Published online Sep 7, 2010. doi: 10.3748/wjg.v16.i33.4200
Revised: April 7, 2010
Accepted: April 14, 2010
Published online: September 7, 2010
AIM: To investigate clinical outcomes of patients with chronic gastric volvulus (GV) who were managed conservatively over a 5-year period.
METHODS: A total of 44 consecutive patients with chronic GV, as diagnosed by barium study between October 2002 and July 2008 were investigated. All of these patients received conservative management initially without anatomical correction. Their clinical manifestations, diagnostic work-ups, and clinical outcomes were analyzed. We sought to identify independent risk factors for poor outcome by using the Cox proportional hazards model.
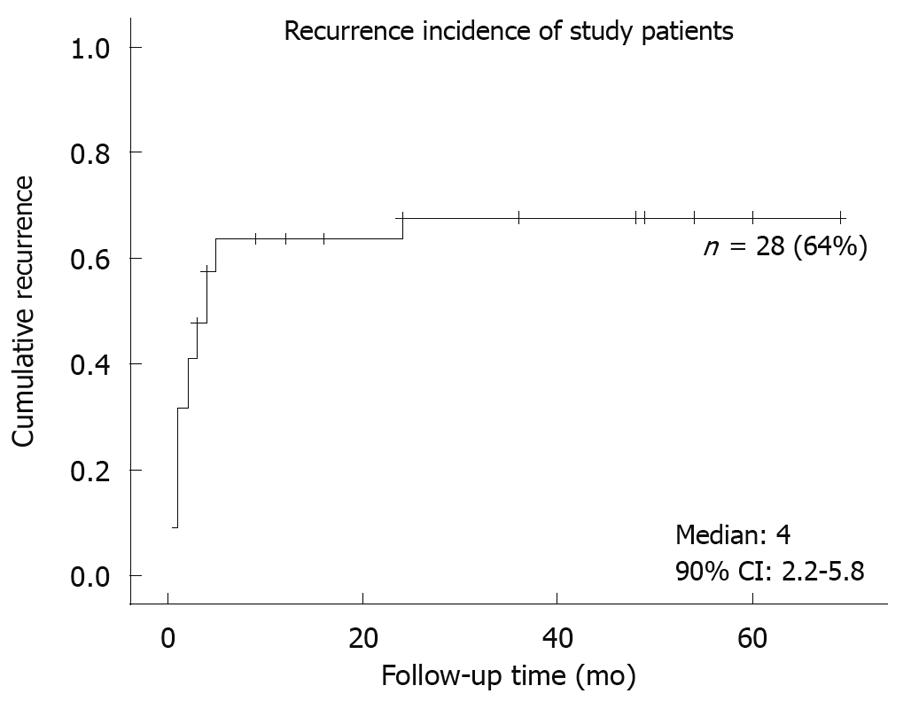
RESULTS: The enrolled patients were predominantly male (n = 37, 84%) and of advanced age (median: 71 years old, interquartile range: 57.5-78 years). Abdominal pain and fullness were the most common presentations. During the follow-up period (median: 16 mo, up to 69 mo), there was no severe complication, but symptomatic recurrence was noted in 28 patients (64%). Only one patient turned to elective surgery for frequent symptoms. Peritoneal adhesion was the only independent risk factor associated with recurrence (hazard ratio: 2.58, 95% CI: 1.08-6.13, P = 0.033).
CONCLUSION: Symptomatic recurrence of chronic GV is very common although serious complications infrequently occur with conservative management. Peritoneal adhesion is independently associated with recurrence.
- Citation: Hsu YC, Perng CL, Chen CK, Tsai JJ, Lin HJ. Conservative management of chronic gastric volvulus: 44 cases over 5 years. World J Gastroenterol 2010; 16(33): 4200-4205
- URL: https://www.wjgnet.com/1007-9327/full/v16/i33/4200.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i33.4200
Gastric volvulus (GV) is a rare condition that is defined as pathological rotation of the stomach. Laxity of gastric attachment constitutes the pathophysiological basis for the development of GV[1]. According to the axis of rotation, GV can be classified into organoaxial (long axis connecting the gastroesophageal junction to the pylorus), mesenteroaxial (short axis bisecting the lesser and greater curvature), and combined type[2].
Although GV has been reported in all ages, it is more often diagnosed in elderly patients[3-6]. Clinical presentations of GV vary widely from incidental radiographic findings to life-threatening catastrophes, depending on its rapidity of onset, degree of rotation, and subsequent extent of obstruction[7-9]. Barium study may be considered as the diagnostic tool of choice, because of its accuracy in demonstrating the abnormal rotation and in estimating the amount of obstruction[4,6,10,11]. Definitive treatment of GV includes reduction of the twisted stomach, percutaneous endoscopic gastrostomy, gastropexy, and repair of the predisposing structural defects[4-6,12-14].
Acute GV usually presents with progressive abdominal (intra-abdominal volvulus) or chest (intra-thoracic volvulus) pain, severe vomiting, and epigastric distention[15]. The classical Borchardt’s triad, which comprises severe epigastric pain, unproductive retching, and inability to pass a nasogastric tube, represent total gastric outlet obstruction[16]. Acute GV can lead to strangulation, necrosis, and perforation of the stomach, and should be regarded as a surgical emergency[7]. In contrast, chronic GV might be completely asymptomatic or manifest with recurrent non-specific symptoms such as vague abdominal pain, abdominal fullness, chest pain, retching, acid reflux, and dysphagia[8,9]. The natural history of chronic GV remains poorly understood. Although theoretically chronic GV can transform into acute volvulus, the actual incidence is unknown. Clinical outcomes of chronic GV patients managed conservatively without anatomical correction has not been investigated.
The aim of the present study was to investigate the manifestations, performance of diagnostic modalities, and specifically clinical outcomes of a large chronic GV cohort who received conservative management.
This was a retrospective study of consecutive chronic GV patients, which was conducted in a tertiary medical center that serves a metropolitan area of 11 million inhabitants in northern Taiwan (Taipei Veterans General Hospital). The Institutional Review Board of the hospital approved the study protocol. We reviewed the medical records and computerized database of all patients with a diagnosis of GV between October 2002 and July 2008. Eligible patients were identified by the following inclusion criteria: (1) unequivocal findings of a twisting stomach on upper gastrointestinal (GI) barium study; (2) age ≥ 20 years; (3) no urgent endoscopic or surgical intervention within 1 mo after diagnosis; and (4) no coexisting malignancy in the upper GI tract. An experienced radiologist (CKC) reviewed each barium study to confirm the diagnosis of GV.
Patients were excluded from analysis if they were not frequently followed up at least every 3 mo after diagnosis. We excluded those who received endoscopic or surgical management within 1 mo after diagnosis, to assure our study subjects were all chronic, instead of acute GV cases. In all enrolled subjects, upper GI endoscopy and computed tomography were performed for regular indication at the discretion of treating physicians. Conservative treatment of chronic GV was defined as no anatomical reduction or correction by endoscopic or surgical procedures, and comprised mainly prokinetic agents, anti-secretory therapy, and life style as well as diet modification.
The primary endpoint of this study was clinical outcome during the follow-up period. We defined three categories of outcome: (1) severe complications including strangulation or perforation of the stomach, complete obstruction, and hypovolemic shock; (2) recurrence of symptoms as documented on the medical records; and (3) no or negligible recurrent symptoms throughout the follow-up period. Pertinent demographic and clinical data of the eligible patients were abstracted, which included age, sex, presenting symptoms, time interval between symptom onset and diagnosis, comorbidity, and findings of other diagnostic modalities (plain radiograph, sonography, and endoscopy). In addition, we determined if predisposing factors for secondary GV were present. Paraesophageal hernia was defined as a stomach protruding through the esophageal hiatus, with the gastroesophageal junction in a normal position at the level of the diaphragm[17], and eventration of the diaphragm as abnormal elevation of an intact hemidiaphragm[18]. Peritoneal adhesion was defined to be present if there was previous abdominal or thoracic surgery, or history of peritonitis[19].
Continuous variables were expressed with median and interquartile range (IQR) and categorical variables with proportions. Mann-Whitney test was used to compare continuous variables and χ2 test was used for univariate analysis of proportions. Fisher’s exact test was applied in the case of expectant values below 10. All statistical analyses were conducted using SPSS for Windows version 14 (SPSS Inc., Chicago, IL, USA), and SAS version 9.1 (Cary, NC, USA). Symptom recurrence was estimated using the Kaplan-Meier method and compared by the log-rank test from the time of diagnosis. Independent risk factors predictive for symptom recurrence were determined by the Cox proportional hazards model. For all tests, P < 0.05 was considered statistically significant.
Fifty-five chronic GV patients were identified by the inclusion criteria, but 11 were excluded from analysis because they were not closely followed up with an interval of at least 3 mo. We contacted these excluded patients or their family by telephone to document the ultimate clinical outcomes. Two had died from unrelated diseases, and no surgical intervention was reported in any of these patients. Baseline demographics of these 44 enrolled patients are summarized in Table 1. They were predominantly male (84%), of advanced age (median: 71 years old, IQR: 57.5-78 years). Common presenting symptoms were abdominal pain (57%), abdominal fullness (55%), chest pain (41%), and nausea (39%). Secondary GV was present in 26 patients, most of whom had more than one etiology (Table 2). Twenty-eight patients (64%) experienced recurrence of symptoms during follow-up (median: 16 mo, IQR: 6-36 mo) (Figure 1). None developed acute complications in this study. Among the 44 enrolled patients, only one underwent elective open surgery for frequent symptoms, and the remaining 43 patients received conservative treatment throughout the study period.
| Enrolled patients (n = 44) | |
| Male sex, n (%) | 37 (84) |
| Age, yr (IQR) | 71 (57.5-78) |
| Duration between symptom onset and diagnosis, mo (IQR) | 1 (0.5-6) |
| Symptoms, n (%) | |
| Abdominal pain | 25 (57) |
| Abdominal fullness | 24 (55) |
| Non-cardiac chest pain | 18 (41) |
| Nausea | 17 (39) |
| Heartburn | 10 (23) |
| Dyspnea | 9 (20) |
| Acid regurgitation | 9 (20) |
| Dysphagia | 8 (18) |
| Vomiting | 5 (11) |
| Hematemesis | 2 (5) |
| Axis of volvulus, n (%) | |
| Organoaxial | 42 (95) |
| Mesenteroaxial | 1 (2) |
| Mixed | 1 (2) |
| Previous thoracic or abdominal surgery, n (%) | 10 (22) |
| Secondary GV, n (%) | 26 (59) |
| Duration of follow-up, mo (IQR) | 16 (6-36) |
| Recurrence-free duration, mo (IQR) | 3 (1-10.5) |
| Patients with secondary GV (n = 26) | |
| Choledocholithiasis | 9 (20) |
| Diaphragm defect | 8 (18) |
| Diaphragm eventration | 13 (30) |
| Gastric ulcer | 9 (20) |
| Paraesophageal hernia | 7 (16) |
| Peritoneal adhesion | 11 (25) |
| Sliding hernia | 10 (23) |
The diagnosis of GV was confirmed by barium study in all patients. The performance of other diagnostic modalities is summarized in Table 3. Plain abdominal and thoracic radiography each suggested GV in one patient, but neither was diagnostic. Sonography failed to disclose clues of GV in any patient. Among the 25 patients who underwent upper GI endoscopy, six (24%) were suspected and two (8%) were confirmed to have GV.
| Ordered | Suggestive | Diagnostic | Unrevealing | |
| Chest radiography | 34 | 2 | 0 | 32 |
| Abdominal radiography | 30 | 2 | 0 | 28 |
| Abdominal sonography | 24 | 0 | 0 | 24 |
| Upper GI endoscopy | 25 | 6 | 2 | 17 |
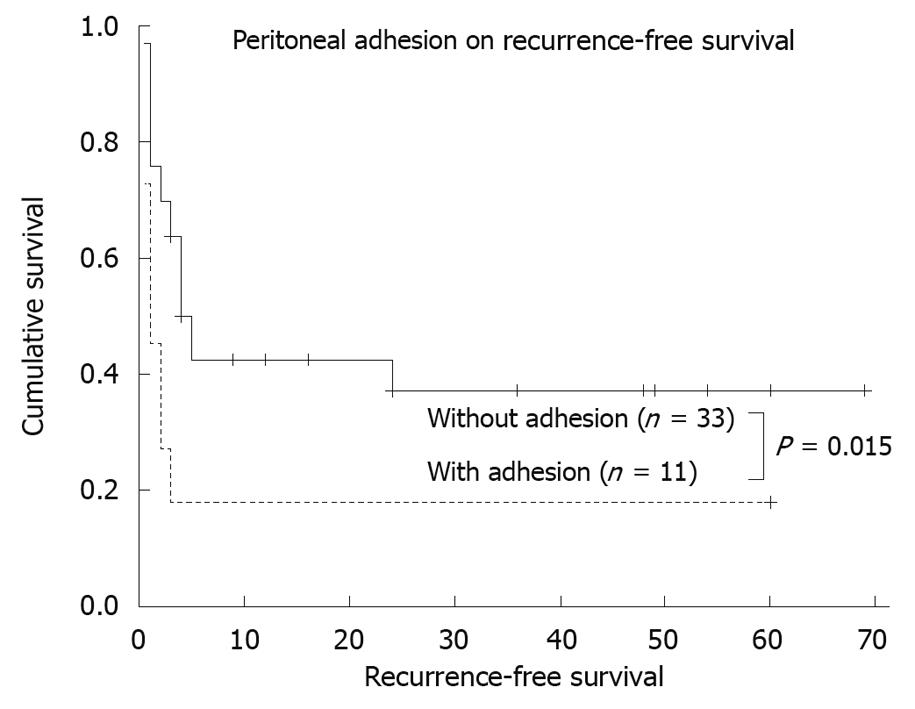
Probable risk factors for recurrence, as determined by univariate analysis, are presented in Table 4. Factors associated with recurrence included longer duration between onset of symptoms and diagnosis (divided by 1.6 mo, P = 0.065), previous thoracic or abdominal operation (P = 0.061), and peritoneal adhesion (P = 0.015, Figure 2). The multivariate regression analysis (Cox proportional hazards model) indentified that peritoneal adhesion (hazard ratio: 2.58, 95% CI: 1.08-6.13, P = 0.033) was independently associated with recurrence.
| Dichotomous variable | Patient number | Median recurrence-free period, mo (95% CI) | P value | |
| Age (yr) | < 70/≥ 70 | 22/22 | 4 (2.2-5.8)/4 (2.9-5.1) | 0.798 |
| Sex | M/F | 37/7 | 4 (2.2-5.8)/3 (0.4-5.6) | 0.158 |
| Delayed diagnosis1 | Yes/no | 20/24 | 2 (0.2-3.8)/24 (NA) | 0.065 |
| Presenting symptoms | ||||
| Abdominal fullness | Yes/no | 24/20 | 2 (0.6-3.4)/5 (3.0-7.0) | 0.101 |
| Abdominal pain | Yes/no | 25/19 | 4 (2.5-5.5)/4 (0-27.0) | 0.751 |
| Acid regurgitation | Yes/no | 9/35 | 5 (1.5-8.5)/4 (2.7-5.3) | 0.680 |
| Non-cardiac chest pain | Yes/no | 18/26 | 2 (0.6-3.4)/5 (3.6-6.4) | 0.096 |
| Dysphagia | Yes/no | 8/36 | 3 (0-7.2)/4 (2.9-5.1) | 0.620 |
| Dyspnea | Yes/no | 9/35 | 4 (NA)/4 (2.6-5.4) | 0.218 |
| Heartburn | Yes/no | 10/34 | 2 (0-4.1)/4 (2.3-5.7) | 0.079 |
| Nausea | Yes/no | 17/27 | 5 (2.9-7.1)/4 (2.8-5.2) | 0.810 |
| Vomiting | Yes/no | 5/39 | 4 (1.9-6.1)/4 (2.3-5.7) | 0.580 |
| Hematemesis | Yes/no | 2/42 | 2 (NA)/4 (2.4-5.6) | 0.332 |
| Previous thoracic or abdominal surgery | Yes/no | 10/34 | 3 (2.1-3.9)/5 (3.3-6.7) | 0.061 |
| Reflux esophagitis | Yes/no | 14/30 | 4 (2.2-5.8)/4 (1.7-6.2) | 0.466 |
| Diaphragm eventration | Yes/no | 13/31 | 3 (0.6-5.4)/5 (3.0-7.0) | 0.126 |
| Diaphragm defect | Yes/no | 8/36 | 4(2.7-5.3)/4 (2.4-5.6) | 0.755 |
| Paraesophageal hernia | Yes/no | 7/37 | 4 (1.4-6.6)/3 (1.4-4.6) | 0.828 |
| Sliding hernia | Yes/no | 10/34 | 4 (2.5-5.5)/3 (0.4-5.3) | 0.510 |
| Peritoneal adhesion | Yes/no | 11/33 | 1 (0-2.0)/5 (3.3-6.7) | 0.015 |
| Choledocholithiasis | Yes/no | 9/35 | 2 (0.5-3.4)/4 (2.7-5.3) | 0.101 |
| Diabetes mellitus | Yes/no | 5/39 | 1 (NA)/4 (1.9-6.1) | 0.307 |
| Gastric ulcer | Yes/no | 9/35 | 4 (1.1-6.9)/4 (1.8-6.2) | 0.926 |
We demonstrate that conservative treatment with watchful observation appears to be a safe therapeutic option in patients with chronic GV. None of the study subjects develop devastating complications during the follow-up period (up to 69 mo), and only one out of 44 patients turned to elective surgery. Nevertheless, symptom recurrence is a common feature in the natural history of chronic GV. This study revealed that 64% of the patients without anatomical correction experienced recurrent symptoms within a median follow-up period of 16 mo. We also identified peritoneal adhesion as an independent risk factor associated with recurrence.
Chronic GV is a disease of recurrent non-specific symptoms in the absence of immediate life-threatening complications. The potential risk of acute strangulation and its associated high mortality rate is regarded as the major indication for surgery[4,12]. Nevertheless, the natural history of chronic GV is poorly understood, and no study has addressed the incidence of chronic GV transforming into acute GV. Catastrophic complications of acute GV result from vascular compromise secondary to strangulation, therefore, transient or partial volvulus might not suffice to bring about severe vascular insufficiency that causes subsequent gastric infarction. With ligamentous attachment, a twisting stomach can spontaneously reduce after intermittent rotation[1]. Therefore, sustained and complete volvulus might not occur in the majority of chronic GV patients as frequently as it was often feared. Consistent with our finding, Al-Salem et al[20] have reported successful conservative treatment in 11 pediatric patients with chronic GV, and have concluded that those with mild to moderate symptoms should be treated conservatively. Similarly, in a large series conducted by Teague et al[4], clinical outcomes were uneventful in the two patients treated conservatively.
Diagnosis of chronic GV is difficult and requires a high index of suspicion and confirmatory upper GI barium study. In the present study, most patients presented with abdominal fullness, abdominal pain, chest pain, and nausea, all of which were non-specific and could be easily misdiagnosed as dyspepsia, peptic ulcer, gastroesophageal reflux disease, or other conditions. Furthermore, usual initial examinations for patients with similar symptoms were not diagnostic (Table 3). Plain radiography might be suggestive in a few patients[21,22], but generally it fails to uncover GV below the diaphragm. Abdominal sonography was not helpful in our study, and did not reveal GV in any of our patients. Even upper GI endoscopy cannot be regarded as an ideal diagnostic tool, because we demonstrated that, in the 25 patients who underwent endoscopy, the diagnosis of GV was missed in 17 (68%). Our results were consistent with previous studies, which reported upper GI barium examination as the most accurate diagnostic procedure, followed by endoscopy[4,6]. As a result of the poor performance of routine examinations, patients with chronic GV might remain undiagnosed unless a barium study is conducted, which is not usually part of routine work-up. As a result, many, and probably most of the chronic GV patients are unrecognized, and the prevalence of this disease is underestimated[23]. Our finding of the infrequent transformation from chronic GV to an emergency situation lends additional support to this postulation, because misdiagnosis would be easy with only mild to moderate, non-specific symptoms.
Our study demonstrates that recurrence is a major feature in the natural history of chronic GV. The median recurrence-free period was only 3 mo. In fact, this was likely to have been an underestimate because mild or unreported symptoms were not categorized as recurrence for the purpose of this study. We further identified peritoneal adhesion as an independent risk factor associated with recurrence. Adhesion within the abdomen might predispose to GV by acting as an axis of rotation[7], and might also hamper spontaneous gastric reduction. Although it has long been reported that chronic GV recurs repeatedly if it is not anatomically corrected[8,9,24], this is believed to be the first study to explore specifically the incidence and risk factors of recurrence. Whether peritoneal adhesion heralds higher risk of gastric strangulation is a serious concern, but was not observed in this study. More studies are necessary to uncover the predictors of acute complications for chronic GV.
Laparoscopic surgery has recently become the trend, with encouraging results for anatomical correction of GV[4,5,25]. Accordingly, management of each chronic GV patient should be carefully individualized. It is important for clinicians to take patients’ age, comorbidity, physical performance, life expectancy, and willingness into consideration. On one hand, chronic GV seems to result in acute complications uncommonly, but on the other, it recurs very frequently. Our results should not be misinterpreted to suggest that we undertook vigilant observation appropriate for every patient with chronic GV, because we did not correct the underlying pathology and recurrence was very common. With current evidence, we suggest that laparoscopic surgery can be regarded as the treatment of choice in good surgical candidates. However, conservative treatment with watchful follow-up appears as a safe alternative for those unwilling to accept or who are unsuitable for invasive procedures. Randomized trials might be necessary to elucidate the most appropriate management in chronic GV patients with different surgical risks, but they are very difficult to conduct.
Several limitations of this study should be noted. First of all, this was an observational study, rather than a comparative trial. We might have demonstrated that conservative management of chronic GV was not a risky alternative, but we did not claim conservative treatment was better than surgery. Second, the retrospective design precluded a standardized protocol of management. For example, not all patients underwent all kinds of diagnostic examinations, and their respective pharmacotherapy was different. Nevertheless, because the primary aim of this study was to explore outcome of conservative management, instead of the efficacy of any specific medication, our conclusion would not have been changed even if we had controlled the medical therapy. Third, the sample size of 44 patients might not permit detection of more predictors for adverse outcomes, although this study was actually a relatively large series of chronic GV patients, in view of the rarity of this disease. Furthermore, since there was no acute complication in our study, it might be controversial whether symptomatic recurrence should be considered as a poor clinical outcome or simply an inevitable part of the natural history. Finally, this was a hospital-based study that consisted of patients with established diagnosis. For reasons of diagnostic difficulty, we assumed there were many undiagnosed chronic GV patients whom we did not have chance to investigate.
In conclusion, clinical presentation of chronic GV is not specific and correct diagnosis remains a challenge. With poor performance of other modalities, upper GI barium study remains the diagnostic tool of choice and should be performed in appropriate settings. Our study reveals that symptomatic recurrence is very common and is independently associated with peritoneal adhesion in chronic GV patients, without anatomical correction. Nevertheless, conservative treatment might be regarded as a safe alternative to surgery, because acute complications infrequently occur in these patients.
Chronic gastric volvulus (GV) usually presents with recurrent non-specific symptoms, but can potentially lead to acute strangulation of the stomach. The natural history of chronic GV remains unknown, and the clinical outcomes of patients managed conservatively without anatomical correction have not been investigated.
It is debatable whether anatomical correction is necessary for all patients with chronic GV, who are usually at an advanced age, with comorbidity.
Symptomatic recurrence is common and independently associated with peritoneal adhesion for chronic GV without anatomical correction, although devastating complications infrequently occur.
Conservative treatment with vigilant follow-up can be regarded as a safe alternative in those patients unwilling to accept or who are unsuitable for invasive procedures.
The aim of the present study was to investigate the natural history of chronic GV in elderly patients. The most significant finding that the authors reported was that peritoneal adhesion was an important risk factor associated with symptomatic recurrence of GV. In addition, the authors have provided some new important data with regard to symptom recurrence in the natural history of GV.
Peer reviewer: Cuong D Tran, PhD, Research Fellow, Affiliate Lecturer, University of Adelaide, Gastroenterology Unit, Children, Youth and Women’s Health Service, 72 King William Rd, North Adelaide, SA 5006, Australia
S- Editor Wang JL L- Editor Kerr C E- Editor Lin YP
| 1. | Dalgaard JB. Volvulus of the stomach case report and survey. Acta Chir Scand. 1952;103:131-153. |
| 3. | Wastell C, Ellis H. Volvulus of the stomach. A review with a report of 8 cases. Br J Surg. 1971;58:557-562. |
| 4. | Teague WJ, Ackroyd R, Watson DI, Devitt PG. Changing patterns in the management of gastric volvulus over 14 years. Br J Surg. 2000;87:358-361. |
| 5. | Palanivelu C, Rangarajan M, Shetty AR, Senthilkumar R. Laparoscopic suture gastropexy for gastric volvulus: a report of 14 cases. Surg Endosc. 2007;21:863-866. |
| 6. | Gourgiotis S, Vougas V, Germanos S, Baratsis S. Acute gastric volvulus: diagnosis and management over 10 years. Dig Surg. 2006;23:169-172. |
| 7. | Carter R, Brewer LA 3rd, Hinshaw DB. Acute gastric volvulus. A study of 25 cases. Am J Surg. 1980;140:99-106. |
| 8. | Patel NM. Chronic gastric volvulus: report of a case and review of literature. Am J Gastroenterol. 1985;80:170-173. |
| 9. | Shivanand G, Seema S, Srivastava DN, Pande GK, Sahni P, Prasad R, Ramachandra N. Gastric volvulus: acute and chronic presentation. Clin Imaging. 2003;27:265-268. |
| 10. | Pistocchi GF. [Radiologic aspects of gastric volvulus]. Ann Radiol Diagn (Bologna). 1966;39:30-60. |
| 11. | Kontorinis N, Waters TE, Zimmerman M, Kaard A. Images of interest. Gastrointestinal: gastric volvulus. J Gastroenterol Hepatol. 2001;16:227. |
| 12. | Haas O, Rat P, Christophe M, Friedman S, Favre JP. Surgical results of intrathoracic gastric volvulus complicating hiatal hernia. Br J Surg. 1990;77:1379-1381. |
| 13. | Tsang TK, Johnson YL, Pollack J, Gore RM. Use of single percutaneous endoscopic gastrostomy in management of gastric volvulus in three patients. Dig Dis Sci. 1998;43:2659-2665. |
| 14. | Tsang TK, Walker R, Yu DJ. Endoscopic reduction of gastric volvulus: the alpha-loop maneuver. Gastrointest Endosc. 1995;42:244-248. |
| 15. | Wasselle JA, Norman J. Acute gastric volvulus: pathogenesis, diagnosis, and treatment. Am J Gastroenterol. 1993;88:1780-1784. |
| 16. | Borchardt M. Zun pathologie and therapie des magnevolvulus. Arch Klin Chir. 1904;74:243-248. |
| 17. | Jeyarajah R, Harford WV. Abdominal Hernias and Gastric Volvulus. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 8th ed. Philadelphia: Saunders 2006; 477-482. |
| 18. | Shah-Mirany J, Schmitz GL, Watson RR. Eventration of the diaphragm. Physiologic and surgical significance. Arch Surg. 1968;96:844-850. |
| 19. | Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260-273. |
| 20. | Al-Salem AH. Acute and chronic gastric volvulus in infants and children: who should be treated surgically? Pediatr Surg Int. 2007;23:1095-1099. |
| 21. | Menuck L. Plain film findings of gastric volvulus herniating into the chest. AJR Am J Roentgenol. 1976;126:1169-1174. |
| 22. | Scott RL, Felker R, Winer-Muram H, Pinstein ML. The differential retrocardiac air-fluid level: a sign of intrathoracic gastric volvulus. Can Assoc Radiol J. 1986;37:119-121. |
| 23. | Llaneza PP, Salt WB 2nd. Gastric volvulus. More common than previously thought? Postgrad Med. 1986;80:279-283, 287-288. |
| 24. | King FP, Bell WH Jr. Gastric volvulus: a case report of organo-axial volvulus with recovery. Ann Intern Med. 1958;48:676-685. |
| 25. | Shono Y, Tsuji T, Horiuchi T, Inoue M, Tabuse K. Laparoscopic gastropexy for chronic gastric volvulus. Hepatogastroenterology. 2007;54:655-656. |