. | Small W Jr, Du Bois A, Bhatnagar S, Reed N, Pignata S, Potter R, Randall M, Mirza M, Trimble E, Gaffney D. Practice patterns of radiotherapy in endometrial cancer among member groups of the gynecologic cancer intergroup. Int J Gynecol Cancer. 2009;19:395-399. |
| 14. | Frobe A, Jones G, Jaksić B, Bokulić T, Budanec M, Iva M, Stancić-Rokotov D, Hrabar D, Bolanca A, Rosenblatt E. Intraluminal brachytherapy in the management of squamous carcinoma of the esophagus. Dis Esophagus. 2009;22:513-518. |
| 15. | Fuwa N, Kodaira T, Tachibana H, Nakamura T, Tomita N, Daimon T. Long-term observation of 64 patients with roentgenographically occult lung cancer treated with external irradiation and intraluminal irradiation using low-dose-rate iridium. Jpn J Clin Oncol. 2008;38:581-588. |
| 16. | Deodato F, Clemente G, Mattiucci GC, Macchia G, Costamagna G, Giuliante F, Smaniotto D, Luzi S, Valentini V, Mutignani M. Chemoradiation and brachytherapy in biliary tract carcinoma: long-term results. Int J Radiat Oncol Biol Phys. 2006;64:483-488. |
| 17. | Syed AM, Puthawala AA, Damore SJ, Cherlow JM, Austin PA, Sposto R, Ramsinghani NS. Brachytherapy for primary and recurrent nasopharyngeal carcinoma: 20 years' experience at Long Beach Memorial. Int J Radiat Oncol Biol Phys. 2000;47:1311-1321. |
| 18. | Corner C, Bryant L, Chapman C, Glynne-Jones R, Hoskin PJ. High-dose-rate afterloading intraluminal brachytherapy for advanced inoperable rectal carcinoma. Brachytherapy. 2010;9:66-70. |
| 19. | Tam TY, Mukherjee S, Farrell T, Morgan D, Sur R. Endoscopic brachytherapy for obstructive colorectal cancer. Brachytherapy. 2009;8:313-317. |
| 20. | Begum N, Asghar AH, N S, Khan SM, Khan A. High dose rate intraluminal brachytherapy in combination with external beam radiotherapy for palliative treatment of cancer rectum. J Coll Physicians Surg Pak. 2003;13:633-636. |
| 21. | Hoskin PJ, de Canha SM, Bownes P, Bryant L, Glynne Jones R. High dose rate afterloading intraluminal brachytherapy for advanced inoperable rectal carcinoma. Radiother Oncol. 2004;73:195-198. |
| 22. | Vuong T, Belliveau PJ, Michel RP, Moftah BA, Parent J, Trudel JL, Reinhold C, Souhami L. Conformal preoperative endorectal brachytherapy treatment for locally advanced rectal cancer: early results of a phase I/II study. Dis Colon Rectum. 2002;45:1486-1493; discussion 1493-1495. |
| 23. | Ishikawa H, Fujii H, Koyama F, Mukogawa T, Matsumoto H, Morita T, Hata M, Terauchi S, Kobayashi T, Nakao T. Long-term results of high-dose extracorporeal and endocavitary radiation therapy followed by abdominoperineal resection for distal rectal cancer. Surg Today. 2004;34:510-517. |
| 24. | Jakobsen A, Mortensen JP, Bisgaard C, Lindebjerg J, Hansen JW, Rafaelsen SR. Preoperative chemoradiation of locally advanced T3 rectal cancer combined with an endorectal boost. Int J Radiat Oncol Biol Phys. 2006;64:461-465. |
| 25. | Tunio MA, Hashmi AH. Capecitabine initially concomitant to radiotherapy then perioperatively administered in locally advanced rectal cancer. In regard to MG Zampino et al. (Int J Radiat Oncol Biol Phys 2009;75:421-427). Int J Radiat Oncol Biol Phys. 2010;76:1275; author reply 1275-1275; author reply 1276. |
| 26. | Abbas A, Tunio MA, Ali N. Neoadjuvant chemo-irradiation in un-resectable carcinoma of rectum. J Ayub Med Coll Abbottabad. 2004;16:24-29. |