Published online Sep 21, 2010. doi: 10.3748/wjg.v16.i35.4410
Revised: May 27, 2010
Accepted: June 3, 2010
Published online: September 21, 2010
AIM: To evaluate the diagnostic value of glypican-3 (GPC3) in serum and liver for primary hepatocellular carcinoma (HCC).
METHODS: Serum levels of GPC3 and α-fetoprotein (AFP) were measured in 75 patients with primary HCC and 32 patients with liver cirrhosis. Expression of GPC3 and AFP in 58 HCC and 12 cirrhotic specimens was detected with immunohistochemical staining.
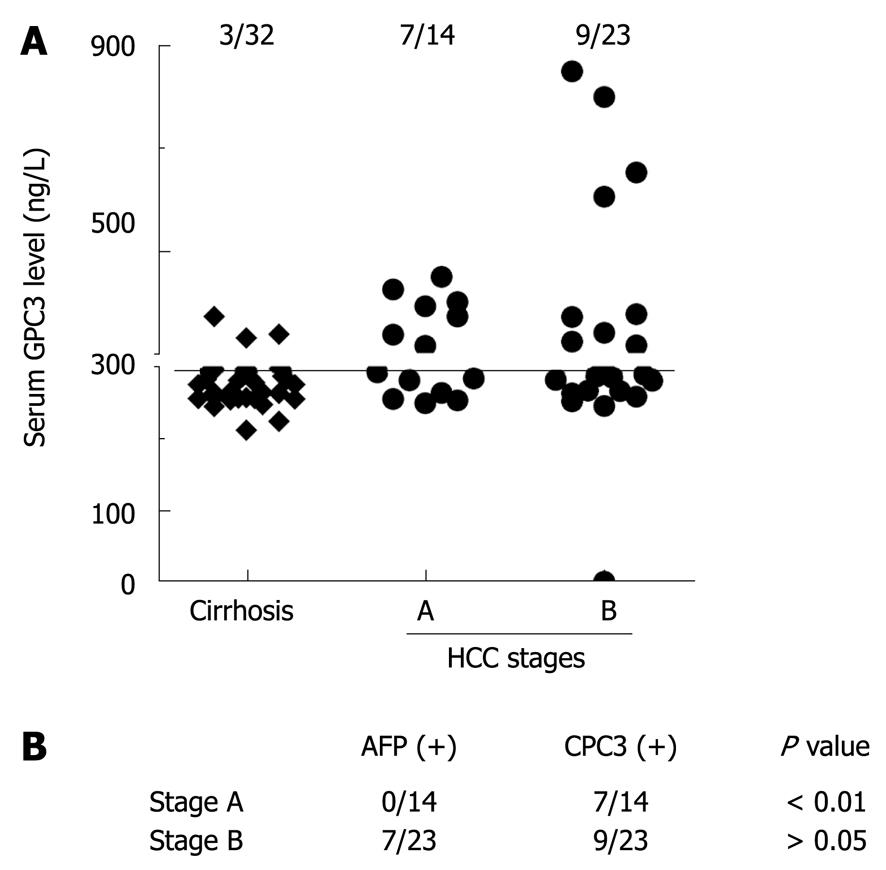
RESULTS: When the cut-off value of serum GPC3 was set at 300 ng/L, its sensitivity and specificity for HCC were 47.0% and 93.5%, respectively. Among the 14 patients with HCC at stage according to the Barcelona Clinic Liver Cancer staging system, the serum GPC3 level was higher than 300 ng/L in 50% (7/14) patients, the serum AFP level was not ≥ 400 μg/L in any patient. Combined serum AFP and GPC3 significantly increased the sensitivity to the diagnosis of HCC. The GPC3 expression was detected in cytoplasm of HCC cells but not in hepatocytes and bile ducts of benign tumors. Among the 58 HCC patients, the GPC3 was expressed in 100% (28/28) patients with their serum AFP level ≥ 400 μg/L, and in 90% (27/30) patients with their AFP level < 400 μg/L, respectively. The GPC3 was weakly or negatively expressed in all paracarcinomatous and cirrhotic tissue samples. AFP positive HCC cells were only found in 1 out of the 58 HCC patients.
CONCLUSION: GPC3 protein is a sensitive and specific serum marker for diagnosis of early HCC. Its expression in liver tissues can be used to discriminate tumor cells from benign hepatic cells.
- Citation: Liu H, Li P, Zhai Y, Qu CF, Zhang LJ, Tan YF, Li N, Ding HG. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol 2010; 16(35): 4410-4415
- URL: https://www.wjgnet.com/1007-9327/full/v16/i35/4410.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i35.4410
The prognosis of patients with primary hepatocellular carcinoma (HCC) is generally very poor with a 5-year survival rate of less than 10%-15% since most of them are diagnosed at their late stage using current strategy[1,2]. Surgical resection is still the most used treatment option for the diagnosed HCC patients[3]. Therefore, early detection and diagnosis of HCC would be of great clinical benefit. Screening strategy is to identify small tumors that may be successfully treated. Currently in China, detection of serum α-fetoprotein (AFP) and ultrasound examination every 6 mo in patients with liver cirrhosis have been recommended[4,5]. Since elevated serum AFP level is correlated with the occurrence of HCC, screening and surveillance for HCC in high-risk patients through periodic measurement of AFP have been proposed since the 1980s[5-7]. The cut-off value for HCC diagnosis in China is set at 400 μg/L according to the guidelines of clinical diagnosis and staging criteria for primary HCC established by Chinese Society of Liver Cancer in 2001[8]. However, 25%-30% of the diagnosed HCC patients have a normal AFP level (< 20 μg/L) or 40%-50% of HCC patients have a lower serum AFP level (> 20 μg/L - < 400 μg/L)[1,2]. On the other hand, the AFP level can reach 2500 μg/L in around 20%-25% of patients with chronic hepatitis, liver cirrhosis and other liver disease[9-11]. Although ultrasonography has been widely used in clinical screening of HCC, it is highly dependent on the experience of its operator[3,5]. Therefore, as AFP and ultrasonography only play a limited role in screening of HCC, some candidate biomarkers can be used in diagnosis of HCC including glypican-3 (GPC3). GPC3 is a heparan sulfate proteoglycan that is bund to the cell surface by glycosylphosphatidylinositol anchors (GPI) and highly expressed in fetal but not in adult liver. It has been shown that GPC3 is closely related to HCC[9,12]. It was reported that the frequency of GPC3 expression in AFP-negative HCC patients is as high as 90%, suggesting that it can be used in diagnostic of HCC[13,14]. However, serum GPC3 and its expression in liver tissue of patients with HCC at different stages have not been fully investigated. Therefore, the value of serum GPC3 expression in tumor/paracarcinomatous tissue for the diagnosis of HCC was explored in this study.
One hundred and seven patients admitted to Beijing Youan Hospital, Capital Medical University from January 2006 to December 2008 were recruited in this study and divided into HCC group (n = 75) and liver cirrhosis group (n = 32) with a male to female ratio of 55:20 and 24:8, respectively. HCC was diagnosed based on at least one of the following criteria in the guidelines of clinical diagnosis and staging for primary hepatocellular carcinoma established by Chinese Society of Liver Cancer, 2001[8]: (1) hepatic space-occupying lesion with a serum AFP level ≥ 400 μg/L; and (2) serum AFP level < 400 μg/L but with new hepatic space occupying lesions, arterial phase enhancement on computed tomography or magnetic resonance imaging. HCC was staged according to the Barcelona Clinic Liver Cancer (BCLC) staging system[15]. Liver cirrhosis was diagnosed based on physical examination, laboratory test, and B-ultrasonography or computed tomography (CT) scan. Patients in liver cirrhosis group were followed up at least for 2 years. The study was approved by the Ethical Committee of Beijing Youan Hospital, Capital Medical University. Investigators explained in detail to all the patients and/or their relatives. Written consent was obtained from all patients when they were recruited.
Serum samples were collected from the patients. HBV and HCV infectious markers were detected in clinical laboratory. HBV infection was determined by the presence of HBsAg using reagents from Roche Diagnostics-China (Shanghai, China) and HBV-DNA from Kehua (Shanghai, China). HCV infection was determined by the presence of anti-HCV using reagents from Diasorin Ltd. (Shanghai, China) and HCV-RNA from Da An Gene Co. Ltd., Sun Yat-Sen University (Guangzhou, China). The serum samples were stored at -20°C for the measurement of serum AFP and GPC3 levels. Liver tissue specimens and needle biopsies from 58 HCC and 12 cirrhotic patients were fixed in 4% polyoxymethylene and embedded in paraffin.
Serum AFP and GPC3 levels were measured by electrochemiluminescence (Abbott, USA) and enzyme-linked immunosorbent assay (ELISA; BioMosaics Company, USA), respectively, following their manufacturer’s instructions. The value of optical density (OD) was detected using a spectrophotometer (Multiskan Ascent V1.24, Switzerland).
Expression of GPC3 in liver tissue and needle liver biopsy samples was detected with immunohistochemistry (IHC) staining. The slides were reviewed to confirm the diagnosis of HCC according to the guidelines of WHO criteria[16]. A mouse monoclonal anti-GPC3 antibody (clone number: sc-65443 1G12, Santa Cruz Inc., USA) and a mouse polyclonal anti-AFP antibody (Neomarker Inc., USA) were used in IHC staining. Normal mouse serum served as a negative control. Briefly, tissue sections were deparaffinized, rehydrated, and antigen was retrieved using heat-induced epitope in a 10 mmol/L citrate buffer, pH 6.0. After blocked with peroxidase, the sections were incubated with primary antibodies diluted at 1:50. The secondary antibody used was a horse-radish peroxidase (HRP)-labeled antibody (Zhongshan Golden Bridge, Beijing, China). Results were evaluated by pathologists at Beijing YouAn Hospital. Five high visual fields were observed with no less than 1000 cells in each visual field. Cells in cytoplasm taking on yellow or brown particles were considered positive.
All analyses were performed using the SPSS for Windows (version 11.5). The data were expressed as mean ± SD. χ2 test and Student t test were used to compare the distribution of categorical and continuous variables, respectively, in the two groups. P < 0.05 was considered statistically significant.
All the 107 patients recruited in this study were Chinese. Their clinical data are shown in Table 1. The patients were diagnosed as HBV-related HCC (84%) and HCV-related HCC (16%) or liver cirrhosis (78.1% and 29.1%), respectively. No significant difference was observed in HBV infection between the two groups. No double HBV and HCV-infected patient was found in the current study. Of the 75 clinically diagnosed HCC patients, 14 were at BCLC-stage A, 23 at BCLC-stage B, 16 at BCLC-stage C, and 22 at BCLC-stage D.
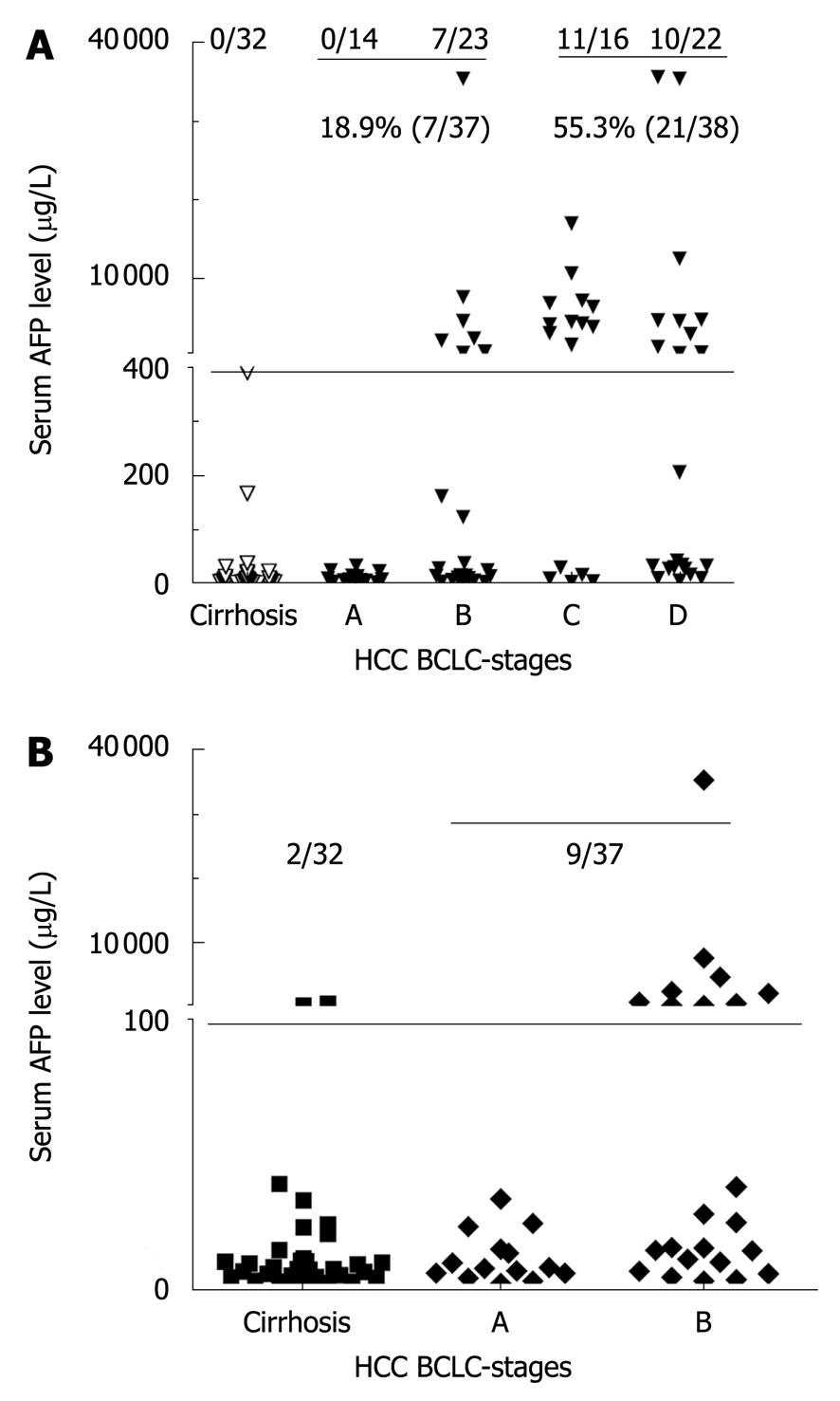
AFP was widely used as a biomarker for the diagnosis of primary HCC. The cut-off value was set at 400 μg/L according to the guidelines of clinical diagnosis and staging criteria for primary hepatocellular carcinoma established by Chinese Society of Liver Cancer in 2001. The serum AFP level was below 400 μg/L in all patients with liver cirrhosis and ≥ 400 μg/L in only 28 patients (37.3%) with HCC (Figure 1A). The serum AFP level was ≥ 400 μg/L in none of the 14 HCC patients at BCLC-stage A and only in 7 patients (30.4%) at BCLC-stage B. The positive rate was only 18.9% in patients at BCLC-stage A and B with their AFP level ≥ 400 μg/L, although it increased to 55.3% in patients at BCLC-stage C and D (Figure 1A). The AFP levels were compared between liver cirrhosis and HCC patients at BCLC-stage A and B (Figure 1B). Among the 32 patients with liver cirrhosis, the AFP level was higher in 2 patients with their liver cirrhosis progressed to HCC than in those with their liver cirrhosis not progressed to HCC. The sensitivity of AFP for the diagnosis of HCC did not significantly increase in HCC patients at different BCLC stages.
The cut-off value of GPC3 was set at 300 ng/L by receiver operating characteristic (ROC) curve analysis. At this cut-off value, the sensitivity and specificity of serum GPC3 for the diagnosis of HCC was 46.7% and 93.5%, respectively. Among the 32 clinically diagnosed liver cirrhosis patients, the serum GPC3 level was > 300 ng/mL in 9.4% (3/32) patients. Among the early HCC patients, 7 (50%) had BCLC-stage A HCC, and 9/23 (39.1%) had BCLC-stage B HCC with their serum GPC3 level > 300 ng/mL (Figure 2A and B). Among the 16 patients with their GPC3 > 300 ng/mL, the serum AFP level was ≥ 400 μg/L in only 3 patients.
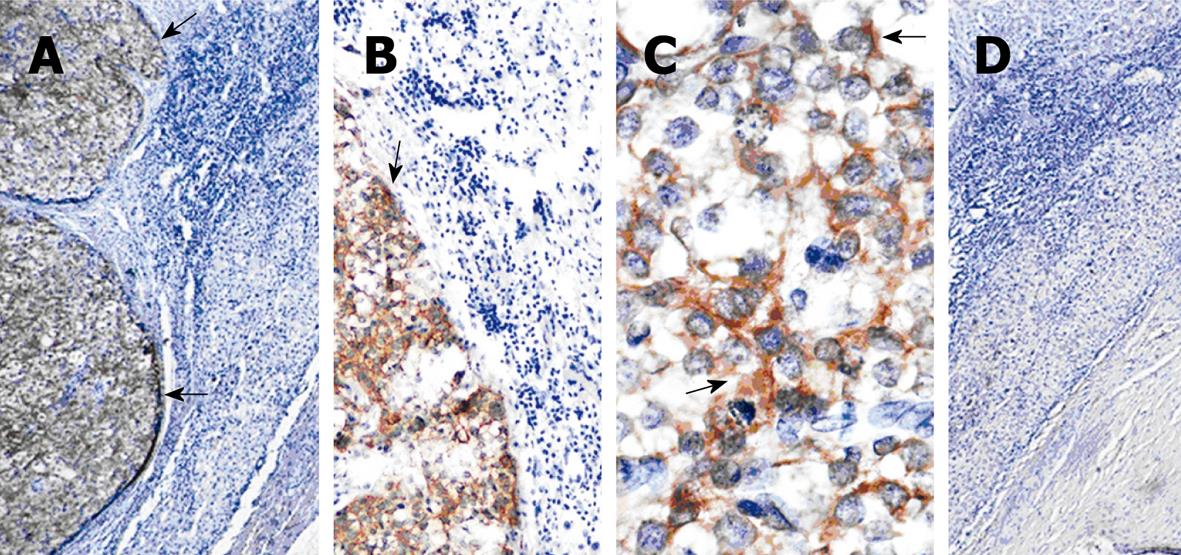
Liver cirrhosis was observed in all the examined specimens. With IHC staining, AFP positive cells were hardly detectable in HCC patients even with their serum AFP level ≥ 400 μg/L (Figure 3) except in 1 tumor tissue sample. However, GPC3 was specifically expressed in cytoplasm of cancer cells but not or weakly expressed in paracarcinomatous tissue samples (Figure 3A-C). GPC3 was expressed in 94.8% (55/58) of HCC tissue samples and over-expressed in all the 28 HCC patients with their serum AFP level ≥ 400 μg/L and in 30 HCC patients with their serum AFP level < 400 μg/L. GPC3-positive cancer cells were found in 90% of the 30 clinically diagnosed HCC patients with their serum AFP level < 400 μg/L. GPC3 was positively expressed in HCC tissue specimens and negatively expressed in all liver cirrhotic specimens.
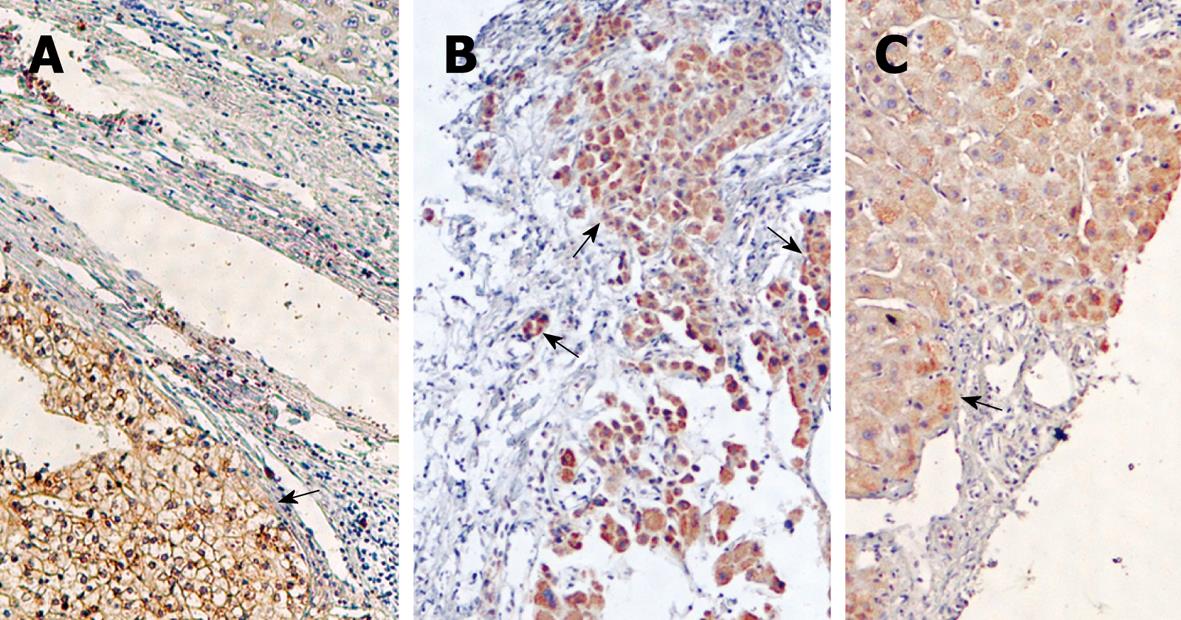
The expression of GPC3 was also detected in needle biopsy samples. After the specimens were stained, some cells were positive for GPC3 but negative for AFP (Figure 4A-C).
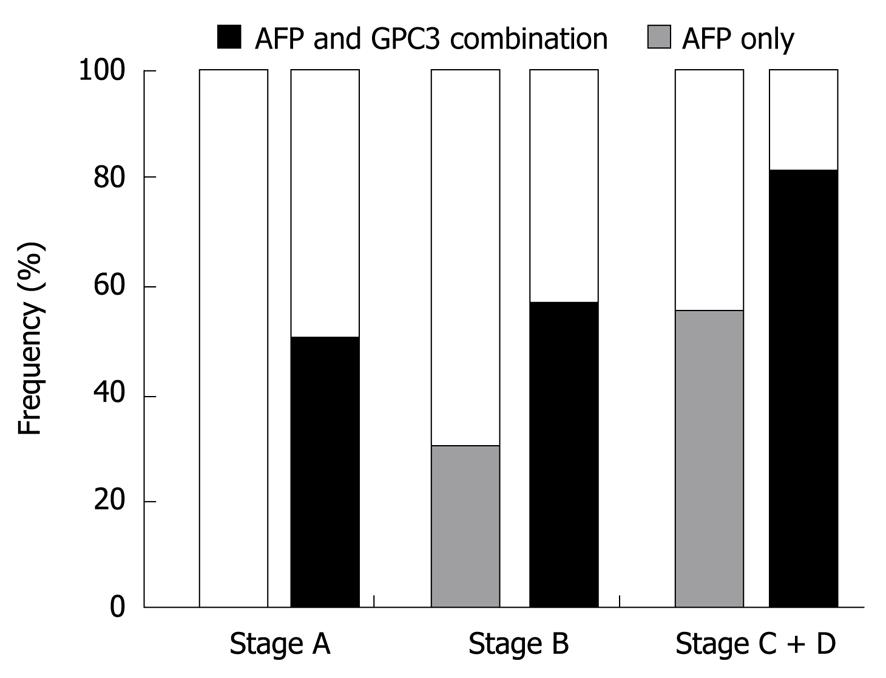
Combined serum GPC3 and AFP significantly increased the sensitivity for HCC diagnosis. The sensitivity of serum AFP increased from 18.9% to 54% for the diagnosis of HCC at BCLC-stage A + B, and from 55.3% to 81.6% for the diagnosis of HCC at BCLC-stage C + D (Figure 5).
The main etiological factor for primary HCC in China is HBV infection[17,18]. However, it has been shown that HCV infection plays a role in pathogenesis of primary HCC[19,20]. Of the 75 clinically diagnosed HCC patients enrolled in this study, 63 had HBV-related HCC. As HCC can develop in patients with HBV infection, chronic HBV carriers are also included in screening of HCC in China with the measurement of AFP level and ultrasonography every 6 mo[5].
Screening of HCC is cost-effective in patients with cirrhosis since its annual incidence exceeds 1.5% per year[3]. However, most liver mass/nodular lesions are benign, such as cirrhotic regenerative nodules, adenoma, and focal nodular hyperplasia, but they may mimic malignant liver lesions[3]. Therefore, the differential diagnosis between HCC and benign mimickers is difficult. AFP is the most commonly used indicator in screening and diagnosis of HCC as well as in follow-up of high-risk population. However, its sensitivity for the diagnosis of HCC is only 30%-40%[2,21]. In the current study, the diagnosis rate of HCC was 37.3% for serum AFP (≥ 400 μg/L), which is consistent with the reported findings[22,23], but lower than that reported by Dr. Yang (44.6%)[24]. The serum AFP level is significantly different in HCC patients with different tumor size, number, stage and etiology[25]. The results of this study confirm that serum AFP (≥ 400 μg/L) alone cannot be used as a diagnostic marker for HCC, especially early HCC[26]. Therefore, all these results indicate that the serum AFP level plays a limited role in diagnosis of HCC, especially early HCC.
It was recently reported that GPC3 is only detected in HCC cells but not in benign liver tissues, and can thus be used as a potential biomarker for the diagnosis of early HCC[9,14,26,27]. The serum GPC3 level was higher than 300 ng/L in 50% of early HCC patients, although their serum AFP level was below 100 μg/L in this study, suggesting that serum GPC3 level plays a potential role in diagnosis of early HCC and can be used in screening of HCC. Therefore, measurement of serum GPC3 level in combination with B-ultrasonography is useful in screening and diagnosis of HCC as well as in follow-up of high-risk population.
In this study, GPC3 was over-expressed in cytoplasm of HCC cells but not expressed in 58 paracarcinomatous and 12 liver cirrhotic tissue samples. GPC3 positive cells were found in 90% of patients with their serum AFP level < 400 μg/L. GPC3 was negatively expressed in only 10% of the examined samples. As all the HCC patients included in this study were accompanied with cirrhosis, the positive GPC3 expression rate was high, which is consistent with the reported data[14], suggesting that expression of GPC3 protein is a sensitive and specific histological marker for the diagnosis of HCC and can thus be used in biopsy in the presence of nodule lesions.
Serum GPC3 and AFP levels were increased in HCC patients included in this study. GPC3 protein was exclusively expressed in cancer cells and serum GPC3 level was increased in early HCC patients with their serum AFP level < 400 g/L. GPC3 protein expression was only detectable in liver cancer cells but not in benign hepatic cells. The sensitivity of combined serum GPC3 and AFP was increased for the diagnosis of HCC at all stages.
In conclusion, GPC3 is a sensitive, specific serum and tissue marker for the diagnosis of early HCC. Over-expression of GPC3 in liver tissues can be used in discriminating tumor cells from benign hepatic cells. Combined AFP and GPC3 should be considered in diagnosis of HCC.
Serum α-fetoprotein (AFP) plays a limited role in and detection and diagnosis of hepatocellular carcinoma (HCC). Some new candidate biomarkers for the diagnosis of HCC have been investigated.
Since 2000, the frequency of hepatic glypican-3 (GPC3) expression in AFP-negative HCC patients has been increased to 90%. However, serum GPC3 and its expression in HCC tissue have not been fully studied.
GPC3 is a sensitive and specific tissue marker for diagnosis of early HCC. Over-expression of GPC3 in liver tissues can be used in discriminating tumor cells from benign hepatic cells. Combined AFP with GPC3 should be considered in detection and diagnosis of HCC.
The results of this study suggest that combined serum GPC3 and AFP is useful in screening and diagnosis of HCC as well as in follow-up of high-risk population. GPC3 protein is a sensitive and specific histological marker for the discrimination between HCC and benign nodule lesion.
GPC3 is a heparan sulfate proteoglycan which is bund to the cell surface by glycosylphosphatidylinositol anchors and highly expressed in fetal but not in normal adult liver.
This is a straightforward well conducted study. The results suggest that combined serum GPC3 and AFP is useful in screening and diagnosis of HCC as well as in follow-up of high-risk population, and the authors showed that GPC3 protein was a sensitive and specific histological marker for the discrimination between HCC and benign nodule lesion, thus making a contribution to diagnosis of early HCC.
Peer reviewer: Emmet B Keeffe, MD, Professor, Chief of Hepatology, Medical Director, Liver Transplant Program, Program Director, Gastroenterology Fellowship, Stanford University Medical Center, 750 Welch Road, Suite 210, Palo Alto, CA 94304, United States
S- Editor Wang YR L- Editor Wang XL E- Editor Ma WH
| 1. | Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Shibata K, Ohta M, Kitano S. Short- and long-term outcomes after hepatic resection for hepatocellular carcinoma with concomitant esophageal varices in patients with cirrhosis. Ann Surg Oncol. 2008;15:1670-1676. |
| 2. | Stefaniuk P, Cianciara J, Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2010;16:418-424. |
| 3. | Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111-1118. |
| 4. | Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, Ryder S, Cramp M, Stein K. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis. Br J Cancer. 2008;98:1166-1175. |
| 5. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. |
| 6. | Ji Z, Sun ZT, Wang LQ, Ding GS, Chu PP, Li FM. [Characteristics of AFP serology in early PHC and the high risk group (author’s transl)]. Zhonghua Zhongliu Zazhi. 1979;1:96-100. |
| 7. | Lopez JB. Recent developments in the first detection of hepatocellular carcinoma. Clin Biochem Rev. 2005;26:65-79. |
| 8. | Yang BH. Chinese Society of Liver Cancer: Clinical diagnosis and staging criteria for primary hepatocellular carcinoma. Zhongguo Ganzangbing Zazhi. 2001;9:324-330. |
| 9. | Libbrecht L, Severi T, Cassiman D, Vander Borght S, Pirenne J, Nevens F, Verslype C, van Pelt J, Roskams T. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 2006;30:1405-1411. |
| 10. | Zinkin NT, Grall F, Bhaskar K, Otu HH, Spentzos D, Kalmowitz B, Wells M, Guerrero M, Asara JM, Libermann TA. Serum proteomics and biomarkers in hepatocellular carcinoma and chronic liver disease. Clin Cancer Res. 2008;14:470-477. |
| 11. | Liaw YF, Tai DI, Chen TJ, Chu CM, Huang MJ. Alpha-fetoprotein changes in the course of chronic hepatitis: relation to bridging hepatic necrosis and hepatocellular carcinoma. Liver. 1986;6:133-137. |
| 12. | Block TM, Marrero J, Gish RG, Sherman M, London WT, Srivastava S, Wagner PD. The degree of readiness of selected biomarkers for the early detection of hepatocellular carcinoma: notes from a recent workshop. Cancer Biomark. 2008;4:19-33. |
| 13. | Zhou XP, Wang HY, Yang GS, Chen ZJ, Li BA, Wu MC. Cloning and expression of MXR7 gene in human HCC tissue. World J Gastroenterol. 2000;6:57-60. |
| 14. | Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol. 2008;21:1011-1018. |
| 15. | Kim KA, Lee JS, Jung ES, Kim JY, Bae WK, Kim NH, Moon YS. [Usefulness of serum alpha-fetoprotein (AFP) as a marker for hepatocellular carcinoma (HCC) in hepatitis C virus related cirrhosis: analysis of the factors influencing AFP elevation without HCC development]. Korean J Gastroenterol. 2006;48:321-326. |
| 16. | Hirohashi S, Ishak KG, Kojiro M, Wanless IR, Theise ND, Tsukuma H, Blum HE, Deugnier Y, Laurent Puig P, Fischer HP. Hepatocellular carcinoma. World Health Organization Classification of Tumours. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press 2000; 159-172. |
| 17. | Ming L, Thorgeirsson SS, Gail MH, Lu P, Harris CC, Wang N, Shao Y, Wu Z, Liu G, Wang X. Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology. 2002;36:1214-1220. |
| 18. | Gao JD, Shao YF, Xu Y, Ming LH, Wu ZY, Liu GT, Wang XH, Gao WH, Sun YT, Feng XL. Tight association of hepatocellular carcinoma with HBV infection in North China. Hepatobiliary Pancreat Dis Int. 2005;4:46-49. |
| 19. | Lu J, Zhou Y, Lin X, Jiang Y, Tian R, Zhang Y, Wu J, Zhang F, Zhang Y, Wang Y. General epidemiological parameters of viral hepatitis A, B, C, and E in six regions of China: a cross-sectional study in 2007. PLoS One. 2009;4:e8467. |
| 20. | Madhoun MF, Fazili J, Bright BC, Bader T, Roberts DN, Bronze MS. Hepatitis C prevalence in patients with hepatocellular carcinoma without cirrhosis. Am J Med Sci. 2010;339:169-173. |
| 21. | Soresi M, Magliarisi C, Campagna P, Leto G, Bonfissuto G, Riili A, Carroccio A, Sesti R, Tripi S, Montalto G. Usefulness of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma. Anticancer Res. 2003;23:1747-1753. |
| 22. | Doffoël M, Bonnetain F, Bouché O, Vetter D, Abergel A, Fratté S, Grangé JD, Stremsdoerfer N, Blanchi A, Bronowicki JP. Multicentre randomised phase III trial comparing Tamoxifen alone or with Transarterial Lipiodol Chemoembolisation for unresectable hepatocellular carcinoma in cirrhotic patients (Fédération Francophone de Cancérologie Digestive 9402). Eur J Cancer. 2008;44:528-538. |
| 23. | Marrero JA, Lok AS. Newer markers for hepatocellular carcinoma. Gastroenterology. 2004;127:S113-S119. |
| 24. | Yang BH, Xia JL, Huang LW, Tang ZY, Chen MS, Li JQ, Liang AM, Mo QG, Lu HS, Dai CL. [Changes of clinical aspect of primary liver cancer in China during the past 30 years--control study for 3,250 cases with primary liver cancer]. Zhonghua Yixue Zazhi. 2003;83:1053-7105. |
| 25. | Baek YH, Lee JH, Jang JS, Lee SW, Han JY, Jeong JS, Choi JC, Kim HY, Han SY. Diagnostic role and correlation with staging systems of PIVKA-II compared with AFP. Hepatogastroenterology. 2009;56:763-767. |
| 26. | Wang DS, Ding HG, Li Z, Xin YM, Zhang B, Li N, Chen DX, Yin JM. The diagnostic value of combination with GPC3 and AFP in hepatocellular carcinomas. Zhonghua Jianyan Yixue Zazhi. 2007;10:1169-1171. |
| 27. | Jia HL, Ye QH, Qin LX, Budhu A, Forgues M, Chen Y, Liu YK, Sun HC, Wang L, Lu HZ. Gene expression profiling reveals potential biomarkers of human hepatocellular carcinoma. Clin Cancer Res. 2007;13:1133-1139. |