Published online Sep 7, 2010. doi: 10.3748/wjg.v16.i33.4145
Revised: May 3, 2010
Accepted: May 10, 2010
Published online: September 7, 2010
AIM: To demonstrate the therapeutic effect of probiotics in patients with ulcerative colitis (UC), and their effect on inflammatory mediators and nuclear factor (NF)-κB activation in these patients.
METHODS: Thirty patients with mild to moderate UC were randomly classified into two groups: sulfasalazine group, who received sulfasalazine 2400 mg/d; and probiotic group, who received sulfasalazine 2400 mg/d with probiotic. The patients were investigated before and after 8 wk of treatment with probiotic (Lactobacillus delbruekii and Lactobacillus fermentum). Colonic activity of myeloperoxidase (MPO) was assayed with UV spectrophotometry, the colonic content of interleukin (IL)-6 was determined by enzyme-linked immunosorbent assay (ELISA), fecal calprotectin was determined by ELISA, and expression of NF-κB p65 and tumor necrosis factor (TNF)-α proteins in colonic tissue was identified by immunohistochemistry and reverse transcription polymerase chain reaction, respectively.
RESULTS: At the start of the study, colonic mucosal injury and inflammation were demonstrated in UC patients by hematoxylin and eosin staining, and an increase in colonic MPO activity, fecal calprotectin, and expression of colonic TNF-α and NF-κB p65 proteins. The use of probiotic for 8 wk significantly ameliorated the inflammation by decreasing the colonic concentration of IL-6, expression of TNF-α and NF-κB p65, leukocyte recruitment, as demonstrated by a decrease in colonic MPO activity, and the level of fecal calprotectin compared to sulfasalazine group and the control group (P < 0.05).
CONCLUSION: Oral supplementation with probiotics could be helpful in maintaining remission and preventing relapse of UC.
- Citation: Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J Gastroenterol 2010; 16(33): 4145-4151
- URL: https://www.wjgnet.com/1007-9327/full/v16/i33/4145.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i33.4145
Ulcerative colitis (UC) is one of the two major forms of inflammatory bowel disease (IBD), and is a chronic and relapsing inflammatory condition that is characterized by colonic tissue edema, increased colonic epithelial permeability, and extensive infiltration of leukocytes in the colon[1]. The pathogenic mechanism of UC involves dysregulation of the intestinal immune response to intestinal environmental antigens, such as intestinal microflora[2-4]. UC occurs in the colon where many intestinal microbes reside[5,6]; it does not significantly develop or progress in germ-free animals, which indicates that intestinal microflora play an important role in initiating and perpetuating colonic inflammation. Normal intestinal microflora comprises an estimated 400 different bacterial species that reach their highest concentrations in the terminal ileum and colon[7,8]. Intestinal microflora produces toxic compounds, such as Gram-negative bacterial endotoxin, and harmful enzymes, such as β-glucuronidase and tryptophanase, which produce cytotoxic or carcinogenic agents[9-11]. Cytotoxins and endotoxins might interact at the apical intestinal surface and induce responses in intestinal epithelial cells, which produce pro-inflammatory cytokines and other mediators that induce inflammatory activation of the mucosal immune system.
At present, medical treatment of UC relies mainly on traditional drugs: aminosalicylates, corticosteroids, and immunosuppressants. These drugs reduce inflammatory injury and attenuate the expression of some pro-inflammatory molecules, but their side effects and systemic activity severely disturb the quality of life of patients, particularly during long-term treatment[12]. Manipulation of the mucosal microbiota to reduce the inflammatory potential of colonizing bacteria is therefore an attractive therapy for UC. One option is to use antibiotics to remove species involved in inducing the inflammatory response[13]. However, antibiotic therapy has had limited success in UC, possibly due to the fact that treatment needs to be customized for individual patients.
An alternative is to use probiotic bacteria that interact with the host epithelium to resolve inflammation. Probiotics have been defined as live microbial food supplements that beneficially affect the host by improving its intestinal microbial balance[14]. The most widely used probiotics in humans are bifidobacteria and lactobacilli. Lactic acid bacteria (LAB) are safe microorganisms that improve disturbances in the indigenous microflora, ameliorate the development of microflora[15], have anti-diabetic and anti-hyperlipidemic effects[16,17], inhibit carcinogenesis[18], have anticolitic effects[18], and induce nonspecific activation of the host’s immune system[19]. Nevertheless, the anticolitic mechanism of LABs has not been thoroughly examined. Therefore, this study was conducted to demonstrate the probable therapeutic effect of probiotics in patients with UC, and to evaluate their effect on the inflammatory mediators and nuclear factor (NF)-κB activation in these patients.
The subjects of this study were selected from UC patients with chronic diarrhea who were seen at the outpatient clinic of Tanta University Hospitals, and some inpatients at the Internal Medicine Department. Patients were newly diagnosed by colonoscopy and biopsy. They were classified as follows: 10 healthy volunteers (six male and four female) as the control group, and 30 patients (23 male and seven female) with mild to moderate UC assessed by Mayo score[20]. Patients were excluded if they had been on corticosteroids or any other immunosuppressant, had colorectal carcinoma, bilharzia, pregnancy, or hepatic or renal dysfunction.
All the patients were subjected to full history taking, thorough clinical examination, endoscopic examination, biopsy and histological examination, and some laboratory investigations (Table 1).
| Parameter | Control (n = 10) | Sulfasalazine group (n = 15) | Probiotic group (n = 15) |
| Age (yr) | 46 ± 1.89 | 48 ± 1.90 | 47 ± 1.59 |
| Sex (M:F) | 6:4 | 11:4 | 12:3 |
| Smoking (yes/no) | 3/7 | 2/13 | 1/14 |
| Mayo score | 0 | 4 | 4 |
| Extent of the disease | |||
| Left side colitis | 0 | 4 | 5 |
| Proctitis | 0 | 5 | 4 |
| Pancolitis | 0 | 6 | 6 |
| ESR (mm/h) | 27 ± 2.1 | 66.3 ± 1.3 | 69.2 ± 1.2 |
| Albumin/globulin ratio | 1.9 ± 0.2 | 1.1 ± 1.3 | 1.2 ± 1.1 |
| Leukocyte count (cells/mm) | 4.3 ± 1.2 | 8.9 ± 1.3 | 8.5 ± 1.1 |
| Hemoglobin (%) | 14 ± 1.5 | 10.5 ± 1.1 | 10.7 ± 1.6 |
| Stool frequency (/d) | 1 | 4 | 4 |
| Blood in stool | - | + | + |
| Fever | - | - | - |
| Pulse rate (/min) | 80 ± 2 | 93 ± 3 | 94 ± 2 |
Informed consent was obtained from all the participants. The protocol of the study was approved by the Ethical Committee of the University.
The patients were randomly sub-divided into two equal groups: the sulfasalazine group, who were treated with oral sulfasalazine 2400 mg/d with placebo (starch) for eight consecutive weeks[21]; and the probiotic group, who were treated with sulfasalazine 2400 mg/d with a probiotic preparation (Lacteol Fort; Rameda, Egypt) for eight consecutive weeks. The probiotic preparation was provided in sachets, which contained powder with 10 billion CFU of Lactobacillus delbruekii and Lactobacillus fermentum, to be dissolved in 50 mL fresh water.
Biopsy samples were obtained from inflamed colonic mucosa. Colon tissues were fixed in 4% paraformaldehyde, dehydrated, paraffin embedded, and stained with hematoxylin and eosin. At the same time, colon samples from the same sites were obtained and snap-frozen at -80°C for subsequent determinations.
Stool samples were collected and suspended in extraction buffer, and homogenized for 25 min. One milliliter of the homogenate was transferred to a tube and centrifuged for 20 min, and the supernatant was collected and frozen at -20°C. Calprotectin was analyzed by enzyme-linked immunosorbent assay (ELISA)[22].
Colon tissues were weighed and homogenized in a solution that contained 0.5% hexadecyl trimethyl ammonium bromide dissolved in 10 mmol/L phosphate buffer (pH 7), and centrifuged for 30 min (20 000 g) at 4°C. An aliquot (50 μL) of the supernatant was added to a reaction mixture of 1.6 mmol/L tetramethylbenzidine and 0.1 mmol/L H2O2, and incubated at 37°C. The absorbance was obtained at 460 nm. Myeloperoxidase (MPO) activity was defined as the quantity of enzyme that degraded 1 μmol/mL of peroxide at 37°C, expressed in units per gram wet tissue[23].
Colon tissues were minced, suspended in 2 mL 10 mmol/L phosphate buffer (pH 7.4) and incubated in a shaking water bath (37°C) for 20 min. The samples were then centrifuged (9000 g for 30 s), and the supernatant was kept at -70°C until interleukin (IL)-6 ELISA[24].
Expression of NF-κB p65 in colonic tissue was assessed by immunohistochemistry. Tissue sections were deparaffinized in xylene and rehydrated in a descending ethanol series. After dewaxing and rehydration, antigen retrieval was done by microwave for 15 min. Endogenous peroxidase activity was blocked by 20 min incubation in 3% H2O2 in methanol at room temperature. The sections were incubated with 1:100 diluted specific polyclonal rabbit anti-rat NF-κB p65 serum (NeoMarkers, Fremont, CA, USA) for 12 h at 4°C, or incubated with 1:100 diluted normal rabbit serum under the same conditions as the negative control. After phosphate buffered saline (PBS) washing, the slides were incubated with a biotinylated horseradish-peroxidase-conjugated secondary antibody and 0.1% diaminobenzidine substrate. Sections prepared by substituting PBS for the primary antibody served as the negative control. Positive expression of NF-κB p65 was shown by brown deposited granules in the cytoplasm and/or nucleus. The results were evaluated semi-quantitatively according to the percentage of positive cells in 10 randomly selected fields under high power microscopy (700 × magnification).
mRNA expression for tumor necrosis factor (TNF)-α was assessed using reverse transcription polymerase chain reaction (RT-PCR) standardization by co-amplification of the housekeeping gene β-actin, which served as an internal control. Total RNA from colonic tissues was isolated using Trizol reagent (Sigma, St. Louis, MO, USA) by the single step method, and was reverse transcribed into cDNA. The resultant cDNA was used as template for subsequent PCR. The rat-specific primers (sense and antisense primers) for TNF-α and β-actin were 5′-CATGATCCGAGATGTGGAACTGGC-3′ and 5′-CTGGCTCAGCCACTCCAGC-3′ (TNF-α, 315 bp) and 5′-ATGGATGACGATATCGCTG-3′ and 5′-ATGAGGTAGTCTGTCAGGT-3′ (β-actin, 568 bp), respectively. Amplification was performed in 30 cycles, with initial incubation at 95°C for 3 min and final extension at 72°C for 7 min; each cycle consisted of denaturation for 30 s at 95°C, annealing for 45 s at 55°C, and extension for 1 min at 72°C. PCR products were separated in 2% agarose gel and stained with ethidium bromide.
Data were statistically analyzed by paired Student’s t test to compare between the results before (baseline) and after treatment within the same group, and unpaired Student’s t test to compare between means of the different groups using the computer program SPSS for Windows version 10 (Chicago, IL, USA). All results were expressed as mean ± SD. The level of significance was set at P < 0.05.
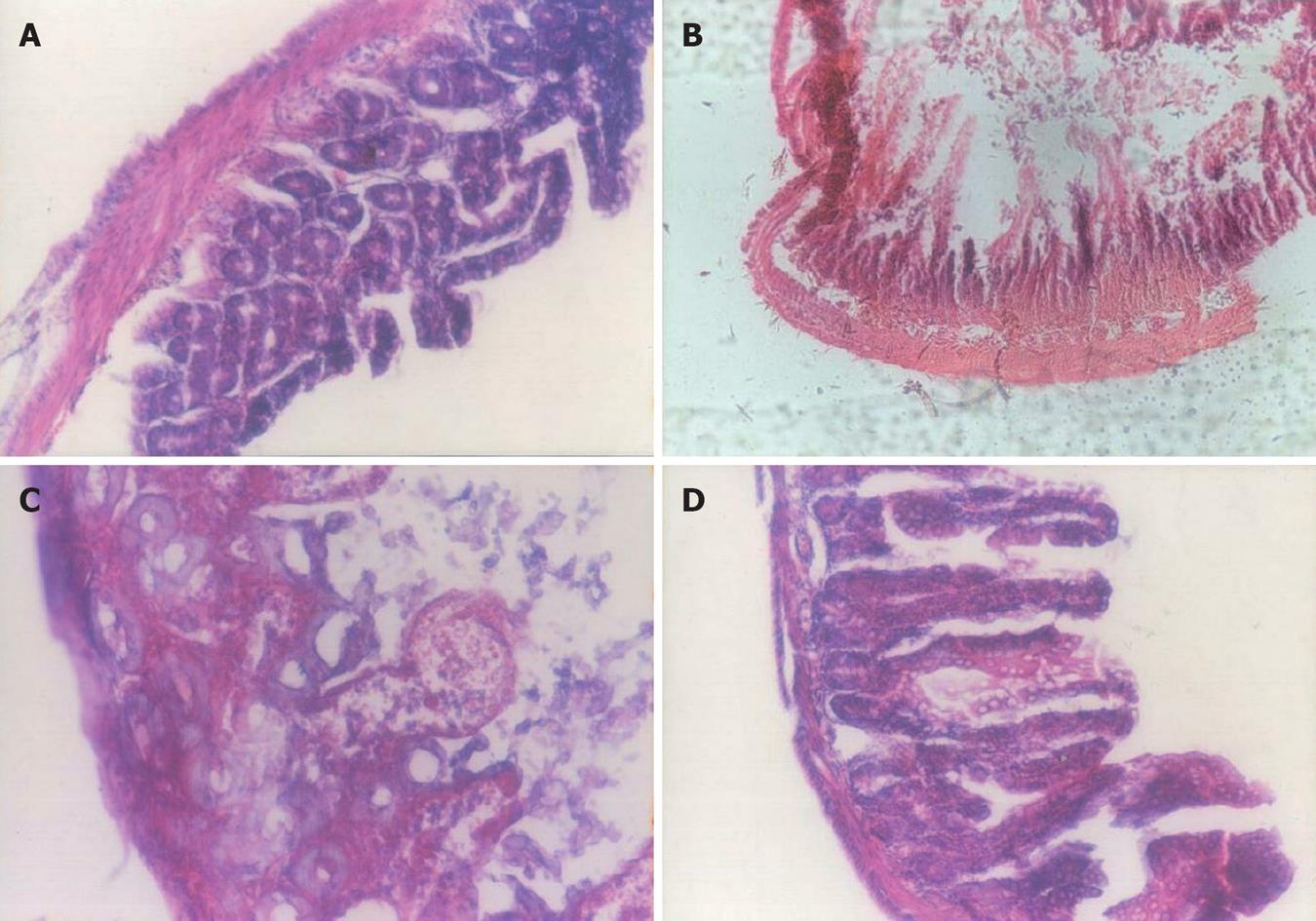
The histological findings of colonic tissues are presented in Figure 1. There was erosion in the mucosa and submucosa, mucosal edema, vascular congestion, focal hemorrhage, and infiltration of polymorphonuclear cells, plasma cells and neutrophils. No changes were observed in the control group. The sulfasalazine group showed attenuation of the extent and severity of the histological signs. Administration of probiotic plus oral sulfasalazine inhibited the extent of inflammation, prevented mucosal injury, and alleviated colitis.
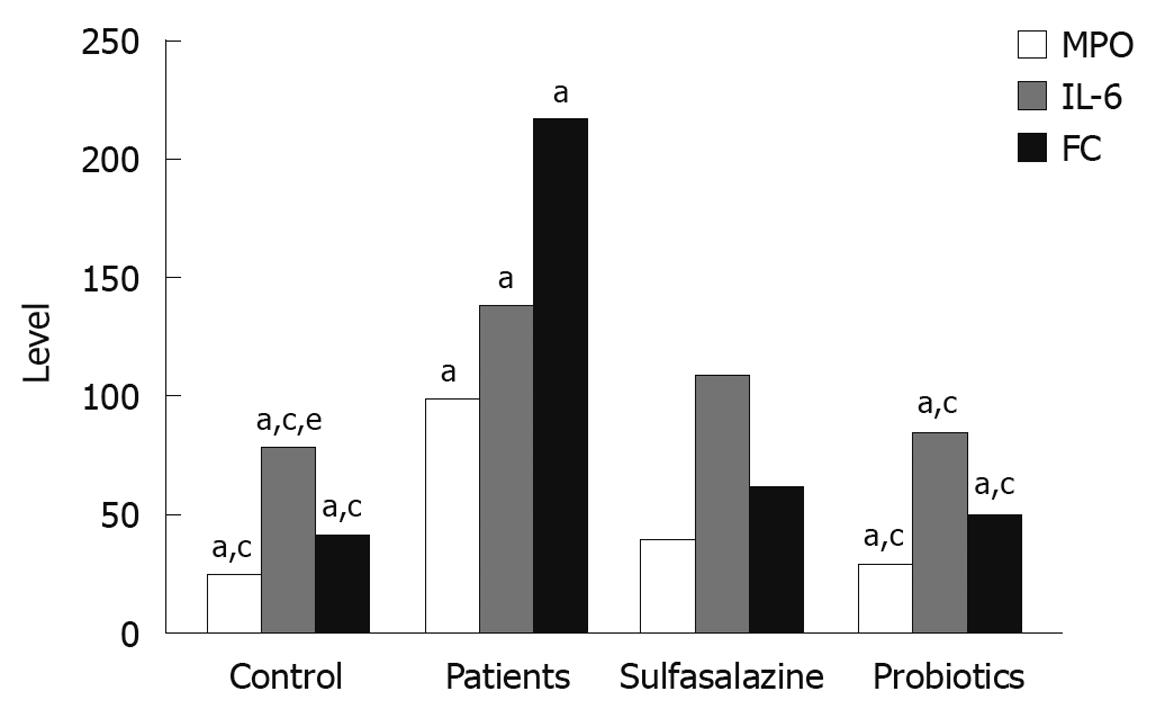
Evaluation of leukocyte recruitment was assessed by measurement of MPO activity. Compared to the control group, MPO activity was significantly increased in the colonic mucosa of UC patients before treatment. Administration of oral sulfasalazine with probiotic decreased MPO activity. The decrease was most significant in the probiotic group (P < 0.05, Figure 2).
As shown in Figure 2, a significant increase in the level of IL-6 was observed in the colon of UC patients. Both the sulfasalazine and probiotic groups showed a significant decrease in the level of IL-6 compared to that before treatment (P < 0.05).
Fecal calprotectin level was significantly higher in UC patients at the beginning of the study. Both the sulfasalazine and probiotic groups showed a significant decrease in calprotectin level compared to the UC patients, and the decrease in the probiotic group was significantly greater than that in the sulfasalazine group (P < 0.05, Figure 2).
Calprotectin showed a significant positive correlation with both MPO (r = 0.93, P < 0.05) and IL-6 (r = 0.91, P < 0.05).
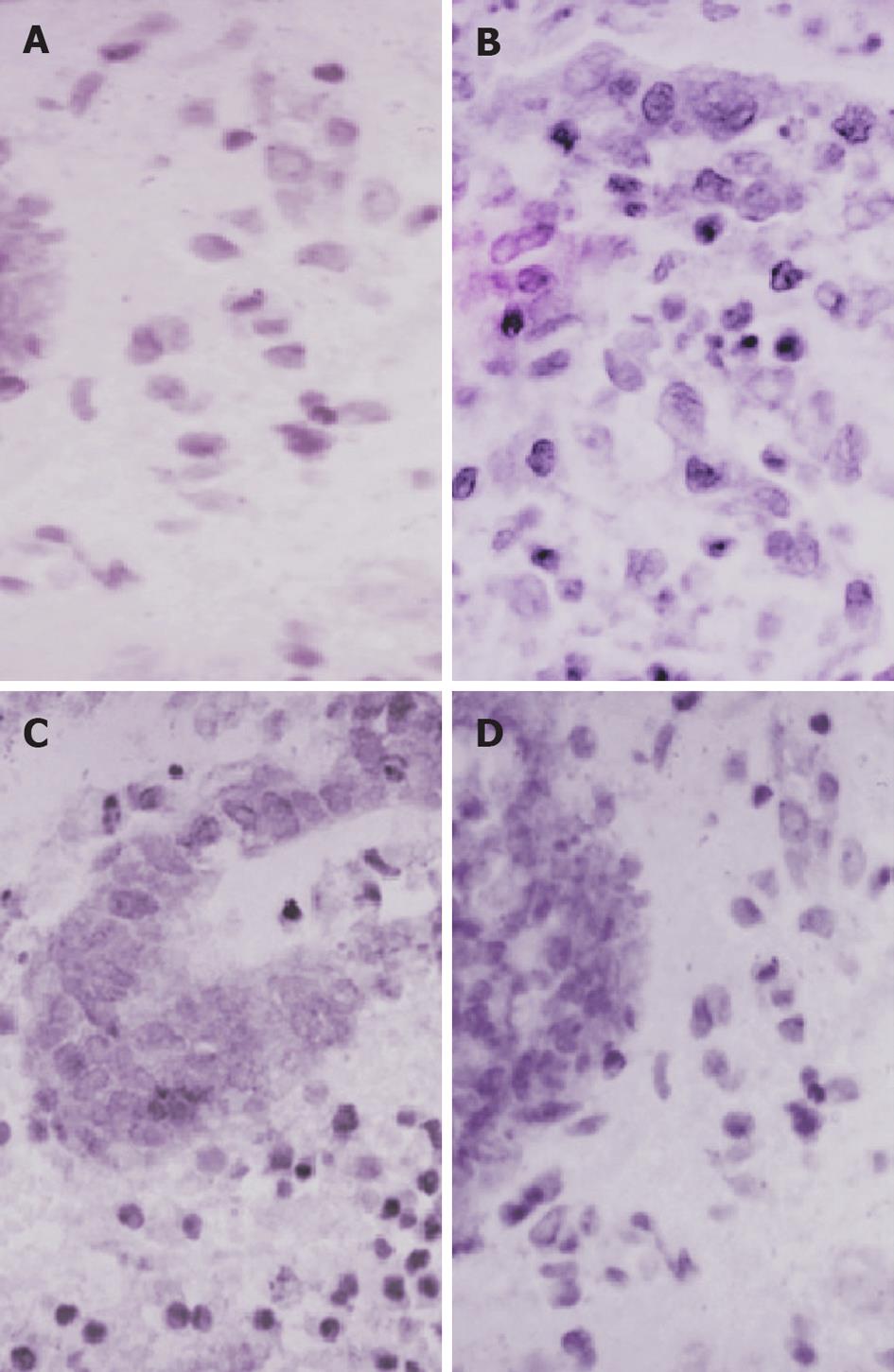
Expression of NF-κB p65 in UC patients before treatment was significantly higher than that in the control group. NF-κB-p65-positive cells were predominantly located within the mucosa and had a brown-yellow cytoplasm. Administration of sulfasalazine resulted in a significant reduction in colonic NF-κB p65 levels. Compared with the patients before treatment, expression of NF-κB p65 in the placebo group weakened significantly, and the expression in the probiotic group was the lowest (Figure 3 and Table 2).
| Reaction | Controls | Patients | Sulfasalazine | Probiotics |
| NF-κB | + (6) | ++++ (6) | +++ (4) | ++ (2) |
| Immunostaining | ++ (2) | + (4) |
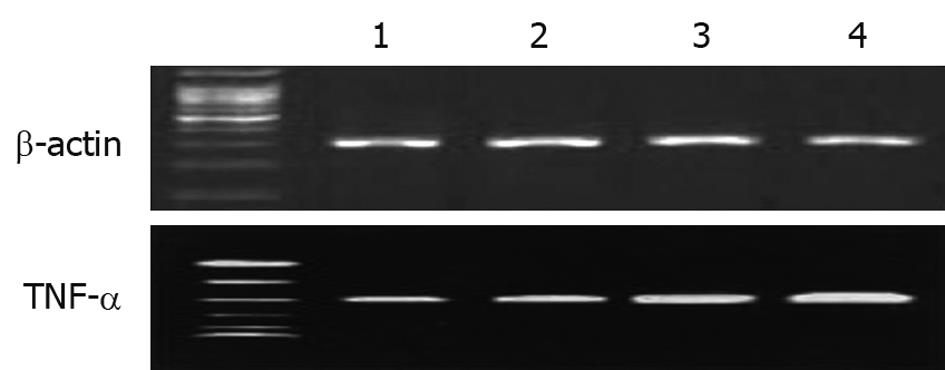
The m-RNA level of TNF-α in UC patients at the beginning of the study was significantly higher than in the control group. TNF-α m-RNA expression was inhibited after treatment with oral sulfasalazine and probiotic. Maximum inhibitory effect was observed with the probiotic preparation (Figure 4).
The pathogenesis of UC remains unknown[25]. Genetic and environmental factors are obviously contributory. Luminal bacteria could play a major role in the initiation and perpetuation of chronic UC[26]. Thousands of endogenous bacteria live in the large intestine and might be an essential factor in certain pathological disorders. In human UC, inflammation is present in parts of the gut that house the highest concentration of bacteria. Enhanced mucosal permeability could play a pivotal role in maintaining a chronic inflammatory state due to genetic predisposition or direct contact with bacteria or their products. Manipulation of the colonic bacteria with antibiotics and probiotics has been shown to be more effective and tolerable than immunosuppressants[27].
It is well known that there is an inflammatory cascade within the gut tissues of UC that is characterized by the recruitment of circulating leukocytes into the gut tissues and the release of pro-inflammatory mediators[1]. In the present study, we observed this cascade of inflammatory cells in colitis. First, our results showed that MPO activity, an index of leukocyte infiltration, was increased significantly in UC patients, which suggested recruitment of leukocytes. Second, the increase in the level of fecal calprotectin represented mucosal neutrophil infiltration. Third, the expression of IL-6 and TNF-α protein was upregulated significantly, as demonstrated by ELISA and PCR. These results are supported by some investigators who have shown the development of such a cascade of inflammatory events in dextran-sulfate-sodium-induced colitis in mice[28]. Probiotic administration for 8 wk reduced MPO activity significantly, as well as the expression of IL-6 and TNF-α. These results are in agreement with those of Federico et al[29], who have found that a symbiotic preparation that contained Lactobacillus paracasei B 20160 restored the serum level and mRNA expression of IL-6, IL-8, and TNF-α in UC patients. Other investigators have found that Lactobacillus HY 7801 blocks tri-nitrobenzene-sulfonic-acid-stimulated MPO activity in the intestine, as well as the expression of IL-1β, and TNF-α[30].
A prominent feature of mucosal histology in patients with active IBD is infiltration by neutrophilic granulocytes[31]. Calprotectin is a major protein of neutrophils and macrophages, and accounts for about 60% of the cytosol of these cells. In the present study, there was a good correlation between fecal calprotectin and MPO and IL-6. This result agrees with that of Wagner et al[32], who have found that fecal calprotectin has the potential to be used as a surrogate marker for successful treatment outcome in IBD patients. Also, they have demonstrated that fecal calprotectin and MPO provide superior discrimination to eosinophil protein X in determining treatment outcome in UC patients.
It is well known that NF-κB plays a pivotal role in expression of inflammatory mediators. NF-κB is usually found in the cytoplasm conjugated to an inhibitory protein IκB. Phosphorylation of IκB by IκB kinase after inflammatory signal transduction leads to degradation of IκB via proteasomes, which results in transfer of NF-κB to the nucleus and its activation. NF-κB regulates transcriptional activity by binding to specific DNA sequences in inflammatory genes that are involved in inflammatory and immune processes[33]. In the present study, the expression of NF-κB p65 was detected by immunohistochemistry. Much more NF-κB p65 protein was activated in the UC patients, and the lowest level was observed in the probiotic group. These data were consistent with the finding that Bifidobacterium longum downregulates TNF-α and IL-8 production and inhibits NF-κB activation of lamina propria mononuclear cells in inflamed mucosa of active UC, without any adverse effect on the viability of colonic cells[34].
Recent data have demonstrated that the mucosal immune response is involved in patients with IBD. NF-κB is a key regulator of inducible expression of many genes that are involved in immune and inflammatory responses in the gut. Stimuli like cytokines (IL-6, TNF-α), bacteria, and viruses can release NF-κB from their cytoplasmic form to the nuclei. NF-κB can activate anti-apoptic genes, including TNF-receptor-related genes, Bcl-2 homologenes, and repress the apoptosis of some inflammatory cells such as neutrophils and activated macrophages, thereby elongating and worsening tissue inflammatory injury[35]. More potent and selective treatment strategies with anti-sense p65 and proteasome inhibitors have been aimed at preventing NF-κB activation in mucosal macrophages and T lymphocytes. However, NF-κB-regulated genes are also involved in survival responses of epithelial cells. Selective inhibition of NF-κB activation in inflammatory cells could be an option for management of IBD[36].
In the present study, administration of probiotics not only decreased the NF-κB DNA binding activity, but also reduced the accumulation of leukocytes, and downregulated IL-6 and TNF-α production, and thereby ameliorated the severity of the colitis. Therefore, supplementation with probiotics could be helpful in maintaining remission and preventing relapse of UC. These results are in agreement with those of Nikfar et al[37] who demonstrated that probiotics can improve the symptoms of irritable bowel syndrome, and can be used as a supplement to standard therapy. Also, other investigators[38,39] have demonstrated the efficacy of probiotics in maintaining remission of human UC and prevention of disease relapse.
Medical treatment of ulcerative colitis (UC) relies mainly on traditional drugs: aminosalicylates, corticosteroids, and immunosuppressants. These drugs reduce inflammatory injury but their side effects and systemic activity severely disturb the quality of life of patients severely, particularly during long-term treatment. An alternative is to use probiotic bacteria that interact with the host epithelium to resolve inflammation. The aim of the present study was to demonstrate the therapeutic effect of probiotics in patients with UC, and to evaluate their effect on the inflammatory mediators and nuclear factor (NF)-κB activation in these patients.
Various in vitro studies have been performed in attempts to suppress the inflammation in experimentally-induced UC. They have suggested that suppression of the activity of NF-κB could control the production of several inflammatory mediators. The present study was undertaken to evaluate the therapeutic role of probiotics in UC patients and their effect on the inflammatory mediators and NF-κB activation in these patients.
The present study suggested that administration of probiotics not only decreased NF-κB DNA binding activity, but also reduced the accumulation of leukocytes, downregulated interleukin-6 and tumor necrosis factor-α production, and thereby ameliorated the severity of the colitis.
These findings suggest that supplementation with probiotics could be helpful in maintaining remission and preventing the relapse of UC. Probiotics are safe microorganisms that protect patients from the side effects of medical treatment that disturbs quality of life.
It is an important paper and can be published after making some revisions.
Peer reviewers: Mohammad Abdollahi, Professor, Faculty of Pharmacy and Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences, Tehran 1417614411, Iran; Laura E Matarese, MS, RD, LDN, FADA, CNSD, Thomas E. Starzl Transplantation Institute, UPMC Montefiore, 7 South, 3459 Fifth Avenue, Pittsburgh, PA 15213, United States
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
| 1. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. |
| 2. | Benno P, Leijonmarck CE, Monsén U, Uribe A, Midtvedt T. Functional alterations of the microflora in patients with ulcerative colitis. Scand J Gastroenterol. 1993;28:839-844. |
| 3. | Berrebi D, Languepin J, Ferkdadji L, Foussat A, De Lagausie P, Paris R, Emilie D, Mougenot JF, Cezard JP, Navarro J. Cytokines, chemokine receptors, and homing molecule distribution in the rectum and stomach of pediatric patients with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2003;37:300-308. |
| 4. | Gorbach SL, Nahas L, Plaut AG, Weinstein L, Patterson JF, Levitan R. Studies of intestinal microflora. V. Fecal microbial ecology in ulcerative colitis and regional enteritis: relationship to severity of disease and chemotherapy. Gastroenterology. 1968;54:575-587. |
| 5. | Binder V. Epidemiology of IBD during the twentieth century: an integrated view. Best Pract Res Clin Gastroenterol. 2004;18:463-479. |
| 6. | Chandran P, Satthaporn S, Robins A, Eremin O. Inflammatory bowel disease: dysfunction of GALT and gut bacterial flora (II). Surgeon. 2003;1:125-136. |
| 8. | Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology. 1984;86:174-193. |
| 9. | Chung KT, Fulk GE, Slein MW. Tryptophanase of fecal flora as a possible factor in the etiology of colon cancer. J Natl Cancer Inst. 1975;54:1073-1078. |
| 10. | Ganguly NK, Kingham JG, Lloyd B, Lloyd RS, Price CP, Triger DR, Wright R. Acid hydrolases in monocytes from patients with inflammatory bowel disease, chronic liver disease, and rheumatoid arthritis. Lancet. 1978;1:1073-1075. |
| 11. | Rhodes JM, Gallimore R, Elias E, Allan RN, Kennedy JF. Faecal mucus degrading glycosidases in ulcerative colitis and Crohn's disease. Gut. 1985;26:761-765. |
| 12. | Katz JA. Advances in the medical therapy of inflammatory bowel disease. Curr Opin Gastroenterol. 2002;18:435-440. |
| 13. | Rahimi R, Nikfar S, Rezaie A, Abdollahi M. A meta-analysis of antibiotic therapy for active ulcerative colitis. Dig Dis Sci. 2007;52:2920-2925. |
| 14. | Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O'neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242-249. |
| 15. | Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. A. m J Clin Nutr. 1999;69:1052S-1057S. |
| 16. | Tabuchi M, Ozaki M, Tamura A, Yamada N, Ishida T, Hosoda M, Hosono A. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2003;67:1421-1424. |
| 17. | Taranto MP, Medici M, Perdigon G, Ruiz Holgado AP, Valdez GF. Evidence for hypocholesterolemic effect of Lactobacillus reuteri in hypercholesterolemic mice. J Dairy Sci. 1998;81:2336-2340. |
| 18. | Perdigon G, de Jorrat WEB, de Petrino SF, Valerde de Budeguer M. Effect of oral administration of Lactobacillus casei on various biological functions of the host. Food Agric Immunol. 1991;3:93-102. |
| 19. | Peran L, Sierra S, Comalada M, Lara-Villoslada F, Bailón E, Nieto A, Concha A, Olivares M, Zarzuelo A, Xaus J. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr. 2007;97:96-103. |
| 20. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. |
| 21. | Nikfar S, Rahimi R, Rezaie A, Abdollahi M. A meta-analysis of the efficacy of sulfasalazine in comparison with 5-aminosalicylates in the induction of improvement and maintenance of remission in patients with ulcerative colitis. Dig Dis Sci. 2009;54:1157-1170. |
| 22. | Tøn H, Brandsnes , Dale S, Holtlund J, Skuibina E, Schjønsby H, Johne B. Improved assay for fecal calprotectin. Clin Chim Acta. 2000;292:41-54. |
| 23. | Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157-167. |
| 24. | Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29-36. |
| 25. | Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79-94. |
| 27. | Israël A. The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol. 2000;10:129-133. |
| 28. | Zhang DK, Cheng LN, Huang XL, Shi W, Xiang JY, Gan HT. Tetrandrine ameliorates dextran-sulfate-sodium-induced colitis in mice through inhibition of nuclear factor -kappaB activation. Int J Colorectal Dis. 2009;24:5-12. |
| 29. | Federico A, Tuccillo C, Grossi E, Abbiati R, Garbagna N, Romano M, Tiso A, Blanco Cdel V, Loguercio C. The effect of a new symbiotic formulation on plasma levels and peripheral blood mononuclear cell expression of some pro-inflammatory cytokines in patients with ulcerative colitis: a pilot study. Eur Rev Med Pharmacol Sci. 2009;13:285-293. |
| 30. | Lee JH, Lee B, Lee HS, Bae EA, Lee H, Ahn YT, Lim KS, Huh CS, Kim DH. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-kappaB activation in experimental colitis. Int J Colorectal Dis. 2009;24:231-237. |
| 31. | Saverymuttu SH, Camilleri M, Rees H, Lavender JP, Hodgson HJ, Chadwick VS. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986;90:1121-1128. |
| 32. | Wagner M, Peterson CG, Ridefelt P, Sangfelt P, Carlson M. Fecal markers of inflammation used as surrogate markers for treatment outcome in relapsing inflammatory bowel disease. World J Gastroenterol. 2008;14:5584-5589; discussion 5588. |
| 33. | Bai AP, Ouyang Q, Xiao XR, Li SF. Probiotics modulate inflammatory cytokine secretion from inflamed mucosa in active ulcerative colitis. Int J Clin Pract. 2006;60:284-288. |
| 34. | Shi XZ, Lindholm PF, Sarna SK. NF-kappa B activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology. 2003;124:1369-1380. |
| 35. | Bengoechea-Alonso MT, Pelacho B, Osés-Prieto JA, Santiago E, López-Moratalla N, López-Zabalza MJ. Regulation of NF-kappaB activation by protein phosphatase 2B and NO, via protein kinase A activity, in human monocytes. Nitric Oxide. 2003;8:65-74. |
| 36. | Dijkstra G, Moshage H, Jansen PL. Blockade of NF-kappaB activation and donation of nitric oxide: new treatment options in inflammatory bowel disease? Scand J Gastroenterol Suppl. 2002;37-41. |
| 37. | Nikfar S, Rahimi R, Rahimi F, Derakhshani S, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51:1775-1780. |
| 38. | Rahimi R, Nikfar S, Rahimi F, Elahi B, Derakhshani S, Vafaie M, Abdollahi M. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn's disease. Dig Dis Sci. 2008;53:2524-2531. |
| 39. | Elahi B, Nikfar S, Derakhshani S, Vafaie M, Abdollahi M. On the benefit of probiotics in the management of pouchitis in patients underwent ileal pouch anal anastomosis: a meta-analysis of controlled clinical trials. Dig Dis Sci. 2008;53:1278-1284. |